Abstract
Although light is essential for photosynthesis, it has the potential to elevate intracellular levels of reactive oxygen species (ROS). Since high ROS levels are cytotoxic, plants must alleviate such damage. However, the cellular mechanism underlying ROS-induced leaf damage alleviation in peroxisomes was not fully explored. Here, we show that autophagy plays a pivotal role in the selective removal of ROS-generating peroxisomes, which protects plants from oxidative damage during photosynthesis. We present evidence that autophagy-deficient mutants show light intensity-dependent leaf damage and excess aggregation of ROS-accumulating peroxisomes. The peroxisome aggregates are specifically engulfed by pre-autophagosomal structures and vacuolar membranes in both leaf cells and isolated vacuoles, but they are not degraded in mutants. ATG18a-GFP and GFP-2×FYVE, which bind to phosphatidylinositol 3-phosphate, preferentially target the peroxisomal membranes and pre-autophagosomal structures near peroxisomes in ROS-accumulating cells under high-intensity light. Our findings provide deeper insights into the plant stress response caused by light irradiation.
Similar content being viewed by others
Introduction
Photosynthesis in plants converts light energy to chemical energy and is accompanied by photorespiration, which involves peroxisomes, mitochondria, and chloroplasts1. Photorespiration is essential for plant survival under high-intensity light and prevents photoinhibition, which damages photosynthetic machinery owing to excess reactive oxygen species (ROS) accumulation2,3. Thus, it is essential to understand how excess ROS are degenerated to protect plants from oxidative damage during photosynthesis under excess light.
Plants have diverse mechanisms to prevent high ROS accumulation under various conditions4,5,6,7,8,9,10, and the relationship between ROS and autophagy has been reported in the previous studies11,12,13,14. ROS accumulation in peroxisomes inhibits catalase (CAT) activity that detoxifies hydrogen peroxide, leading to the oxidation of peroxisomes5,9,10. We have previously shown that oxidatively damaged peroxisomes are accumulated in autophagy-deficient mutants11,14.
A set of autophagy (ATG) genes has been discovered to be involved in the specific degradation of peroxisomes, namely pexophagy in yeasts and animals5f, g, and Supplementary Fig. 26). This was also seen in isolated vacuoles (Fig. 6 and Supplementary Figs.27). ATG7 plays a role in the maturation of ATG8-PE as a ubiquitin-activating enzyme-like protein for generating autophagosomes56,57. We have previously revealed that ATG8a localises to degraded peroxisomes in atg2(p1) and atg5 as dot structures11,14. In this study, we showed that ATG8e co-localises with ATG18a on peroxisome aggregates in atg2(p1) and atg7(p4) (Supplementary Figs. 6 and 7); thus, suggesting that ATG8 acts in concert with ATG18a. Leaf damage and peroxisome aggregation in atg9 are reduced compared to those in atg2, atg5, and atg7 under high-intensity (Supplementary Figs. 20–22) and normal light conditions11,14, suggesting that the contribution of ATG9 in plant macropexophagy is reduced, unlike in yeast and mammal pexophagy18,19,49,50. ATG9 might not have a specific role in pexophagy, although it is generally required for autophagy in plants.
We discovered that the vacuolar membrane forms large cavities to surround peroxisome aggregates in atg7(p4) under high-intensity light conditions (Fig. 5f–j and Supplementary Fig. 26). Furthermore, some of the peroxisomes and peroxisome aggregates on the surface of the isolated vacuole in wild-type and atg7(p4) cells were also surrounded by the vacuolar membrane (Fig. 6 and Supplementary Figs. 27 and Supplementary Movies. 18–21). These direct actions of the vacuolar membrane indicate the involvement of the microautophagy process during the incorporation of degraded peroxisomes into the vacuole. In plants, microautophagy is induced in sucrose-starved root cells58. Microautphagy contributes to the accumulation of anthocyanin aggregate in vacuoles59 and damaged chloroplast degradation under high-intensity light irradiation60,61. Microautophagic degradation of peroxisomes (micropexophagy) was reported in yeast, where it is accompanied by a micropexophagy-specific apparatus (MIPA)22, but not yet in plants. Taken together, these findings suggest that micropexophagy is induced following high-intensity light exposure resulting in the degradation of oxidized peroxisomes and their aggregates. Since the microautophagic degradation of peroxisome aggregate seems incomplete in atg7(p4), ATG7 and ATG8-PE are probably required for microautophagy.
