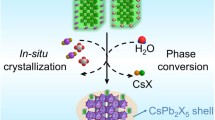
Abstract
A novel chemical vapor method is developed to synthesize ultrastable lead halide perovskite-zeolite (ZSM-5) composites, in which CsPbX3 (X = Cl, Br, I) perovskite quantum dots (QDs) are grown in situ in the nanopores of the ZSM-5 substrate. The key chemical reaction between PbBr2 vapor and the Si–O network in ZSM-5 leads to collapse of the initial zeolite crystal structure, realizing effective confinement and encapsulation of CsPbBr3 QDs and boosting their stability under harsh conditions, including heat, water, polar solvents, and ultraviolet (UV) light. At the same time, the acquired encapsulation structure possesses the channels needed for halogen exchange to regulate the halide ratios of the CsPbX3-ZSM-5 composites. The synthesized CsPbX3-ZSM-5 composites exhibit tunable emission from 400 to 700 nm and narrow full-widths at half-maximum (FWHM). To demonstrate the commercial potential, CsPbX3-ZSM-5 composites synthesized on a large scale are applied in white light-emitting diodes (WLEDs) and multicolor-coded anti-counterfeiting inks.
Similar content being viewed by others
Introduction
CsPbX3 (X = Cl, Br, I) QDs are emerging semiconductor materials and have attracted increasing attention due to their high defect tolerances, charge carrier mobilities, tunable bandgaps, and long carrier diffusion lengths1,2. Numerous recent reports have demonstrated the advantages of CsPbX3 QDs over conventional semiconducting materials3. In particular, their high photoluminescence quantum yields (PLQYs), narrow emission widths, and wide color range make them competitive candidates for lighting, backlight display, and anti-counterfeiting applications3. As photoluminescent layers, CsPbX3 QDs have been used in WLEDs and anti-counterfeiting coatings exhibiting high performance4. To accelerate practical use of CsPbX3 QDs, a great deal of research on crystal structures and fabrication strategies has been carried out, and remarkable progress has been achieved5. However, it is still challenging to use CsPbX3 QDs as commercial materials because of the drawbacks of prevalent synthetic methods, which usually involve large amounts of organic solvents, ligands, inert gas protection, and tedious cleaning and purification processes, thereby rendering them unsuitable for environmentally friendly and scalable production6,7. In addition, the stabilities of colloid QDs prepared by using solvents are generally mediated by large organic ligands, but unfortunately, the highly dynamic surfaces of QDs might cause desorption of ligands, resulting in structural damage and performance degradation of the ionic CsPbX3 QDs due to moisture or thermal attack8,9.
Great effort has been expended to overcome the instability issues, and various passivation strategies have been proposed, such as surface engineering and matrix encapsulation10. Mohammed et al. found that ligands with –NH3+ groups strongly bound to Br− ions on specific surfaces of CsPbBr3 (110) and enhanced the PL intensity and stability11. Compared with ligand modification methods, the inert encapsulation technique showed greater potential for preparing ultrastable perovskite composites. Oxides (SiO212, SiO2/AlOx13, molecular sieves14,15,16), semiconductors (ZnS17 and Pb4S3Br218), polymers (polydimethylsiloxane19 and polystyrene20), and metal-organic frameworks (PCN-333(Fe)21 and UiO-66(NH2)22) have served as effective matrix materials. Y Lin et al. reported the preparation of CsPbX3/SiOx by high-temperature sintering synthesis, and the humidity and heat resistance were significantly improved12. Wang et al. successfully synthesized an ultrastable perovskite composite based on the aluminophosphate AIPO-5, which provided confinement for growth of the nanocrystals, defect passivation, and robust barrier surroundings15. In addition to the enhanced stability, the absolute PL intensities of CsPbX3-agZIF-62 composites are often at least two orders of magnitude higher than those of the corresponding pure CsPbX323. In view of the significant breakthroughs in the stabilities of CsPbX3 QDs, the problems with scalable preparation should be considered because complicated postprocessing steps are inevitable in the strategies mentioned above24.
