Abstract
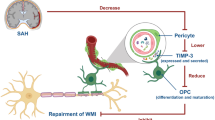
Oligodendrocyte progenitor cells (OPCs) differentiate to myelin-producing mature oligodendrocytes and enwrap growing or demyelinated axons during development and post central nervous diseases. Failure of remyelination owing to cell death or undifferentiation of OPCs contributes to severe neurologic deficits and motor dysfunction. However, how to prevent the cell death of OPCs is still poorly understood, especially in hemorrhagic diseases. In the current study, we injected autologous blood into the mouse lateral ventricular to study the hemorrhage-induced OPC cell death in vivo. The integrity of the myelin sheath of the corpus callosum was disrupted post intraventricular hemorrhage (IVH) assessed by using magnetic resonance imaging, immunostaining, and transmission electron microscopy. Consistent with the severe demethylation, we observed massive cell death of oligodendrocyte lineages in the periventricular area. In addition, we found that ferroptosis is the major cell death form in Hemin-induced OPC death by using RNA-seq analysis, and the mechanism was glutathione peroxidase 4 activity reduction-resulted lipid peroxide accumulation. Furthermore, inhibition of ferroptosis rescued OPC cell death in vitro, and in vivo attenuated IVH-induced white matter injury and promoted recovery of neurological function. These data demonstrate that ferroptosis is an essential form of OPC cell death in hemorrhagic stroke, and rescuing ferroptotic OPCs could serve as a therapeutic target for stroke and related diseases.
Similar content being viewed by others
Introduction
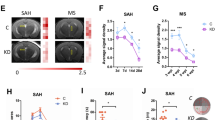
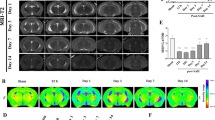
Intracerebral hemorrhage (ICH) accounts for 10–30% of all stroke subtypes, but with mortality of 50% at 1 year [1, 2]. The most frequent occurrence location of ICH is basal ganglia and thalamus, which are rich in white matter fibers [3]. When ICH occurs, blood enters the brain parenchyma from the ruptured blood vessel, leading to primary and secondary brain injury, both of which impact the structure and function of white matter fibers [4, 5]. Studies have reported that white matter injury (WMI) often occurs after ICH, and is considered an important predictor of the outcome [6]. However, most of the previous treatments for ICH focus on neuronal protection but paid insufficient attention to the protection of WMI. Therefore, the repair of WMI is expected to become a new treatment strategy for ICH.
Oligodendrocyte progenitor cells (OPCs) present as a pool of migratory and proliferative progenitor cells in the adult central nervous system (CNS), which differentiate into mature oligodendrocytes and re-myelinate damaged axons in diseases such as multiple sclerosis and stroke [7]. However, OPCs and oligodendrocytes are both vulnerable to cytotoxic and excitotoxic factors, and in vivo, how to differentiate OPCs to mature oligodendrocytes and remyelinate damaged axons has always been a difficult problem to overcome [8, 9]. Therefore, inhibiting OPC cell death and maintaining the stability of the OPC pool is the prerequisite for myelin repair.
Ferroptosis is a newly identified type of cell death, driven by iron-dependent phospholipid peroxidation [10]. This unique cell death is regulated by multiple cellular metabolic events, including iron metabolism, redox homeostasis, amino acid metabolism, mitochondrial function, and numerous signaling pathways [11]. Glutathione peroxidase 4 (GPx4) is an antioxidant enzyme catalyzing the glutathione (GSH)-dependent reduction of membrane lipid hydroperoxides (L-OOH) to lipid alcohols (L-OH), which limits the lipid peroxide in the cell membrane. Cystine/glutamate antiporter system χc–/GSH/GPX4 axis is a classical pathway protecting against ferroptosis, in which GPx4 is considered to be the key regulator in cancer cells and neurons [12,13,14,15]. Although we and others have demonstrated that ferroptosis is an important cell death form in cancers and CNS diseases, such as stroke [10, 14,15,8]. Mature oligodendrocytes are derived from oligodendrocyte progenitor cells (OPCs), which originate from neuroepithelial progenitor cells of neuroepithelium in the embryonic neural tube and the periventricular germinal area (retain in adult animals and known as the subventricular zone, SVZ) during embryogenesis and early postnatal period [27]. The previous research has proved that OPCs originate in the SVZ and migrated into corpus callosum (CC), striatum, and fimbria fornix to differentiate into mature oligodendrocytes in adult mice [28], indicated that OPCs in the SVZ of the brain contribute to oligodendrogenesis throughout life and rescuing OPC cell death could be an efficient strategy to treat myelin defects-related CNS diseases.
In this study, we injected autologous blood into the lateral ventricle of mice to study the pathological process of hemorrhage on OPCs and to prevent the interference of neuronal ferroptosis in outcome elevations in vivo. After intraventricular stroke (IVH), blood enters the lateral ventricle, which first produces huge pressure and space-occupying effect on the structures around the ventricle, increases intracranial pressure, reduces blood flow and oxygen metabolism, and may destroy the permeability of the blood–brain barrier and blood-cerebrospinal fluid barrier [32]. Combined with recent research that hemoglobin causes damage to mitochondrial function in primary cultured OPCs and inhibits OPC proliferation [33], our work further emphasizes the importance of protecting OPC against cell death after hemorrhagic stroke to reduce WMI.