Collectively, our data suggest that ATG2, ATG5, ATG7, ATG8, and ATG18a work cooperatively to generate complete pexophagosomes and degrade them in vacuoles via macro- and micropexophagy. Based on these results, we propose the following model for macropexophagy (Fig. 7c): (1) peroxisomes with inactive catalase accumulate high levels of ROS, and PtdIns3P is generated on the peroxisome membrane or phagophores formed adjacent to peroxisome and ER; (2) ATG18a targets the PtdIns3P on the damaged peroxisomes; (3) pexophagosomes are formed by ATG18a and PtdIns3P along with other autophagy factors; (4) pexophagosomes completely sequester damaged peroxisomes; (5) pexophagosomes are incorporated into the vacuole. We provided the scheme of the process of degradation and formation of the peroxisome aggregates and their degradation in wild type, atg2(p1), and atg7(p4) via macro- and micropexophagy (Supplementary Fig. 35). Under normal-intensity light conditions, the atg7(p4) mutant showed a higher number of peroxisome aggregates compared to the other atg mutants (Fig. 1 and Supplementary Fig. 1). The difference may reflect the function of ATG2 and ATG7 protein in macropexophagosome formation (Figs. 2, 7c and Supplementary Figs. 9, 35). Conversely, no difference was observed in the degree of peroxisome aggregation under high-intensity light conditions between atg2 and atg7 (Supplementary Figs. 20–22); thus, suggesting that ATG2 and ATG7 play equally important roles in micropexophagy.
We speculate that ROS generation is responsible for the induction of pexophagy; however, it is yet unclear how ROS generated in the peroxisome matrix are recognised for pexophagy. In human pexophagy, ataxia-telangiectasia mutated protein on the peroxisomal membrane senses ROS inside the peroxisome to induce pexophagy by mediating mTORC1 suppression and peroxin 5 (PEX5) phosphorylation12,62. Plant pexophagy might also involve sensor protein(s) along with plant PEX proteins on the peroxisome membrane to induce pexophagy12,22,62. In yeasts, receptors such as PpAtg30 and ScAtg36 interact with PEX3 and PEX14 to recognise peroxisomes to be degraded in pexophagy; however, orthologues of these receptors were not identified in plants12,22,23,49,51,62,63. Alternatively, oxidised lipids on the peroxisome membrane may represent the signal to induce pexophagosome formation as they are the hallmark of oxidised peroxisomes. PtdIns3P accumulation takes place in both peroxisomes and phagophores. This is supported by the fact that multiple pathways for the accumulation of PtdIns3P are activated in autophagy46,48,64. During mitophagy in mammalian cells, activation of phosphoinositide 3-kinase and inactivation of PTEN, a phosphatase removing the phosphate in the D3 position of the inositol ring, occur on the membrane of initial phagophores, namely omegasomes41,42,45,47, which are derived from the ER as platforms for mitophagy65,66. Here, we showed that phosphoinositide 3-kinase is involved in pexophagosome formation (Supplementary Fig. 19). The future direction of this study is to find the ROS or oxidative lipid sensor protein(s) on the peroxisome membrane for activating the phosphoinositide 3-kinase to induce pexophagy and clarify the involvement of PEXs in plant pexophagy.
We demonstrated that ATG18a-GFP selectively targets and surrounds peroxisomes to be degraded, which is the first observation of pexophagosomes forming from phagophores in plant cells. Hence, our analysis provides deep insight into the mechanism underlying autophagosome formation. Furthermore, our findings allow a better understanding of how plants reduce ROS production via autophagy to improve photosynthetic efficiency and thus increase crop yield.
Methods
Plant material and growth conditions
Wild-type and transgenic plants were grown in a 16 h light/8 h dark cycle at 23 °C in an incubator (MLR-351, Sanyo Electric Co., Ltd., Japan)11,14. Arabidopsis thaliana (L.) Heynh (Columbia, Col-0) and that expressing GFP-PTS1 (the GFP-PTS1 plant) or RFP-PTS1 (the RFP-PTS1 plant)67 were used as controls. The atg2(p1), atg18a(p2), and atg7(p4) (peups) plants were previously screened as pexophagy mutants11, and T-DNA insertion lines atg2-1 (SALK_076727), atg5-1 (SAIL_129B07), atg7-2 (GABI_655B06), and atg9-3 (SALK_130796)14 were used for plant growth analysis. The peups expressing RFP-PTS1 (RP) were generated from F3 lines by crossing peups with the RFP-PTS1 plants. We produced the RFP-PTS1 plants expressing ATG18a-GFP or GFP-2×FYVE using the floral dip method68 with Agrobacterium tumefaciens (EHA101) harbouring the binary vector pGWB451-ATG18a or pGWB452-2×FYVE. More than three independent lines that showed normal growth phenotypes similar to Col-0 were selected (Supplementary Fig. 18c, d). The peups (RP) expressing ATG18a-GFP or GFP-2×FYVE were generated by crossing RFP-PTS1 plants expressing ATG18a-GFP or GFP-2×FYVE with peups (RP). These lines theoretically express the transgenes at the same level. Transgenic Arabidopsis expressing Venus-VAM3 (vacuolar membrane marker)58,69 or Mt-GFP (mitochondrial marker)70 were crossed with the RFP-PTS1 plant and peups (RP), respectively, to generate T3 homozygous lines.
