Abstract
Acute kidney injury (AKI) is one of the serious clinical syndromes with high morbidity and mortality. Despite substantial progress in understanding the mechanism of AKI, no effective drug is available for treatment or prevention. In this study, we identified that a ligand-activated transcription factor aryl hydrocarbon receptor (AhR) was abnormally increased in the kidneys of cisplatin-induced AKI mice or tubular epithelial TCMK-1 cells. The AhR inhibition by BAY2416964 and tubular conditional deletion both alleviated cisplatin-induced kidney dysfunction and tubular injury. Notably, inhibition of AhR could improve cellular senescence of injured kidneys, which was indicated by senescence-associated β-galactosidase (SA-β-gal) activity, biomarker p53, p21, p16 expression, and secretory-associated secretory phenotype IL-1β, IL-6 and TNFα level. Mechanistically, the abnormal AhR expression was positively correlated with the increase of a methyltransferase EZH2, and AhR inhibition suppressed the EZH2 expression in cisplatin-injured kidneys. Furthermore, the result of ChIP assay displayed that EZH2 might indirectly interact with AhR promoter region by affecting H3K27me3. The direct recruitment between H3K27me3 and AhR promoter is higher in the kidneys of control than that of cisplatin-treated mice, suggesting EZH2 reversely influenced AhR expression through weakening H3K27me3 transcriptional inhibition on AhR promoter. The present study implicated that AhR and EZH2 have mutual regulation, which further accelerated tubular senescence in cisplatin-induced AKI. Notably, the crucial role of AhR is potential to become a promising target for AKI.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is a common clinical syndrome and a major health issue, which refers to a rapid decline of kidney function in a short period of time caused by a variety of factors, such as renal hypoperfusion, trauma, sepsis, and toxic drugs, etc [1,2,3]. Approximately 13.3 million people suffer from AKI every year [4], and 30 ~ 70% of AKI patients could develop into chronic kidney disease (CKD) or end-stage kidney disease (ESKD) [5], and about 1.7 million of the deaths are caused by AKI [6, 7]. As we known, cisplatin is an antitumor chemotherapy drug, but one-third of cancer patients receiving cisplatin chemotherapy are susceptible to AKI [8]. However, practical strategies for treating cisplatin-induced AKI are still lacking. Therefore, focusing on the mechanism of cisplatin-induced AKI is of great significance for drug discovery and the improvement in the quality of the population associated with cisplatin nephrotoxicity.
The increasing studies reported that cisplatin could stimulate oxidative stress and induce cellular senescence [7h). These results indicated that the inhibition of H3K27me3 on AhR gene promoters was weakened, and the expression of AhR was up-regulated in cisplatin-induced AKI mice.
Effect of EZH2 inhibition on AhR-mediated tubular epithelial cell senescence
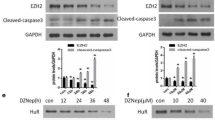
As previously mentioned, AhR regulated cellular senescence and affected EZH2 expression in injured kidneys. However, whether EZH2 participates in the process of AhR-mediated senescence is unknown. To solve the problem, we assessed anti-senescent effect of EZH2 inhibitor zld1039 with AhR agonist FICZ in TCMK-1 cells [24, 27]. Cisplatin triggered the upregulation of SAGs (p16, p21, p53), and EZH2 inhibitor zld1039 repressed senescent mRNA level (Fig. 8a). Furthermore, whether AhR affected senescence through the direct or indirect regulation of EZH2 remain unclear. Here, we used to FICZ agonist activate AhR with the inhibition of EZH2 by zld1039 in TCMK-cells. Consequently, the senescent mRNA level of p16, p21 and p53 were increased following AhR agonist, while EZH2 inhibitor suppressed their corresponding expression (Fig. 8b). These results indicated that the upregulation of EZH2 is necessary for AhR to accelerate cisplatin-induced senescence.
a The mRNA level of senescence related p16, p21, and p53 in cisplatin-induced AKI with or without EZH2 inhibitor zld1039 (EZH2i, n = 4). b The mRNA level of senescence related p16, p21 and p53 in AhR activation (AhRa) by FICZ with zld1039 (n = 4). Data are represented as means ± SDs. cis, cisplatin. ****P < 0.0001, ***P < 0.001, **p < 0.01, *p < 0.05.
