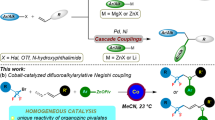
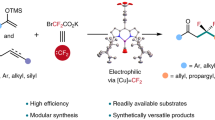
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Article
ADS
Google Scholar
Mikami, K., Itoh, Y. & Yamanaka, M. Fluorinated carbonyl and olefinic compounds: basic character and asymmetric catalytic reactions. Chem. Rev. 104, 1–16 (2004).
Article
CAS
Google Scholar
Uneyama, K., Katagiri, T. & Amii, H. α-trifluoromethylated carbanion synthons. Acc. Chem. Res. 41, 817–829 (2008).
Article
CAS
Google Scholar
Ma, J.-A. & Cahard, D. Asymmetric fluorination, trifluoromethylation, and perfluoroalkylation reactions. Chem. Rev. 104, 6119–6146 (2004).
Article
CAS
Google Scholar
Zhang, C.-P., Chen, Q.-Y., Guo, Y., **ao, J.-C. & Gu, Y.-C. Progress in fluoroalkylation of organic compounds via sulfinatodehalogenation initiation system. Chem. Soc. Rev. 41, 4536–4559 (2012).
Article
CAS
Google Scholar
Liang, T., Neumann, C. N. & Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 52, 8214–8264 (2013).
Article
CAS
Google Scholar
Ni, C., Hu, M. & Hu, J. Good partnership between sulfur and fluorine: sulfur-based fluorination and fluoroalkylation reagents for organic synthesis. Chem. Rev. 115, 765–825 (2015).
Article
CAS
Google Scholar
Belhomme, M.-C., Besset, T., Poisson, T. & Pannecoucke, X. Recent progress toward the introduction of functionalized difluoromethylated building blocks onto C(sp2) and C(sp) centers. Chem. Eur. J. 21, 12836–12865 (2015).
Article
CAS
Google Scholar
Feng, Z., **ao, Y.-L. & Zhang, X. Transition-metal (Cu, Pd, Ni)-catalyzed difluoroalkylation via cross-coupling with difluoroalkyl halides. Acc. Chem. Res. 51, 2264–2278 (2018).
Article
CAS
Google Scholar
Nagib, D. A., Scott, M. E. & MacMillan, D. W. C. Enantioselective α-trifluoromethylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 131, 10875–10877 (2009).
Article
CAS
Google Scholar
Shibata, N., Mizuta, S. & Kawai, H. Recent advances in enantioselective trifluoromethylation reactions. Tetrahedron.: Asymmetry 19, 2633–2644 (2008).
Article
CAS
Google Scholar
Cho, E. J. et al. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science 328, 1679–1681 (2010).
Article
ADS
CAS
Google Scholar
Guo, C., Wang, R.-W. & Qing, F.-L. Palladium catalyzed direct α-arylation of α,α-difluoroketones with aryl bromides. J. Fluor. Chem. 143, 135–142 (2012).
Article
CAS
Google Scholar
Feng, Z., Min, Q.-Q., **ao, Y.-L., Zhang, B. & Zhang, X. Palladium-catalyzed difluoroalkylation of aryl boronic acids: a new method for the synthesis of aryldifluoromethylated phosphonates and carboxylic acid derivatives. Angew. Chem. Int. Ed. 53, 1669–1673 (2014).
Article
CAS
Google Scholar
Min, Q.-Q., Yin, Z., Feng, Z., Guo, W.-H. & Zhang, X. Highly selective gem-difluoroallylation of organoborons with bromodifluoromethylated alkenes catalyzed by palladium. J. Am. Chem. Soc. 136, 1230–1233 (2014).
Article
CAS
Google Scholar
Shao, C., Shi, G., Zhang, Y., Pan, S. & Guan, X. Palladium-catalyzed C-H ethoxycarbonyldifluoromethylation of electron-rich heteroarenes. Org. Lett. 17, 2652–2655 (2015).
Article
CAS
Google Scholar
Gu, Y., Leng, X. & Sheng, Q. Cooperative dual palladium/silver catalyst for direct difluoromethylation of aryl bromides and iodides. Nat. Commun. 5, 5405–5411 (2014).
Article
ADS
CAS
Google Scholar
Feng, Z., Min, Q.-Q., Fu, X.-P., An, L. & Zhang, X. Chlorodifluoromethane-triggered formation of difluoromethylated arenes catalysed by palladium. Nat. Chem. 9, 918–923 (2017).