Plant growth analysis under high-intensity light conditions
One week after germination on 0.8 % (w/v) agar plates containing half-strength MS medium and 1% (w/v) sucrose at 23 °C in a 16 h light (50 μmol m−2 s−1)/8 h dark photoperiod, plants were transferred to rockwool inserted into the soil under 50 μmol m−2 s−1 white light (OSRAM FL25W White, Hitachi, Japan) at the same photoperiod for 2 weeks and then placed in incubators with white light at 50, 100, and 200 μmol m−2 s−1 for plant growth analysis (Supplementary Fig. 2). After the plants were grown at 23 °C in the 16 h light (normal white light, 100 μmol m−2 s−1)/8 h dark cycle for 3 weeks on 0.8% (w/v) agar containing 1% (w/v) sucrose and 1× MS salt, plant samples were used for the plant growth and biochemical analysis. The plant growth analysis (Figs. 4–7 and Supplementary Figs. 20–34) involved irradiation with blue (450 nm) and red (640 nm) light using an LED equipment (ISC-150 × 150-H4RB45; CCS, Japan) with a power supply (ISC-201-2; CCS, Japan) in low and high-intensity light conditions at 100 and 1000 μmol m−2 s−1 for 16 h, respectively.
Vector construction
Binary vectors pGWB451-ATG18a-G3-GFP and pGWB452-G3-GFP-2×FYVE were constructed using the Gateway system (Thermo Fisher Scientific, Waltham, MA, USA) to transform RFP-PTS1 plants. Adapter-tagged cDNA of AtATG18a (At3g62770, accession no. NM_116142) was generated by PCR by amplifying the corresponding region using the following primer set: F: 5′-TACAAAAAAGCAGGCTTCATGGCCACCGTATCTTCTTC-3′, R: 5′-GTACAAGAAAGCTGGGTTGAAAACTGAAGGCGGTTTCAGA-3′ for ATG18a, which was then recombined with the pDONR™221 vector71.
Adapter-tagged cDNA of the FYVE domain32,72 was generated using PCR by amplifying the corresponding region using the following primer set: attB1-adapter, 5′-GGGGACAAGTTTGTACAAAAAAGCAG GCTTC-3′; and attB2-adapter, 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTT-3′ using the pBluescript KS (-) (Stratagene) vector containing the nucleotide sequence of the 2×FYVE domain with attB1 and attB2 as templates and then recombining with the pDONR™221 vector. The nucleotide sequences of two FYVE domains, attB1-FYVE and FYVE-attB2, were separately inserted in the same pBluescript KS (-) vector in two steps using the FYVE region of Mus musculus HGF-regulated tyrosine kinase substrate (Hgs; accession no.: NM_001159328) as the cDNA template32,72. Two primer sets, F1: 5′-AAGTCGACTACAAAAAAGCAGGCYYCGAAA GTGATGCCATGTTCGCTG-3′ and R1: 5′-AAAAGCTTGACCTTGTGCCTTCTTGTTCAGCTGCTCATA-3′ for attB1-FYVE, and F2: 5′-AAAAAGCTTCTGAAAGTGATGCCATGTTCGCTGCTGAAA-3′ and R2: 5′-AAGATTCGTACAAGAAAGCTGGGTGCCTTCTTGTTCAGCTGCTCATA-3′ for FYVE-attB2 were used to amplify the corresponding regions.