The solvent-free chemical vapor method is commonly applied in scalable syntheses of functional films, nanotubes, and catalysts due to its relatively low reaction temperatures, flexible product compositions, broad application range, and extraordinary diffusion ability25,26. Molecular sieves are typical active porous materials, and they allow gases and small molecules to pass through their unique channel structures; these are ideal accessory ingredients and support materials in various synthetic reactions27,28. Herein, a novel chemical vapor method is designed and demonstrated for scalable production of CsPbX3 composites with superior optical properties and ultrahigh stabilities. By employing ZSM-5 as the porous template and taking advantage of special reactions between PbBr2 vapor and the Si–O network of ZSM-5, in situ growth of CsPbX3 QDs confined in the nanometer-scale space is achieved without organic solvents and ligands. Additionally, the acquired encapsulation structure has the channels needed for halogen exchange to regulate the halide ratios of the CsPbX3-ZSM-5 composites. Consequently, the CsPbX3-ZSM-5 composites can be mass-produced with high PLQYs and narrow emission FWHM. In addition, owing to the protection and isolation provided by inert ZSM-5, the CsPbX3 QDs are prevented from agglomerating and regrowing, and the composites exhibit exceptional stability under harsh conditions, including heat, water, polar solvents, and UV light. To demonstrate practical viability, the composites are applied in WLEDs with a large color range and multicolor-coded anti-counterfeiting inks.
Materials and methods
Materials and chemicals
PbBr2 (lead bromide, 99%, Shanghai Aladdin Biochemical Technology Co., Ltd., China), CsBr (cesium bromide, 99.5%, Aladdin), ZSM-5 molecular sieves (SiO2/Al2O3, molar ratio ~ 40–50, Aladdin), tetrabutylammonium chloride (TBAC, 97%, Aladdin), and tetrabutylammonium iodide (TBAI, 99%, Aladdin) were used as received without further purification.
Synthesis of CsPbX3-ZSM-5 composites
The green-emission composite was synthesized via a facile one-step vapor diffusion method. CsBr and PbBr2 were weighed to give a 1:1 stoichiometric ratio, and an appropriate amount of ZSM-5 was added (designed mass ratio of (CsBr+PbBr2): ZSM-5 = 1:2). The mixture was calcined at 650 °C for 300 min with a heating rate of 10 °C min−1 and then cooled to 30 °C in a muffle furnace in air. The processes used to prepare materials with different proportions were similar. CsPbBrxCl3−x-ZSM-5 and CsPbBrxI3−x-ZSM-5 with different halogen compositions were synthesized via ion exchange by mixing and grinding the CsPbBr3-ZSM-5 powder with a certain amount of tetrabutylammonium iodide or tetrabutylammonium chloride and then calcining at 250 °C for 300 min to obtain CsPbBrxCl3−x-ZSM-5 and CsPbBrxI3−x-ZSM-5.
Syntheses of ZSM-650, CsBr-ZSM, PbBr2-ZSM
ZSM-650 was prepared by annealing ZSM-5 at 650 °C. CsBr-ZSM was prepared by mixing CsBr with ZSM-5 (mass ratios from 1:2 to 2:1) and then annealing at 650 °C. Similarly, PbBr2-ZSM was prepared by mixing PbBr2 with ZSM-5 (mass ratios from 1:4 to 1:1) and then annealing at 650 °C.
Characterization
The morphologies and microstructures of the CsPbX3-ZSM-5 composites were examined by scanning electron microscopy (SEM, Zeiss GeminiSEM 300) and transmission electron microscopy (TEM, FEI Talos F200X) at 200 kV. The compositions of the composites were determined by energy-dispersive X-ray spectroscopy (EDS) using an accessory manufactured by Oxford Instruments. To characterize structures, powder X-ray diffraction (XRD) studies were performed with a Rigaku Smartlab 3 kW X-ray diffractometer with Cu Kα radiation (λ = 1.54056 Å, 40 kV, 30 mA, 10° min−1 from 5 to 80°). X-ray photoelectron spectroscopy (XPS) was carried out on a Thermo ESCALAB 250** excitons of CsPbX3 (Fig. S16 in the Supplementary Information)38. All of the tested composites (Blue-Z, Green-Z, and Red-Z) showed only approximately 50% PL loss during heating from 20 to 100 °C, and 10% of the PL intensity remained even at 200 °C, indicating that the excitons in the CsPbX3-ZSM-5 composites were more thermally stable than those of pure CsPbX3 QDs39. As shown in Fig. 5d–f, the PL decrease caused by thermal quenching was recoverable, and the PL intensity was nearly equal to the initial value after cooling to room temperature. In addition, reversible PL was observed when heating the samples from 20 to 200 °C for 20 cycles. The outstanding thermal stability can be ascribed to three main factors. First, ZSM-5 has low thermal conductivity and protects the internal CsPbX3 QDs from high temperatures. Second, confinement within the porous structure of ZSM-5 hinders the aggregation of CsPbX3 QDs at a high temperature. Finally, no ligands were used in the syntheses of the composites, thus avoiding fluorescence damage caused by oxidation and delamination of organic ligands14.