Article
CAS
Google Scholar
Aikawa, K., Serizawa, H., Ishii, K. & Mikami, K. Palladium-catalyzed Negishi cross-coupling reaction of aryl halides with (difluoromethyl)zinc reagent. Org. Lett. 18, 3690–3693 (2016).
Article
CAS
Google Scholar
Oishi, M., Kondo, H. & Amii, H. Aromatic trifluoromethylation catalytic in copper. Chem. Commun. 45, 1909–1911 (2009).
Article
Google Scholar
Feng, Z., Chen, F. & Zhang, X. Copper catalyzed cross-coupling of iodobenzoates with bromozinc-difluorophosphonate. Org. Lett. 14, 1938–1941 (2012).
Article
CAS
Google Scholar
Belhomme, M.-C., Poisson, T. & Pannecoucke, X. Copper-catalyzed direct C-2 difluoromethylation of furans and benzofurans: access to C-2 CF2H derivatives. J. Org. Chem. 79, 7205–7211 (2014).
Article
CAS
Google Scholar
Jiang, X., Chu, L. & Qing, F.-L. Copper-mediated oxidative difluoromethylenation of aryl boronic acids with α-silyldifluoromethylphosphonates: a new method for aryldifluorophosphonates. New J. Chem. 37, 1736–1741 (2013).
Article
CAS
Google Scholar
Serizawa, H., Ishii, K., Aikawa, K. & Mikami, K. Copper-catalyzed difluoromethylation of aryl iodides with (difluoromethyl)zinc reagent. Org. Lett. 18, 3686–3689 (2016).
Article
CAS
Google Scholar
Gao, X., **ao, Y.-L., Wan, X. & Zhang, X. Copper-catalyzed highly stereoselective trifluoromethylation and difluoroalkylation of secondary propargyl sulfonates. Angew. Chem. Int. Ed. 57, 3187–3191 (2018).
Article
CAS
Google Scholar
Ivanova, M. V., Bayle, A., Besset, T., Poisson, T. & Pannecoucke, X. Copper-mediated formation of aryl, heteroaryl, vinyl and alkynyl difluoromethylphosphonates: a general approach to fluorinated phosphate mimics. Angew. Chem. Int. Ed. 54, 13406–13410 (2015).
Article
CAS
Google Scholar
Jiang, X., Chu, L. & Qing, F.-L. Copper-mediated oxidative cross-coupling reaction of terminal alkynes with α-silyldifluoromethylphosphonates: an efficient method for α,α-difluoropropargylphosphonates. Org. Lett. 14, 2870–2873 (2014).
Article
Google Scholar
Belhomme, M.-C., Poisson, T. & Pannecoucke, X. Copper catalyzed β-difluoroacetylation of dihydropyrans and glycals by means of direct C–H functionalization. Org. Lett. 15, 3428–3431 (2013).
Article
CAS
Google Scholar
Zhu, J., Ni, C., Gao, B. & Hu, J. Copper(0)-mediated fluoroalkylation of iodobenzene with 2-bromo-1,1,2,2-tetrafluoroethyl compounds: investigation on the influence of R substituent on the reactivity of RCF2Cu species. J. Fluor. Chem. 171, 139–147 (2015).
Article
CAS
Google Scholar
He, Z. et al. Copper-mediated trifluoromethylation of propiolic acids: facile synthesis of α-trifluoromethyl ketones. Chem. Sci. 4, 3478–3483 (2013).
Article
CAS
Google Scholar
Qi, Q., Shen, Q. & Lu, L. Copper-mediated aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides at room temperature. J. Am. Chem. Soc. 134, 6548–6551 (2012).
Article
CAS
Google Scholar
Hu, M., Ni, C. & Hu, J. Copper-mediated trifluoromethylation of α-diazo esters with TMSCF3: the important role of water as a promoter. J. Am. Chem. Soc. 134, 15257–15260 (2012).
Article
CAS
Google Scholar
Moselage, M., Li, J. & Ackermann, L. Cobalt-catalyzed C–H activation. ACS Catal. 6, 498–525 (2016).
Article
CAS
Google Scholar
Cahiez, G. & Moyeux, A. Cobalt-catalyzed cross-coupling reactions. Chem. Rev. 110, 1435–1462 (2010).
Article
CAS
Google Scholar
Su, B., Cao, Z.-C. & Shi, Z.-J. Exploration of earth-abundant transition metals (Fe, Co, and Ni) as catalysts in unreactive chemical bond activations. Acc. Chem. Res. 48, 886–896 (2015).