Imaging analysis
A confocal laser scanning microscope (LSM 510, LSM880, Zeiss, Germany) with a ×40 or ×63 objective was used for imaging analyses of peroxisomes and for determining the intracellular distribution of fluorescent proteins as described previously11,40. Images were obtained from the top surface to the middle depth region, “Top”, or the middle depth to the bottom region, “Bottom”, of a 3-week-old plant leaf. We used a slice from the z-axis scanning image taken at every 1 µm thickness with a pinhole size of 1 Airy unit. The excitation and emission wavelengths for the images were 488 and 492−570 nm, respectively, for GFP, and 516 and 600−625 nm, respectively, for RFP. Time-lapse images were obtained for 250−300 s with a temporal resolution of 5 s, and movie files were generated using Fiji (ImageJ, NIH public domain). The number of cells and organelles was counted using the Analyze Particles and Cell Counter plugins equipped in Fiji73. The size of peroxisome aggregates was measured manually using the polygon selection tool in Fiji after the images were magnified three-fold for precise selection of the periphery. The pexophagosome around peroxisomes in atg7(p4) targeted by ATG18a-GFP (Fig. 2h) was identified by conducting mathematical morphology analyses28 based on time-lapse images. Fluorescence intensity (Figs. 2g, 3g, 5e, 7a, and Supplementary Fig. 31b) was measured using the Plot Profile plugin equipped in Fiji. FRAP analysis (Supplementary Figs. 11, 12) was performed using LSM880 with an Ar laser (488 nm) at 50% intensity to induce photobleaching. Images were obtained every 1 s, and fluorescence intensity was then measured using Fiji.
Measurement of chlorophyll content and photosynthetic efficiency
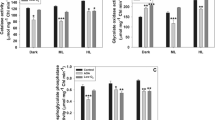
Chlorophyll content (Supplementary Figs. 2b, 20b, 32b) was measured as previously described74 using the rosette leaves adapted to each light intensity. Photosynthetic efficiency (Supplementary Fig. 2c) was measured as the maximum yield of photosynthesis system II using a photosynthesis yield analyser (MINI-PAM; Walz, Effeltrich, Germany)75 using at least three leaves from five plants after they were adapted to each light intensity. Three independent experiments were performed.
Electron microscopy analysis
Electron microscopy analysis was performed following previous works11,76. Three-week-old wild-type and atg7(p4) plants were analysed for catalase accumulation (Supplementary Figs. 3, 15c), chloroplast and peroxisome membranes (Supplementary Fig. 5e), and mitochondria (Supplementary Fig. 25b). Plant leaves were fixed in 4% (w/v) paraformaldehyde, 1% (w/v) glutaraldehyde, and 0.06 M sucrose in 0.05 M cacodylate buffer (pH 7.4) for immunoelectron microscopy analyses with antibodies against peroxisomal proteins [malate synthase (MS), isocitrate lyase (ICL), glycolate oxidase (GO), hydroxypyruvate reductase (HPR), and catalase (CAT)] (Supplementary Figs. 3, 15c)11,76. Three-days-old wild-type and atg2(p1) plants expressing GFP-2×FYVE and ATG18a-GFP were analyzed to detect the locations of PtdIns3P and ATG18 (Supplementary Figs. 15b, 16, 17)11,76. For immunoelectron microscopy analyses with antibodies against GFP, cotyledons were frozen with a high-pressure freezing machine (HPM-010, Bal-Tec, Balzer, FL) and dehydrated by freeze-substitution methods. Samples were embedded in LR white. Sections were treated with anti-GFP antibodies (1:100–1000 (v/v)) for 1 h at room temperature, and then treated with protein A-gold (15 nm, BBI international) for 30 min. Sections were stained with 4% (w/v) uranyl acetate for 10 min at room temperature and examined under a transmission electron microscope (H-7650, Hitachi High-Tech Co.) at 80 kV11,76.
NBT and H2-DCF staining
Nitro blue tetrazolium (NBT) and 2ʹ7ʹ-dichlorodihydrofluorescein (H2-DCF) staining were performed as follows: rosette leaves of GFP-PTS1, atg2(p1), and atg7(p4) were immediately submerged in NBT (Sigma-Aldrich, St. Louis, MO, USA) solution for 1 h, and chlorophyll was then repeatedly removed with 100% ethanol in 95 °C water for 10 min and washed with pure water. In the case of H2-DCF staining, the leaves were submerged in 10 µM H2-DCF-DA (Thermo Fisher Scientific) for 10 min and then washed once with pure water33,34. At least three independent experiments were performed. We carefully selected leaf mesophyll cells from similar regions and depths within the leaves and used the same confocal microscope setting of exposure time and dynamic range across images. The mean intensity of fluorescence from H2-DCF inside peroxisome and chloroplast was measured using Image J. The area of peroxisome and chloroplast was determined by surrounding them with the “Polygon selections tool” in Image J.