Discussion
Illustrating the potential mechanism of AhR-mediated cellular senescence is of great significance in identifying the cisplatin-induced AKI therapeutic targets. In this study, a novel finding was proposed that AhR-associated cellular senescence was involved in cisplatin-induced AKI. We found that the abnormal expression of AhR was positively associated with cisplatin-induced senescence. Inhibition of AhR by BAY2416964 or tubule-specific gene deletion significantly suppressed cellular senescence and alleviated cisplatin-induced kidney injury. Notably, further studies indicated that EZH2, a histone methyltransferase, was a pivotal factor in AhR-mediated cellular senescence in kidney injury.
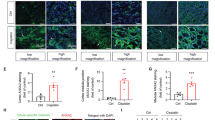
Cellular senescence is a proven process of AKI maladaptive repair and kidney fibrosis [10]. A previous study has demonstrated that repeated low-dose cisplatin led to cell cycle arrest at the G2/M phase and cellular senescence, which indicated cellular senescence played a role in cisplatin-induced kidney injury [28]. Here, an increase of β-galactosidase activity, a known characteristic of senescent cells, SAGs (p16, p21, p53) and SASPs (IL-1β, IL-6, TNF-α) were also observed in the kidneys of cisplatin group compared with that of control mice. These results are consistent with those of Li et al. who confirmed that cisplatin-induced cellular senescence in tubular epithelium, could accelerate the progression of renal fibrosis [9].
AhR is an essential ligand-activated transcription factor [29]. A distinct time-dependent and tissue-specific AhR activation is displayed in different mouse models of kidney diseases [30]. Growing evidence presented that AhR activation by uremic toxins, like indoxyl sulfate, plays a harmful role in CKD progression [31, 32]. Whereas, the role of AhR in AKI remains controversial [17, 18]. Tao et al. thought that AhR activation alleviated renal injury in rhabdomyolysis and IR-induced mice by inhibiting inflammation and apoptosis [17]. Eleftheriadis et al. demonstrated that AhR pathway activation enhanced DNA damage response and promoted primary proximal renal tubular epithelial cells senescence, eventually leading to IR-induced kidney injury [18]. Therefore, the contradictory effect of AhR was explored in our study. Here, our results revealed that AhR activation accelerated the progression of kidney injury through a cellular senescence-related mechanism in a cisplatin-induced AKI mice. The AhR inhibition by BAY2416964 or tubule-specific gene deletion repressed cisplatin-induced cellular senescence, which implied that AhR might be one of the causative mechanisms of cisplatin-associated cellular senescence, and inhibition of AhR may be a promising therapeutic strategy against AKI.
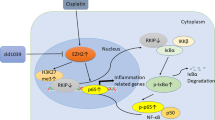
Although the relationship between AhR and tubular senescence was observed in our study, we cannot clear the possible mechanisms of AhR-mediated tubular senescence in kidneys of cisplatin-induced mice. EZH2, a catalytic subunit of polycomb repressive complex 2 (PRC2), is an H3K27 histone methyltransferase [33]. Another point noticed is that H3K27me3 is mainly responsible for silencing genes, so it usually acts as a transcriptional suppressor [34]. It has been reported that EZH2 played a significant role in multiple tumor progression by affecting cellular senescence [35, 36]. In particular matter-induced skin keratinocytes, skin senescence depended on AhR-induced ROS as well as the decrease in EZH2 and H3K27me3 [37]. Meanwhile, AhR activation could enhance the EZH2 activity and increase its epigenetic silencing activity, which is a risk factor for environmental toxicant-associated pancreatitis and pancreatic cancer [26]. Not only that, EZH2 binds to the AhR promoter to repress the expression of AhR gene [38]. From this, we thought that EZH2 might play a role in AhR-mediated cellular senescence. Herein, we firstly investigated the correlation between AhR and EZH2. As expected, our results revealed that the mRNA level of AhR and EZH2 were significantly correlated in kidney RNA-sequencing data of cisplatin-stimulated mice. Cisplatin stimulation concurrently up-regulated AhR and EZH2 expression, and AhR inhibitor BAY2416964 or tubule specific AhR deficiency suppressed the expression of EZH2. In vitro, the expression of AhR and EZH2 were increased in cisplatin-treated TCMK-1 cells. The AhR knockdown reversed the elevation of AhR as well as EZH2, suggesting that EZH2 may be a potential mediator in AhR-mediated cellular senescence. Furthermore, we found that EZH2 inhibitor zld1039 indeed improved cisplatin-induced cellular senescence. Activating AhR by agonist FICZ and inhibiting EZH2 by zld1039 repressed the mRNA level of senescent p16, p21 and p53 in cisplatin-stimulated TCMK-1 cells, suggesting that EZH2 is necessary for AhR to accelerate cisplatin-induced cellular senescence.