Article
CAS
Google Scholar
Liang, Y. & Fu, G. C. Catalytic asymmetric synthesis of tertiary alkyl fluorides: Negishi cross-couplings of racemic α,α-dihaloketones. J. Am. Chem. Soc. 136, 5520–5524 (2016).
Article
Google Scholar
Jiang, X., Sakthivel, S., Kulbitski, K., Nisnevich, G. & Gandelman, M. Efficient synthesis of secondary alkyl fluorides via suzuki cross-coupling reaction of 1-Halo-1-fluoroalkanes. J. Am. Chem. Soc. 136, 9548–9551 (2014).
Article
CAS
Google Scholar
Su, Y.-M., Feng, G.-S., Wang, Z.-Y., Lan, Q. & Wang, X.-S. Nickel-catalyzed monofluoromethylation of aryl boronic acids. Angew. Chem. Int. Ed. 54, 6003–6007 (2015).
Article
CAS
Google Scholar
Schwaebe, M. K., McCarthy, J. R. & Whitten, J. P. Nickel(0)-catalyzed coupling of vinylzirconiums to α-bromo-α,α-difluoro esters Convenient generation of a functionalized allyldifluoro moiety. Tetrahedron Lett. 41, 791–794 (2000).
Article
CAS
Google Scholar
Sheng, J., Ni, H.-Q., Liu, G., Li, Y. & Wang, X.-S. Combinatorial nickel-catalyzed monofluoroalkylation of aryl boronic acids with unactivated fluoroalkyl iodides. Org. Lett. 19, 4480–4483 (2017).
Article
CAS
Google Scholar
Sheng, J., Ni, H.-Q., Zhang, H.-R., Wang, Y.-N. & Wang, X.-S. Nickel-catalyzed reductive cross-coupling of aryl halides with monofluoroalkyl halides for late-stage monofluoroalkylation. Angew. Chem. Int. Ed. 57, 7634–7639 (2018).
Article
CAS
Google Scholar
Gu, J.-W., Min, Q.-Q., Yu, L.-C. & Zhang, X. Tandem difluoroalkylation-arylation of enamides catalyzed by nickel. Angew. Chem. Int. Ed. 55, 12270–12274 (2016).
Article
CAS
Google Scholar
**ao, Y.-L., Guo, W.-H., He, G.-Z., Pan, Q. & Zhang, X. Nickel-catalyzed cross-coupling of functionalized difluoromethyl bromides and chlorides with aryl boronic acids: a general method for difluoroalkylated arenes. Angew. Chem. Int. Ed. 53, 9909–9913 (2014).
Article
CAS
Google Scholar
Xu, C. et al. Difluoromethylation of (hetero)aryl chlorides with chlorodifluoromethane catalyzed by nickel. Nat. Commun. 9, 1170–1179 (2018).
Article
ADS
Google Scholar
An, L., **ao, Y.-L., Zhang, S. & Zhang, X. Bulky diamine ligand promotes cross-coupling of difluoroalkyl bromides by iron catalysis. Angew. Chem. Int. Ed. 57, 6921–6925 (2018).
Article
CAS
Google Scholar
Lin, X., Zheng, F. & Qing, F.-L. Iron-catalyzed cross-coupling reactions between arylzinc reagents and alkyl halides bearing β-fluorines. Organometallics 31, 1578–1582 (2012).
Article
CAS
Google Scholar
Miao, W. et al. Iron-catalyzed difluoromethylation of arylzincs with difluoromethyl 2-pyridyl sulfone. J. Am. Chem. Soc. 140, 880–883 (2018).
Article
CAS
Google Scholar
Ohtsuka, Y. & Yamakawa, T. Direct ethoxycarbonyldifluoromethylation of aromatic compounds using Fenton reagent. Tetrahedron 67, 2323–2331 (2011).
Article
CAS
Google Scholar
Araki, K. & Inoue, M. Cobalt-catalyzed cross-coupling reaction of arylzinc reagents with ethyl bromodifluoroacetate. Tetrahedron 69, 3913–3918 (2013).
Article
CAS
Google Scholar
Ohtsuka, Y. & Yamakawa, T. Cobalt/diamine-catalyzed 1,1-difluoroethylation and 2,2,2-trifluoroethylation of aryl Grignard reagents with corresponding fluoroalkyl halides. J. Fluor. Chem. 185, 96–102 (2016).