Immunoblot analysis
Immunoblotting was performed following a previous work11. Total proteins of wild-type, peups, atgs, and various transgenic plants grown under different light intensities for 1–2 days were extracted with the extraction buffer containing 10 mM HEPES–KOH (pH 8.0) and a protease inhibitor cocktail (Roche). Total proteins were then fractionated into supernatant and pellet by centrifugation at 20,000×g for 10 min at 4 °C. The pellet was washed with extraction buffer twice, followed by solubilisation with extraction buffer containing 1% (w/v) SDS. Each 10 µg of total protein was separated by SDS–PAGE and transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA) in a semidry electroblotting system (BioCraft). Immunoblot analyses were subsequently performed using antibodies against peroxisomal proteins CAT, peroxin 14 (PEX14), GO, ascorbate peroxidase (APX), and HPR11, as well as against mitochondrial proteins cytochrome c oxidase 2 (COXII) (Agrisera, Sweden) and serine hydroxymethyltransferase (SHMT) (Agrisera, Sweden). We captured immunoblot images with a CCD camera by using precisely the same parameters for all conditions (exposure time, contrast, and background intensity). Signal intensities of bands in the immunoblot image were quantified using Dot Blot Analysis in Fiji. The CAT, COXII, and SHMT amounts in the supernatant and pellet fractions were calculated using volume-based normalisation of the extraction buffer (Source Data: Fig.4 and Supplementary Figs. 22, 25).
Mass spectrum analysis
Total protein was extracted from atg2(p1) expressing ATG18a-GFP or GFP grown under normal light conditions with 1 mL of lysis buffer [50 mM HEPES–KOH (pH 7.5), 0.15 M NaCl, 0.5% (v/v) Triton X-100, and 0.1% (v/v) Tween 20]. ATG18a-GFP-binding proteins were obtained through immunoprecipitation using µMACS Anti-GFP MicroBeads and µMACS columns (Miltenyi Biotec, Gaithersburg, MD, USA)77. The eluted fraction was assessed by immunoblot analysis using an anti-GFP antibody to detect GFP or ATG18a-GFP (Supplementary Fig. 13a). ATG18a-GFP/GFP-binding proteins were subjected to SDS–PAGE following in-gel digestion77. The obtained proteins were electrophoresed briefly until the BPB dye band was 2 mm from the well. A 4-mm piece of gel centred on the dye band was cut out and digested with trypsin78,79. Collected peptides were analysed using nano-LC–MS/MS (LTQ Orbitrap XL; Thermo Fisher Scientific)78,79. The obtained spectra were searched against the TAIR 10 Arabidopsis protein database (version 20101214) with MASCOT server (version 2.3.02, Matrix Science, London, UK)78,79. The list of identified proteins is shown in Supplementary Table 2. The experiments were repeated three times.
Lipid binding assay
The binding ability of ATG18a-GFP to PtdIns3P was determined using PIP Strips P-6001 (Echelon Biosciences Inc., Salt Lake City, UT, USA) according to the manufacturer’s instructions and Tamura et al. (2013)31. Crude extracts from atg2(p1) expressing ATG18a-GFP and wild type (Col-0) expressing GFP were incubated with PIP strips for 3 h at 23 °C after removing debris by centrifugation at 1000×g for 5 min. After two washes with TBS containing 0.1% (v/v) Tween 20, the binding of ATG18a-GFP to lipids was detected using an antibody against GFP and ImageQuant LAS4000 (GE Healthcare) at high sensitivity mode.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The source data for Figs. 1–7 and Supplementary Figs. 1–34 are provided with this paper as a Source Data file. Other data and materials of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper. Proteome data were deposited in PRIDE with accession number (PXD038480).
References
Oikawa, K., Hayashi, M., Hayashi, Y. & Nishimura, M. Re‐evaluation of physical interaction between plant peroxisomes and other organelles using live‐cell imaging techniques. J. Integr. Plant Biol. 61, 836–852 (2019).
Kozaki, A. & Takeba, G. Photorespiration protects C3 plants from photooxidation. Nature 384, 557–560 (1996).
Takahashi, S., Bauwe, H. & Badger, M. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol. 144, 487–494 (2007).
Apel, K. & Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399 (2004).
Corpas, F. J., Barroso, J. B. & del Rı́o, L. A. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 6, 145–150 (2001).
del Río, L. A. & López-Huertas, E. ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol. 57, 1364–1376 (2016).
Foyer, C. H., Bloom, A. J., Queval, G. & Noctor, G. Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 60, 455–484 (2009).
Noctor, G. & Foyer, C. H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 171, 1581–1592 (2016).
Sandalio, L. M. & Romero-Puertas, M. C. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann. Bot. 116, 475–485 (2015).
Willekens, H. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 16, 4806–4816 (1997).
Shibata, M. et al. Highly oxidized peroxisomes are selectively degraded via autophagy in Arabidopsis. Plant Cell 25, 4967–4983 (2013).