Importantly, considering the crucial role of EZH2 in epigenetic regulation and combining with the previous results of Ko et al. [38], we further explored the influence of EZH2 knockdown on the expression of AhR. Surprisingly, EZH2 silencing reversely repressed the upregulation of AhR in cisplatin-treated TCMK-1 cells. To illustrate how EZH2 regulates AhR, we used a ChIP assay to examine the enrichment between EZH2 or H3K27me3 and the AhR promoter regions. Consequently, H3K27me3 is responsible for exerting transcriptional inhibition effect in kidneys of control mice, because of the rich enrichment between H3K27me3 and AhR promoter region, which repressed the expression of AhR. Nevertheless, the weaken enrichment of them has been described in kidneys of cisplatin mice, indicating that the rare enrichment of H3K27me3 and AhR promoter might cause cisplatin-induced AhR expression. These results identify that EZH2 also is one of the positive regulators of AhR expression by affecting the enrichment between H3K27me3 and AhR promoter.
In conclusion, our finding demonstrated that AhR was abnormally expressed in kidneys of cisplatin-induced mice and AhR inhibition alleviated cisplatin-induced cellular senescence and tubular injury against AKI. Mechanistically, our study indicated that AhR and EZH2 have mutual regulation, which accelerated tubular senescence in cisplatin-induced AKI. Notably, the crucial role of AhR is the potential to become a promising target for AKI.
Material and methods
Agents and antibodies
Cisplatin and BAY 2416964 were purchased from Synguider (Chengdu, China) and Selleck Chemicals (America), zld1039 was presented by the State Key of Laboratory of Biotherapy, Sichuan University (Sichuan, China), and FICZ was purchased from Selleck Chemicals (America), respectively. All primary antibodies were displayed in Supplementary Table 1.
Animal experiments
Animal experimental procedures were licensed and permitted by the Animal Care and Use Ethics Committee of Sichuan University (2020205 A). Male C57BL/6 J mice were clarified previously [24]. C57BL/6 J mice were administered cisplatin (20 mg/kg) with or without BAY 2416964 (20 mg/kg) by intraperitoneal injection. Tubule specific AhR knockout mice were obtained from the GemPharmatech Co.,Ltd. (Jiangsu, China). AhR tecKO mice were injected cisplatin intraperitoneally to induce AKI.
Cell culture and treatment
Mouse renal tubular epithelial cells (TCMK-1) were purchased from ATCC agency Shanghai Limai Biological Engineering Co., Ltd (Shanghai, China) and were cultured in MEM medium (G4550-500ML, Sercicebio) containing 10% fetal bovine serum (FBS) (SH30084.03, Hyclone) at 37 °C under 5% CO2-95% air environment. TCMK-1 cells were starved in 0.5% FBS medium for 6 h and then treated with 10 μg/ml cisplatin for another 24 h. The siRNAs were used to knock down AhR and EZH2. The transfection procedure is detailed in the riboFECT™ CP transfection kit instruction. AhR-siRNA, EZH2-siRNA and negative control (NC) siRNA were designed and synthesized by GenePharma (Shanghai, China). The detailed transfection sequences information of them is provided in Supplementary Table 2.
Public single-cell RNA-seq analysis
The single-cell RNA sequencing database from http://humphreyslab.com/SingleCell/ was used for single-cell data analysis. According to the database, we checked the single-cell RNA sequencing data about AhR in the healthy adult humans, healthy mice, and ischemia-reperfusion injury mouse kidneys.