In the solvent resistance tests, the Blue-Z, Green-Z, and Red-Z composites were immersed in different solvents to monitor PL evolution. Figure 5g shows that after immersion in water for 30 days, Blue-Z, Green-Z, and Red-Z maintained 84, 87, and 86% of their initial PL intensities, respectively. Stability in other solvents was also assessed, as shown in Figs. S17, S18, and S19 in the Supplementary Information. The samples were immersed in ethanol, isopropanol, glycol, dimethylformamide, dimethyl sulfoxide, and acetylacetone, and all of them maintained strong emission intensities after 180 days. The effects of aging are presented in Fig. 5h. The PL intensity of the CsPbX3-ZSM-5 composite was still higher than 90% of the initial value after 30 days of aging at 80 °C under 80% relative humidity (abbreviated as “80 °C, 80% R.H.”). To further evaluate the stability under other harsh conditions, the samples were exposed to continuous UV radiation at 365 nm, and the PL intensities exhibited nearly no changes after 500 h (Fig. 5i). The excellent resistance of the CsPbX3-ZSM-5 composites to water, polar solvents, aging, and UV radiation was attributed to protection from ZSM-5, which precluded direct exposure of the CsPbX3 QDs to these external factors and inhibited interparticle fusion of the perovskite nanoparticles. These results provided strong evidence showing that the proposed strategy overcame the inherent instability of CsPbX3 QDs.
Applications for WLEDs and anti-counterfeiting
Because of the advantages of the synthetic strategy, scalable preparation (Fig. 6a) can be easily realized and makes commercialization a possibility. In practice, the use of composites commonly involves organic polymers such as polydimethylsiloxane (PDMS) and polyurethane (PU); therefore, the practicability of these perovskite composites was investigated. Good compatibility with PDMS is indicated in Fig. 6b, and the uniform fluorescent disks emitted bright fluorescence under UV light excitation. In addition, a large-area film measuring 35 cm × 25 cm and made of the Green-Z composite and PU was prepared by screen printing (Fig. S20 in the Supplementary Information).
a Photographs of 1 kg of the CsPbBr3-ZSM-5 composite under ambient and UV illumination. b Photographs of CsPbX3-ZSM-5/PDMS disks with tunable emission under ambient and UV light. c Emission spectrum of the WLED fabricated by depositing the Green-Z and Red-Z composites on blue LED chips (the inset shows a photograph of the device operated at 20 mA). d CIE chromaticity coordinates and color range for the WLED. e Fluorescent images of the patterns prepared by silk printing and demonstration of anti-counterfeiting coding with CsPbX3-ZSM-5-based fluorescent security inks.