Zhang, J. et al. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nat. Cell Biol. 15, 1186–1196 (2013).
Pérez-Pérez, M. E., Lemaire, S. D. & Crespo, J. L. Reactive oxygen species and autophagy in plants and algae. Plant Physiol. 160, 156–164 (2012).
Yoshimoto, K. et al. Organ-specific quality control of plant peroxisomes is mediated by autophagy. J. Cell Sci. https://doi.org/10.1242/jcs.139709 (2014).
**e, Z. & Klionsky, D. J. Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 9, 1102–1109 (2007).
Mizushima, N., Yoshimori, T. & Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 (2011).
Liu, Y. & Bassham, D. C. Autophagy: pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 63, 215–237 (2012).
Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 24, 9–23 (2014).
Farré, J.-C. & Subramani, S. Mechanistic insights into selective autophagy pathways: lessons from yeast. Nat. Rev. Mol. Cell Biol. 17, 537–552 (2016).
Nair, U., Cao, Y., **e, Z. & Klionsky, D. J. Roles of the lipid-binding motifs of Atg18 and Atg21 in the cytoplasm to vacuole targeting pathway and autophagy. J. Biol. Chem. 285, 11476–11488 (2010).
Obara, K. & Ohsumi, Y. PtdIns 3-kinase orchestrates autophagosome formation in yeast. J. Lipids 2011, 1–9 (2011).
Oku, M. & Sakai, Y. Pexophagy in yeasts. Biochim. Biophys. Acta - Mol. Cell Res. 1863, 992–998 (2016).
Anding, A. L. & Baehrecke, E. H. Cleaning house: selective autophagy of organelles. Dev. Cell 41, 10–22 (2017).
Filomeni, G., De Zio, D. & Cecconi, F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 22, 377–388 (2015).
Sinclair, A. M., Trobacher, C. P., Mathur, N., Greenwood, J. S. & Mathur, J. Peroxule extension over ER-defined paths constitutes a rapid subcellular response to hydroxyl stress. Plant J. 59, 231–242 (2009).
Brunkard, J. O., Runkel, A. M. & Zambryski, P. C. Chloroplasts extend stromules independently and in response to internal redox signals. Proc. Natl Acad. Sci. USA 112, 10044–10049 (2015).
**ong, Y., Contento, A. L., Nguyen, P. Q. & Bassham, D. C. Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol. 143, 291–299 (2007).
Kimori, Y., Hikino, K., Nishimura, M. & Mano, S. Quantifying morphological features of actin cytoskeletal filaments in plant cells based on mathematical morphology. J. Theor. Biol. 389, 123–131 (2016).
**ong, Y., Contento, A. L. & Bassham, D. C. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J. 42, 535–546 (2005).
Krick, R., Tolstrup, J., Appelles, A., Henke, S. & Thumm, M. The relevance of the phosphatidylinositolphosphat-binding motif FRRGT of Atg18 and Atg21 for the Cvt pathway and autophagy. FEBS Lett. 580, 4632–4638 (2006).
Tamura, N. et al. Atg18 phosphoregulation controls organellar dynamics by modulating its phosphoinositide-binding activity. J. Cell Biol. 202, 685–698 (2013).
Vermeer, J. E. M. et al. Visualization of PtdIns3 P dynamics in living plant cells. Plant J. 47, 687–700 (2006).
de Torres Zabala, M. et al. Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat. Plants 1, 15074 (2015).
Yamauchi, S. et al. Autophagy controls reactive oxygen species homeostasis in guard cells that is essential for stomatal opening. Proc. Natl Acad. Sci. USA 116, 19187–19192 (2019).
Liu, Y., **ong, Y. & Bassham, D. C. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 5, 954–963 (2009).
Luo, L. et al. Autophagy is rapidly induced by salt stress and is required for salt tolerance in Arabidopsis. Front. Plant Sci. 8, 1459 (2017).
Foyer, C. H. & Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant 119, 355–364 (2003).
Feierabend, J. & Engel, S. Photoinactivation of catalase in vitro and in leaves. Arch. Biochem. Biophys. 251, 567–576 (1986).
Shang, W. & Feierabend, J. Dependence of catalase photoinactivation in rye leaves on light intensity and quality and characterization of a chloroplast-mediated inactivation in red light. Photosynth. Res. 59, 201–213 (1999).
Oikawa, K. et al. Physical interaction between peroxisomes and chloroplasts elucidated by in situ laser analysis. Nat. Plants 1, 15035 (2015).
Polson, H. E. J. et al. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy 6, 506–522 (2010).