Renal function
An automatic biochemical analyzer (Mindray BS-240) was taken to assess Scr and BUN. We defined that the cisplatin-induced AKI mouse model was successfully established, when the Scr value in the cisplatin group was higher than twice that in the control group.
Pathological examination
The kidney tissues were fixed, embedded, and sectioned for Periodic Acid-Schiff (PAS) staining. Renal tubular damage semi-quantitatively scores were used to assess the pathological injury. The specific score standards and detailed rules have been shown in previous study [24].
Immunofluorescence staining
Paraffin kidney tissue sections were firstly deparaffinized and dehydrated. Then, using the microwave method repairs the antigen. After antigen retrieval, the sections were sealed with 1× horse serum containing 0.3% Triton (Sigma, America) for 1 h at 37 °C. And then, they were incubated with primary antibody (the concentration is determined according to the instructions) overnight at 4 °C. Washing the sections and incubating the corresponding secondary antibody and lectin (1:400 dilution) for 1 h at room temperature. Then, the sections were rewashed. DAPI (D8200; Solarbio) was used to stain nuclei for 5 min. Finally, 50% glycerin was used to seal the sections. Photographs were collected from ZEN 2012 microscopy software [39].
Senescence β-galactosidase staining
The senescence β-galactosidase staining kit (Cell Signaling Technology) was used to detect β-galactosidase activity, a known characteristic of senescent cells. For SA β-Gal staining of frozen renal tissues, frozen sections were fixed with 1× fixative solution for 10–15 min at room temperature. Washing the sections with 1×PBS. Then, added the β-galactosidase staining solution to the sections and incubated them at 37 °C overnight in a dry incubator (no CO2). The senescent cells showed blue color under a microscope.
ChIP assay
Proteins and DNA interaction was evaluated by ChIP-qPCR using the ChIP assay kit (Millipore, MA, USA). The experiment protocols were according to the manufacturer’s instructions. The antibodies used for the ChIP assay were as follows: anti-H3k27me3 (Cell Signaling Technology) and control IgG (Millipore). The primers used for ChIP were as follows: AhR-F 5’-GTCAACGACATTTGCGTCCT-3’, AhR-R 5’-TCCCCTTAAGAATTTCAACTGTCC-3’. The calculation formula for enrichment efficiency was elaborated on previously [24].
Western blot analysis
Western blotting was carried out as described earlier [24]. Densitometry analysis was evaluated by using ImageJ 6.0 software (National Institutes of Health, Bethesda, MD, USA). Gray density was normalized using internal reference proteins GAPDH or Histone 3. To ensure the repeatability of the experiment, all immunoblot bands were repeated three times.
Quantitative real-time PCR
Total RNA separation and purification steps and RT-qPCR protocols were displayed as previously shown [24]. The corresponding gene primers were listed in Table S2. The 2−ΔΔCt method was used to calculate the relative gene quantities, and GAPDH was used as the internal reference gene.
Statistical analysis
All quantitative data were presented as mean ± standard deviation (SD). Statistical difference comparisons between the two groups were performed using the T-test. Comparisons between three or multiple groups were performed using a one-way analysis of variance (Tukey’s test). Prism 9.0 (GraphPad Software, San Diego, CA, USA) was used to draw statistical graphs, and P-value less than 0.05 was considered statistically significant.
Data availability
All data supporting this research has been included in this manuscript and its supplementary information files. The RNA-sequencing data for this study are available in the Gene Expression Omnibus database under accession number GSE106993. Additionally, the single-cell RNA sequencing data are from http://humphreyslab.com/SingleCell/.
References
Wen X, Murugan R, Peng Z, Kellum JA. Pathophysiology of acute kidney injury: A new perspective. Contrib Nephrol. 2010;165:39–45.
Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: An increasing global concern. Lancet 2013;382:170–9.
Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet 2019;394:1949–64.
Bawaskar PH, Bawaskar PH, Bawaskar HS. Eliminating acute kidney injury by 2025: An achievable goal for India. Lancet 2015;386:855.
Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z. Rodent models of AKI-CKD transition. Am J Physiol Ren Physiol. 2018;315:F1098–106.