LEDs are widely recognized as mainstream devices in next-generation backlit displays20. Due to their compatibility with polymers, light conversion LED chips can be fabricated by packaging CsPbX3-ZSM-5 composites into commercial LED chips. The tolerance of Green-Z and Red-Z packed in the single-wavelength LED chips was evaluated by monitoring the output spectra produced by different driving currents under working conditions. As shown in Fig. S21 in the Supplementary Information, the light intensity had a linear relationship with the current applied. Accordingly, WLEDs were fabricated by combining the Green-Z and Red-Z composites with blue LED chips (430 nm). The emission spectrum of the WLED is presented in Fig. 6c, and the triangle for the CIE color coordinates in the CIE 1931 chromaticity diagram is displayed in Fig. 6d. Three obvious emission peaks were observed at 430, 517, and 687 nm, and the CIE chromaticity coordinates were (0.17, 0.01), (0.13, 0.77), and (0.73, 0.27), respectively. The luminous efficiency of the WLED was 6.8 lm/W at a current of 20 mA. The chromaticity coordinate of the WLED was (0.32, 0.33), and the color temperature (CCT) was 6062 K, close to that of standard white light. The area of the triangle was calculated to be 138% of the National Television System Committee (NTSC) standard and 103% of the ITU-R Recommendation BT.2020 (Rec.2020.), demonstrating an ultrabroad color range.
The CsPbX3-ZSM-5 composites with narrow emission bands and tunable colors also have ample potential for use in security printing technology. Except for the intrinsic UV-excited on/off phenomenon, secondary anti-counterfeiting coding is also quite useful. As mentioned above, the WLEDs exhibited three discrete primary color peaks, thereby providing the possibility of anti-counterfeiting responses coded with the three colors. As shown in Fig. 6e, patterns were printed by blending Blue-Z, Green-Z, and Red-Z with epoxy resin. Similar white emissions with different emission spectra were achieved by mixing the three composites in different ratios, which made it difficult for the naked eye to discern the differences. Therefore, the concept of white light-coded anti-counterfeiting is proposed. The rules for coding were enacted and shown in Fig. 6e. First, blue, green, and red emissions were denoted as “0”, “1”, and “2”, respectively. Second, one of the white light spectra was selected as a reference and encoded as (0 1 2). Finally, the relative increase in a specific peak intensity compared to the reference was denoted as “∧” on the top of the corresponding number; otherwise, it was denoted as “∨”. For example, white light with a stronger blue emission peak would be noted as (\(\hat 0\) 1 2). To assess the practicality, a batch of fluorescent inks exhibiting white emission was prepared, and based on the aforementioned coding rules, symbols were printed with the four different inks, as shown in Fig. 6e. Although all parts of the symbols showed white emission, they had different spectra. These results revealed the excellent potential of the composites for use in anti-counterfeiting coding.
Conclusions
A novel chemical vapor method was designed and demonstrated for large-scale syntheses of CsPbX3-ZSM-5 composites without the need for organic solvents, organic ligands or an inert environment. Confined growth of CsPbX3 inside the nanopores of ZSM-5 was observed because of reactions between PbBr2 vapor and the Si–O network of ZSM-5, and the resulting encapsulation structure provided the channels needed for halogen exchange. This method offered scalable production of extremely stable composites that exhibited tunable emissions with high PLQYs, narrow emission FWHMs, heat resistance up to 200 °C and radiation resistance, as demonstrated by continuous UV radiation, for 500 h. After immersion in water for 30 days or polar solvents for 180 days, the PL intensity exhibited almost no change. Furthermore, the composites survived aging (80 °C, 80% R.H.) and retained 90% of the initial PL intensity after 30 days. The narrow emissions, high PLQYs and outstanding stability make the CsPbX3-ZSM-5 composites promising for lighting and display applications, especially WLEDs with large color ranges and multicolor-coded anti-counterfeiting inks. This facile, organic-free and ambient atmosphere process indicates the probability for commercial production of these perovskite composites with robust stability.
References
Smock, S. R., Chen, Y., Rossini, A. J. & Brutchey, R. L. The surface chemistry and structure of colloidal lead halide perovskite nanocrystals. Acc. Chem. Res. 54, 707–718 (2021).
Yu, J. et al. Perovskite CsPbBr3 crystals: Growth and applications. J. Mater. Chem. C 8, 6326–6341 (2020).
Kovalenko, M. V., Protesescu, L. & Bodnarchuk, M. I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 358, 745–750 (2017).
Yu, X. et al. Hydrochromic CsPbBr3 nanocrystals for anti-counterfeiting. Angew. Chem., Int. Ed. 59, 14527–14532 (2020).
Smith, M. D., Connor, B. A. & Karunadasa, H. I. Tuning the luminescence of layered halide perovskites. Chem. Rev. 119, 3104–3139 (2019).