Proikas-Cezanne, T., Takacs, Z., Dönnes, P. & Kohlbacher, O. WIPI proteins: essential PtdIns3 P effectors at the nascent autophagosome. J. Cell Sci. https://doi.org/10.1242/jcs.146258 (2015).
Izumi, M., Ishida, H., Nakamura, S. & Hidema, J. Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell 29, 377–394 (2017).
Tekirdag, K. & Cuervo, A. M. Chaperone-mediated autophagy and endosomal microautophagy: joint by a chaperone. J. Biol. Chem. 293, 5414–5424 (2018).
Mizushima, N. & Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 147, 728–741 (2011).
Cheng, J. et al. Yeast and mammalian autophagosomes exhibit distinct phosphatidylinositol 3-phosphate asymmetries. Nat. Commun. 5, 3207 (2014).
Roberts, R. & Ktistakis, N. T. Omegasomes: PI3P platforms that manufacture autophagosomes. Essays Biochem. 55, 17–27 (2013).
Nascimbeni, A. C., Codogno, P. & Morel, E. Phosphatidylinositol‐3‐phosphate in the regulation of autophagy membrane dynamics. FEBS J. 284, 1267–1278 (2017).
Stolz, A., Ernst, A. & Dikic, I. Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 16, 495–501 (2014).
Carlsson, S. R. & Simonsen, A. Membrane dynamics in autophagosome biogenesis. J. Cell Sci. https://doi.org/10.1242/jcs.141036 (2015).
Young, P. G. & Bartel, B. Pexophagy and peroxisomal protein turnover in plants. Biochim. Biophys. Acta - Mol. Cell Res. 1863, 999–1005 (2016).
Hamasaki, M. et al. Autophagosomes form at ER–mitochondria contact sites. Nature 495, 389–393 (2013).
Le Bars, R., Marion, J., Le Borgne, R., Satiat-Jeunemaitre, B. & Bianchi, M. W. ATG5 defines a phagophore domain connected to the endoplasmic reticulum during autophagosome formation in plants. Nat. Commun. 5, 4121 (2014).
Zhuang, X. et al. ATG9 regulates autophagosome progression from the endoplasmic reticulum in Arabidopsis. Proc. Natl Acad. Sci. USA 114, E426–E435 (2017).
Kotani, T., Kirisako, H., Koizumi, M., Ohsumi, Y. & Nakatogawa, H. The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc. Natl Acad. Sci. USA 115, 10363–10368 (2018).
Mizushima, N. et al. A protein conjugation system essential for autophagy. Nature 395, 395–398 (1998).
Komatsu, M. et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434 (2005).
Goto-Yamada, S. et al. Sucrose starvation induces microautophagy in plant root cells. Front. Plant Sci. 10, 1604 (2019).
Chanoca, A. et al. Anthocyanin vacuolar inclusions form by a microautophagy mechanism. Plant Cell 27, 2545–2559 (2015).
Nakamura, S. & Izumi, M. Regulation of chlorophagy during photoinhibition and senescence: lessons from mitophagy. Plant Cell Physiol. 59, 1135–1143 (2018).
Sieńko, K., Poormassalehgoo, A., Yamada, K. & Goto-Yamada, S. Microautophagy in plants: consideration of its molecular mechanism. Cells 9, 887 (2020).
Subramani, S. A mammalian pexophagy target. Nat. Cell Biol. 17, 1371–1373 (2015).
Motley, A. M., Nuttall, J. M. & Hettema, E. H. Pex3-anchored Atg36 tags peroxisomes for degradation in Saccharomyces cerevisiae. EMBO J. 31, 2852–2868 (2012).
Maehama, T., Taylor, G. S. & Dixon, J. E. PTEN and myotubularin: Novel phosphoinositide phosphatases. Annu. Rev. Biochem. 70, 247–279 (2001).
Nguyen, T. N., Padman, B. S. & Lazarou, M. Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol. 26, 733–744 (2016).
Harper, J. W., Ordureau, A. & Heo, J.-M. Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 19, 93–108 (2018).
Mano, S. et al. Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: Dynamic morphology and actin-dependent movement. Plant Cell Physiol. 43, 331–341 (2002).
Zhang, X., Henriques, R., Lin, S.-S., Niu, Q.-W. & Chua, N.-H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1, 641–646 (2006).
Ebine, K. et al. A SNARE complex unique to seed plants is required for protein storage vacuole biogenesis and seed development of Arabidopsis thaliana. Plant Cell 20, 3006–3021 (2008).
Arimura, S., Yamamoto, J., Aida, G. P., Nakazono, M. & Tsutsumi, N. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc. Natl Acad. Sci. USA 101, 7805–7808 (2004).