Coelho S, Cabral G, Lopes JA, Jacinto A. Renal regeneration after acute kidney injury. Nephrology 2018;23:805–14.
Guo C, Dong G, Liang X, Dong Z. Epigenetic regulation in AKI and kidney repair: Mechanisms and therapeutic implications. Nat Rev Nephrol. 2019;15:220–39.
Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78:743–50.
Li C, **e N, Li Y, Liu C, Hou FF, Wang J. N-acetylcysteine ameliorates cisplatin-induced renal senescence and renal interstitial fibrosis through sirtuin1 activation and p53 deacetylation. Free Radic Bio Med. 2019;130:512–27.
Sears SM, Siskind LJ. Potential therapeutic targets for cisplatin-induced kidney injury: Lessons from other models of AKI and fibrosis. J Am Soc Nephrol. 2021;32:1559.
Andrade L, Rodrigues CE, Gomes SA, Noronha IL. Acute kidney injury as a condition of renal senescence. Cell Transpl. 2018;27:739–53.
Johnson AC, Zager RA. Plasma and urinary p21: Potential biomarkers of AKI and renal aging. Am J Physiol Ren Physiol. 2018;315:F1329–35.
Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, et al. Sirtuin 3–dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest. 2015;125:715–26.
Wang W-G, Sun W-X, Gao B-S, Lian X, Zhou H-L. Cell cycle arrest as a therapeutic target of acute kidney injury. Curr Protein Pept Sc. 2017;18:1224–31.
Wang Z, Li T, Mao C, Liu W, Tao Y. IL4I1-driven AHR signature: A new avenue for cancer therapy. Sig Transduct Target Ther. 2021;6:118.
Zhu Q, Ma Y, Liang J, Wei Z, Li M, Zhang Y, et al. AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Sig Transduct Target Ther. 2021;6:299.
Tao S, Guo F, Ren Q, Liu J, Wei T, Li L, et al. Activation of aryl hydrocarbon receptor by 6-formylindolo[3,2-b]carbazole alleviated acute kidney injury by repressing inflammation and apoptosis. J Cell Mol Med. 2021;25:1035–47.
Eleftheriadis T, Pissas G, Filippidis G, Liakopoulos V, Stefanidis I. The role of Indoleamine 2,3-Dioxygenase in renal tubular epithelial cells senescence under anoxia or reoxygenation. Biomolecules 2021;11:1522.
Späth MR, Bartram MP, Palacio-Escat N, Hoyer KJR, Debes C, Demir F, et al. The proteome microenvironment determines the protective effect of preconditioning in cisplatin-induced acute kidney injury. Kidney Int. 2019;95:333–49.
Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;15:264–76.
Ren F, Ji C, Huang Y, Aniagu S, Jiang Y, Chen T. AHR-mediated ROS production contributes to the cardiac developmental toxicity of PM2.5 in zebrafish embryos. Sci Total Environ. 2020;719:135097.
Yang H, Fogo AB. Cell senescence in the aging kidney. J Am Soc Nephrol. 2010;21:1436.
Yabuuchi N, Hou H, Gunda N, Narita Y, Jono H, Saito H. Suppressed hepatic production of Indoxyl Sulfate Attenuates Cisplatin-induced acute kidney injury in Sulfotransferase 1a1-deficient mice. Int J Mol Sci. 2021;22:1764.
Wen L, Tao S-H, Guo F, Li L-Z, Yang H-L, Liang Y, et al. Selective EZH2 inhibitor zld1039 alleviates inflammation in cisplatin-induced acute kidney injury partially by enhancing RKIP and suppressing NF-κB p65 pathway. Acta Pharm Sin. 2021;43:2067–80.
Tao S, Yang L, Wu C, Hu Y, Guo F, Ren Q, et al. Gambogenic acid alleviates kidney fibrosis via epigenetic inhibition of EZH2 to regulate Smad7-dependent mechanism. Phytomedicine 2022;106:154390.
Lee J-E, Cho S-G, Ko S-G, Ahrmad SA, Puga A, Kim K. Regulation of a long noncoding RNA MALAT1 by aryl hydrocarbon receptor in pancreatic cancer cells and tissues. Biochem Bioph Res Comm. 2020;532:563–9.