Article
CAS
Google Scholar
Chou, T. S. et al. Stereospecific synthesis of 2-deoxy-2,2-difluororibonolactone and its use in the preparation of 2′-Deoxy-2′,2′-difluoro-β-D-ribofuranosyl pyrimidine nucleosides: the key role of selective crystallization. Synthesis 6, 565–570 (1992).
Article
Google Scholar
Yue, X., Qiu, X.-L. & Qing, F.-L. Synthesis of 2′,3′-dideoxy-6′,6′-difluoro-3′-azanucleosides. J. Fluor. Chem. 129, 866–874 (2008).
Article
CAS
Google Scholar
Nakayama, K. et al. Synthesis and antifungal activity of rhodopeptin analogues. 2. Modification of the west amino acid moiety. Org. Lett. 2, 977–980 (2000).
Article
CAS
Google Scholar
Uoto, K. et al. Synthesis and structure-activity relationships of novel 2’,2’-difluoro analogues of docetaxel. Chem. Pharm. Bull. 45, 1793–1804 (1997).
Article
CAS
Google Scholar
Fujikawa, K., Fujioka, Y., Kobayashi, A. & Amii, H. A new method for aromatic difluoromethylation: copper-catalyzed cross-coupling and decarboxylation sequence from aryl iodides. Org. Lett. 13, 5560–5563 (2011).
Article
CAS
Google Scholar
Ge, S., Arlow, S. I., Mormino, M. G. & Hartwig, J. F. Pd-catalyzed α-arylation of trimethylsilyl enolates of α,α-difluoroacetamides. J. Am. Chem. Soc. 136, 14401–14404 (2014).
Article
CAS
Google Scholar
Xu, L. & Vicic, D. A. Direct difluoromethylation of aryl halides via base metal catalysis at room temperature. J. Am. Chem. Soc. 138, 2536–2539 (2016).
Article
CAS
Google Scholar
Aikawa, K., Maruyama, K., Nitta, J., Hashimoto, R. & Mikami, K. Siladifluoromethylation and difluoromethylation onto C(sp3), C(sp2), and C(sp) centers using ruppert–prakash reagent and fluoroform. Org. Lett. 18, 3354–3357 (2016).
Article
CAS
Google Scholar
An, L., Xu, C. & Zhang, X. Highly selective nickel-catalyzed gem-difluoropropargylation of unactivated alkylzinc reagents. Nat. Commun. 8, 1460–1468 (2017).
Article
ADS
Google Scholar
Cinderella, A. P., Vulovic, B. & Watson, D. A. Palladium-catalyzed cross-coupling of silyl electrophiles with alkylzinc halides: a Silyl-Negishi reaction. J. Am. Chem. Soc. 139, 7741–7744 (2017).
Article
CAS
Google Scholar
Luo, Z. et al. Synthesis and evaluation of multi-target-directed ligands against Alzheimer’s disease based on the fusion of donepezil and ebselen. J. Med. Chem. 56, 9089–9099 (2013).
Article
CAS
Google Scholar
Chen, Q.-A., Kim, D. K. & Dong, V. M. Regioselective hydroacylation of 1,3-Dienes by cobalt catalysis. J. Am. Chem. Soc. 136, 3772–3775 (2014).
Article
CAS
Google Scholar
Liebeskind, L. S., Baysdon, S. L., South, M. S., Iyer, S. & Leeds, J. P. The development of an organotransition metal synthesis of quinones. Tetrahedron 41, 5839–5853 (1985).
Article
CAS
Google Scholar
Fillon, H., Gosmini, C. & Périchon, J. New chemical synthesis of functionalized arylzinc compounds from aromatic or thienyl bromides under mild conditions using a simple cobalt catalyst and zinc dust. J. Am. Chem. Soc. 125, 3867–3870 (2003).
Article
CAS
Google Scholar
**, M.-Y. & Yoshikai, N. Cobalt−Xantphos-catalyzed, LiCl-mediated preparation of arylzinc reagents from aryl iodides, bromides, and chlorides. J. Org. Chem. 76, 1972–1978 (2011).
Article
CAS
Google Scholar
Brennan, M. R., Kim, D. & Fout, A. R. A synthetic and mechanistic investigation into the cobalt(I) catalyzed amination of aryl halides. Chem. Sci. 5, 4831–4839 (2014).
Article
CAS
Google Scholar