Nakagawa, T. et al. Improved gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71, 2095–2100 (2007).
Sankaran, V. G., Klein, D. E., Sachdeva, M. M. & Lemmon, M. A. High-affinity binding of a FYVE domain to phosphatidylinositol 3-phosphate requires intact phospholipid but not FYVE domain oligomerization. Biochemistry 40, 8581–8587 (2001).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Asakura, Y. et al. Maize mutants lacking chloroplast FtsY exhibit pleiotropic defects in the biogenesis of thylakoid membranes. Plant Cell 16, 201–214 (2004).
Yamaguchi, K. & Nishimura, M. Reduction to below threshold levels of glycolate oxidase activities in transgenic tobacco enhances photoinhibition during irradiation. Plant Cell Physiol. 41, 1397–1406 (2000).
Hayashi, Y., Hayashi, M., Hayashi, H., Hara-Nishimura, I. & Nishimura, M. Direct interaction between glyoxysomes and lipid bodies in cotyledons of the Arabidopsis thaliana ped1 mutant. Protoplasma 218, 83–94 (2001).
Tamura, K., Fukao, Y., Iwamoto, M., Haraguchi, T. & Hara-Nishimura, I. Identification and characterization of nuclear pore complex components in Arabidopsis thaliana. Plant Cell 22, 4084–4097 (2011).
Takahashi, D., Li, B., Nakayama, T., Kawamura, Y. & Uemura, M. Shotgun proteomics of plant plasma membrane and microdomain proteins using nano-LC–MS/MS. Methods Mol. Biol. 1072, 481–498 (2014).
Takahashi, D., Kawamura, Y. & Uemura, M. Cold acclimation is accompanied by complex responses of glycosylphosphatidylinositol (GPI)-anchored proteins in Arabidopsis. J. Exp. Bot. 67, 5203–5215 (2016).
Acknowledgements
We thank Dr. Shunichi Takahashi (National Institute for Basic Biology) and Dr. Murray Badger (Australian National University) for the helpful discussion about photoinhibition and photosynthetic efficiency in pexophagy mutants. We also thank Dr. Tsuyoshi Nakagawa (University of Shimane) for providing the Gateway vectors pGWB451 and pGWB452, Dr. Shinichi Arimura (University of Tokyo), and Dr. Tomohiro Uemura (Ochanomizu University) for kindly providing the transgenic line expressing Mt-GFP and the plasmid hovering VAM3 gene, and the Bioimaging Facility in National Institute for Basic Biology (NIBB) as well as the NIBB BioResource Center for technical support. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas to M.N. (no. 22120007) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); by Grants-in-Aid for Scientific Research to Y.H. and K.O. (no. 17K07467), to M.N. (no. 20370024), to S.M. (nos. 26440157 and 20570045), and to I.H.-N. (nos. 15H05776 and 22000014) from Japan Society for the Promotion of Science (JSPS); by the Japan Science and Technology Agency Exploratory Research for Advanced Technology program (JST-ERATO) to K.N. (no. JPMJER1602); by a SONATA-BIS Grant to S.G.-Y. (UMO-2019/34/E/NZ3/00299) from National Science Centre Poland; by a TEAM Grant to K.Ya. (TEAM/2017-4/41) from the Foundation for Polish Science; by the Wyeth Foundation to M.N. and I.H.-N.; and by the Hirao Taro Foundation of KONAN GAKUEN for Academic Research to I.H.-N. The open-access publication of this article was funded by the BioS Priority Research Area under the program “Excellence Initiative—Research University” at the Jagiellonian University in Krakow.
Author information
Authors and Affiliations
Contributions
K.O., M.S., K.Yo., Y.H., S.G.-Y., S.M., K.N., K.Ya., Y.O., and M.N. designed the study. K.O. performed most of the experiments. Y.H., M.K., and K.S. performed EM analysis. Y.K. performed mathematical morphology analyses. D.T. and M.U. performed protein mass spectrometry analyses. K.O., S.G.-Y., K.Yo., A.T., A.K., H.U., I.H.-N., K.H., and S.M. generated transgenic plants and performed plant growth analyses. All the authors analysed the data and wrote the manuscript. K.O., S.G.-Y., S.M., K.Ya., and M.N. mainly performed revised experiments and wrote revised manuscripts.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oikawa, K., Goto-Yamada, S., Hayashi, Y. et al. Pexophagy suppresses ROS-induced damage in leaf cells under high-intensity light. Nat Commun 13, 7493 (2022). https://doi.org/10.1038/s41467-022-35138-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-35138-z
- Springer Nature Limited