Song X, Gao T, Wang N, Feng Q, You X, Ye T, et al. Correction: Corrigendum: Selective inhibition of EZH2 by ZLD1039 blocks H3K27methylation and leads to potent anti-tumor activity in breast cancer. Sci Rep. 2016;6:24893.
Sharp CN, Doll MA, Dupre TV, Shah PP, Subathra M, Siow D, et al. Repeated administration of low-dose cisplatin in mice induces fibrosis. Am J Physiol-Ren. 2016;310:F560–8.
Vondráček J, Machala M. Environmental ligands of the Aryl hydrocarbon receptor and their effects in models of adult liver progenitor cells. Stem Cells Int. 2016;2016:4326194.
Walker JA, Richards S, Belghasem ME, Arinze N, Yoo SB, Tashjian JY, et al. Temporal and tissue-specific activation of aryl hydrocarbon receptor in discrete mouse models of kidney disease. Kidney Int. 2020;97:538–50.
Dou L, Poitevin S, Sallée M, Addi T, Gondouin B, McKay N, et al. Aryl hydrocarbon receptor is activated in patients and mice with chronic kidney disease. Kidney Int. 2018;93:986–99.
Ren Q, Cheng L, Guo F, Tao S, Zhang C, Ma L, et al. Fisetin improves hyperuricemia-induced chronic kidney disease via regulating gut microbiota-mediated tryptophan metabolism and aryl hydrocarbon receptor activation. J Agr Food Chem. 2021;69:10932–42.
Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell 2007;128:735–45.
Sauvageau M, Sauvageau G. Polycomb group proteins: Multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7:299–13.
Liu C, Liu L, Yang M, Li B, Yi J, Ai X, et al. A positive feedback loop between EZH2 and NOX4 regulates nucleus pulposus cell senescence in age-related intervertebral disc degeneration. Cell Div. 2020;15:2.
Chen Y, Pan K, Wang P, Cao Z, Wang W, Wang S, et al. HBP1-mediated regulation of p21 protein through the Mdm2/p53 and TCF4/EZH2 pathways and its impact on cell senescence and tumorigenesis*. J Biol Chem. 2016;291:12688–705.
Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM, Bossis G. et al. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp Mol Med. 2019;51:1–14.
Ko C-I, Wang Q, Fan Y, **a Y, Puga A. Pluripotency factors and Polycomb Group proteins repress aryl hydrocarbon receptor expression in murine embryonic stem cells. Stem Cell Res. 2014;12:296–308.
Wang B, Xu J, Ren Q, Cheng L, Guo F, Liang Y, et al. Fatty acid-binding protein 4 is a therapeutic target for septic acute kidney injury by regulating inflammatory response and cell apoptosis. Cell Death Dis. 2022;13:333.
Acknowledgements
This study was supported by a grant from the National Key R&D Program of China (No. 2020YFC2005000), National Natural Science Foundation of China (82070711), and Science/Technology Project of Sichuan province (No. 2020YFQ0055).
Author information
Authors and Affiliations
Contributions
LM, and LW designed experiments. LW, QR, FG, YL, XD, PF, and LM performed experiments. LW and LM analyzed the data. LW, and LM wrote the draft of the manuscript. All authors have read the manuscript and approved the submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Our research was approved by the Experimental Animal Ethics Committee of West China Hospital of Sichuan University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Boris Zhivotovsky
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wen, L., Ren, Q., Guo, F. et al. Tubular aryl hydratocarbon receptor upregulates EZH2 to promote cellular senescence in cisplatin-induced acute kidney injury. Cell Death Dis 14, 18 (2023). https://doi.org/10.1038/s41419-022-05492-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-05492-3
- Springer Nature Limited
Keywords
This article is cited by
-
Nucleic acid and protein methylation modification in renal diseases
Acta Pharmacologica Sinica (2024)
-
Cellular senescence of renal tubular epithelial cells in acute kidney injury
Cell Death Discovery (2024)
-
Network pharmacology combined with molecular docking and dynamics to assess the synergism of esculetin and phloretin against acute kidney injury-diabetes comorbidity
Molecular Diversity (2024)