Pan, A. et al. Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: the role of organic acid, base, and cesium precursors. ACS Nano 10, 7943–7954 (2016).
Shamsi, J., Urban, A. S., Imran, M., De Trizio, L. & Manna, L. Metal halide perovskite nanocrystals: synthesis, post-synthesis modifications, and their optical properties. Chem. Rev. 119, 3296–3348 (2019).
Zhang, S. et al. Barrier designs in perovskite solar cells for long-term stability. Adv. Energy Mater. 10, 2001610 (2020).
Tong, Y. et al. One-pot synthesis of CsPbX3 (X = Cl, Br, I)@zeolite: A potential material for wide-color-gamut backlit displays and upconversion emission. Adv. Opt. Mater. 9, 2100012 (2021).
Wei, Y., Cheng, Z. & Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 48, 310–350 (2019).
Yin, J. et al. Luminescence and stability enhancement of inorganic perovskite nanocrystals via selective surface ligand binding. ACS Nano 15, 17998–18005 (2021).
Lin, Y. et al. All-inorganic encapsulation for remarkably stable cesium lead halide perovskite nanocrystals: toward full-color display applications. J. Mater. Chem. C 9, 12303–12313 (2021).
Lin, Y. et al. Remarkable black-phase robustness of CsPbI3 nanocrystals sealed in solid SiO2/AlOx sub-micron particles. Small 17, 2103510 (2021).
Zhang, Q. et al. Ceramic-like stable CsPbBr3 nanocrystals encapsulated in silica derived from molecular sieve templates. Nat. Commun. 11, 31 (2020).
Wang, P. et al. Ultrastable perovskite–zeolite composite enabled by encapsulation and in situ passivation. Angew. Chem., Int. Ed. 59, 23100–23106 (2020).
Sun, J.-Y. et al. Facile two-step synthesis of all-inorganic perovskite CsPbX3 (X = Cl, Br, I) zeolite-Y composite phosphors for potential backlight display application. Adv. Funct. Mater. 27, 1704371 (2017).
Ravi, V. K., Saikia, S., Yadav, S., Nawale, V. V. & Nag, A. CsPbBr3/ZnS core/shell type nanocrystals for enhancing luminescence lifetime and water stability. ACS Energy Lett. 5, 1794–1796 (2020).
Imran, M. et al. Halide perovskite–lead chalcohalide nanocrystal heterostructures. J. Am. Chem. Soc. 143, 1435–1446 (2021).
Wang, Z. et al. One-step polymeric melt encapsulation method to prepare CsPbBr3 perovskite quantum dots/polymethyl methacrylate composite with high performance. Adv. Funct. Mater. 31, 2010009 (2021).
Yang, W. et al. CsPbBr3-quantum-dots/polystyrene@silica hybrid microsphere structures with significantly improved stability for white LEDs. Adv. Opt. Mater. 7, 1900546 (2019).
Qiao, G.-Y. et al. Perovskite quantum dots encapsulated in a mesoporous metal–organic framework as synergistic photocathode materials. J. Am. Chem. Soc. 143, 14253–14260 (2021).
Wan, S., Ou, M., Zhong, Q. & Wang, X. Perovskite-type CsPbBr3 quantum dots/Uio-66(NH2) nanojunction as efficient visible-light-driven photocatalyst for CO2 reduction. Chem. Eng. J. 358, 1287–1295 (2019).
Hou, J. et al. Liquid-phase sintering of lead halide perovskites and metal-organic framework glasses. Science 374, 621–625 (2021).
Jang, J. et al. Extremely stable luminescent crosslinked perovskite nanoparticles under harsh environments over 1.5 years. Adv. Mater. 33, 2005255 (2021).
Li, X. et al. Chemical vapor deposition for N/S-doped single Fe site catalysts for the oxygen reduction in direct methanol fuel cells. ACS Catal. 11, 7450–7459 (2021).
Wang, H. & Yu, G. Direct CVD graphene growth on semiconductors and dielectrics for transfer-free device fabrication. Adv. Mater. 28, 4956–4975 (2016).
Almutairi, S. M. T., Mezari, B., Magusin, P. C. M. M., Pidko, E. A. & Hensen, E. J. M. Structure and reactivity of Zn-modified ZSM-5 zeolites: The importance of clustered cationic Zn complexes. ACS Catal. 2, 71–83 (2012).
Ou, X. et al. Hierarchical Fe-ZSM-5/SiC foam catalyst as the foam bed catalytic reactor (FBCR) for catalytic wet peroxide oxidation (CWPO). Chem. Eng. J. 362, 53–62 (2019).
Kübel, C. et al. Recent advances in electron tomography: TEM and HAADF-STEM tomography for materials science and semiconductor applications. Microsc. Microanal. 11, 378–400 (2005).
Coudurier, G., Naccache, C. & Vedrine, J. C. Uses of I.R. spectroscopy in identifying ZSM zeolite structure. J. Chem. Soc., Chem. Commun. 24, 1413–1415 (1982).
Isernia, L. F. FTIR study of the relation, between extra-framework aluminum species and the adsorbed molecular water, and its effect on the acidity in ZSM-5 steamed zeolite. Mater. Res. 16, 792–802 (2013).
Wang, H. et al. Encapsulating silica/antimony into porous electrospun carbon nanofibers with robust structure stability for high-efficiency lithium storage. ACS Nano 12, 3406–3416 (2018).
Selim, A. Q. et al. Statistical physics-based analysis of the adsorption of Cu2+ and Zn2+ onto synthetic cancrinite in single-compound and binary systems. J. Environ. Chem. Eng. 7, 103217 (2019).
Brennan, M. C. et al. Universal size-dependent Stokes shifts in lead halide perovskite nanocrystals. J. Phys. Chem. Lett. 11, 4937–4944 (2020).
Nedelcu, G. et al. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 15, 5635–5640 (2015).
Imran, M. et al. Shape-pure, nearly monodispersed CsPbBr3 nanocubes prepared using secondary aliphatic amines. Nano Lett. 18, 7822–7831 (2018).
Huang, X. et al. Reversible 3D laser printing of perovskite quantum dots inside a transparent medium. Nat. Photonics 14, 82–88 (2020).
Li, X. et al. CsPbBr3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins, and white light-emitting diodes. Adv. Funct. Mater. 26, 2435–2445 (2016).
Wei, Y. et al. Epitaxial growth of CsPbX3 (X = Cl, Br, I) perovskite quantum dots via surface chemical conversion of Cs2GeF6 double perovskites: a novel strategy for the formation of leadless hybrid perovskite phosphors with enhanced stability. Adv. Mater. 31, 1807592 (2019).
Acknowledgements
The work was jointly supported by the National Natural Science Foundation of China No. 21975280, Guangdong Provincial Natural Science Foundation No. 2020A1515110831, Shenzhen Science and Technology Research Funding No. JCYJ20180507182047316, Shenzhen Science and Technology Research Funding No. RCJC20200714114435061, National Natural Science Foundation of China No. 62004091, Natural Science Foundation of Guangdong Province No. 2022A1515011959, and City University of Hong Kong Strategic Research Grant No. 7005505.
Author information
Authors and Affiliations
Contributions
H.H. and X.-F.Y. conceived the original research idea. Experiments, including CsPbX3-ZSM composite syntheses, CsPbX3-ZSM-5/PDMS disks, LED packing, anti-counterfeiting ink preparation and stability tests, were performed by T.Y.S. and X.C., Y.H.D., H.H., J.H.W., R.H., and Y.L.L. X.C.H. carried out the characterizations, and J.L. assisted with data analysis. T.Y.S., X.C., H.H., P.K.C., and X.-F.Y. wrote this manuscript. All authors discussed the results, interpreted the findings, and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41427_2022_433_MOESM1_ESM.doc
Scalable synthesis of ultrastable lead halide perovskite-zeolite composites via a chemical vapor method in air Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, T., Chen, X., Deng, Y. et al. Scalable synthesis of ultrastable lead halide perovskite-zeolite composites via a chemical vapor method in air. NPG Asia Mater 14, 87 (2022). https://doi.org/10.1038/s41427-022-00433-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-022-00433-0
- Springer Japan KK