On the other hand, we observed a small number of other cells colocalized with PI, suggesting that cell death in OPCs is an important but not exclusive mechanism for WMI after IVH. Although a mechanistic contribution of OPC-specific cell death to WMI is reported here, it is conceivable that the crosstalk of OPCs and other cells, such as microglia/macrophages, astrocytes, and neurons, in the pathogenesis of WMI is also of significant importance and requires further studies.
Ferroptosis, a new form of iron‐dependent programmed cell death, has been shown to be involved in a range of CNS diseases, such as stroke, traumatic brain injury, neural degeneration, and autoimmune demyelination in the context of multiple sclerosis and its mouse model of EAE [14, 34,35,Hindlimb placing test We tested each mouse for Hindlimb placing test on days 1, 3, 7, 14, and 28 post IVH as described previously [14]. The mice were placed on the edge of the table, and the contralateral hind limbs were gently pulled below the edge of the table to observe the retraction of the animal limbs. Quickly retract and place on the edge of the table accurately, scored 0 point; delayed pullback, scored 1 point; no retraction of the limbs, scored 2 points. A total of 10 successful experiments were performed and the total score was recorded for each mouse. Gait analysis was performed using the Catwalk gait analysis system (Noldus Information Technology, The Netherlands) as described previously [54, 55]. In brief, the mice were placed in the dark experimental environment for 0.5 h for adaption before each experiment. At the beginning of the trial, the mouse was placed individually on the walkway and walked freely traverse from one side to another of the walkway glass plate where has a goal box. The videos of footprint images were recorded by a camera positioned under the walkway. The records were converted into digital signatures and processed using CatWalk XT 10.6 software. All mice completed three times of runs with an interval of 10 min. Mice were trained for 3 days prior to the record of baseline (the day before surgery), and the gait was assessed at −1 (baseline), 14, and 28 days after IVH/sham surgery. Data are presented as mean ± SD, all statistical analyses were carried out with GraphPad Prism 7.0 Software. P values were calculated with a two-tailed Student’s unpaired t test for two groups. One-way analysis of variance (ANOVA) was used for comparisons within multiple groups. Two-way ANOVA was used to evaluate groups differentially regulated by the time factor. Tukey’s post hoc test or Dunnett’s post hoc test was used to determine specific differences between groups. P values of <0.05 indicated statistically significant differences.Gait analysis
Statistical analysis
Data and materials availability
All data are available in the main text or supplementary materials.
References
Schrag M, Kirshner H. Management of intracerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol 2020;75:1819–31.
Samarasekera N, Fonville A, Lerpiniere C, Farrall AJ, Wardlaw JM, White PM, et al. Influence of intracerebral hemorrhage location on incidence, characteristics, and outcome: population-based study. Stroke 2015;46:361–8.
Jolink WMT, Wiegertjes K, Rinkel GJE, Algra A, de Leeuw FE, Klijn CJM. Location-specific risk factors for intracerebral hemorrhage: Systematic review and meta-analysis. Neurology 2020;95:e1807–e18.
Zuo S, Pan P, Li Q, Chen Y, Feng H. White matter injury and recovery after hypertensive intracerebral hemorrhage. Biomed Res Int 2017;2017:6138424.
Kang M, Yao Y. Oligodendrocytes in intracerebral hemorrhage. CNS Neurosci Ther 2019;25:1075–84.
Jiang YB, Wei KY, Zhang XY, Feng H, Hu R. White matter repair and treatment strategy after intracerebral hemorrhage. CNS Neurosci Ther 2019;25:1113–25.
Nishiyama A, Boshans L, Goncalves CM, Wegrzyn J, Patel KD. Lineage, fate, and fate potential of NG2-glia. Brain Res 2016;1638:116–28.
Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells. 2019;8:1424.
van Tilborg E, Heijnen CJ, Benders MJ, van Bel F, Fleiss B, Gressens P, et al. Impaired oligodendrocyte maturation in preterm infants: Potential therapeutic targets. Prog Neurobiol 2016;136:28–49.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012;149:1060–72.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 2021;22:266–82.
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 2014;16:1180–91.
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014;156:317–31.
Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2017;2:e90777.
Ratan RR. The chemical biology of ferroptosis in the central nervous system. Cell Chem Biol 2020;27:479–98.
**ao Z, Shen D, Lan T, Wei C, Wu W, Sun Q, et al. Reduction of lactoferrin aggravates neuronal ferroptosis after intracerebral hemorrhagic stroke in hyperglycemic mice. Redox Biol 2022;50:102256.
**e BS, Wang YQ, Lin Y, Mao Q, Feng JF, Gao GY, et al. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci Ther 2019;25:465–75.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009;373:1632–44.
Mandeville ET, Ayata C, Zheng Y, Mandeville JB. Translational MR neuroimaging of stroke and recovery. Transl Stroke Res 2017;8:22–32.
Yang J, Li Q, Wang Z, Qi C, Han X, Lan X, et al. Multimodality MRI assessment of grey and white matter injury and blood-brain barrier disruption after intracerebral haemorrhage in mice. Sci Rep. 2017;7:40358.
Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 2017;48:1033–43.
Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of mitochondria in ferroptosis. Mol Cell 2019;73:354–63. e3
Chapple SJ, Cheng X, Mann GE. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol 2013;1:319–31.
Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med 2020;152:175–85.
Li Q, Weiland A, Chen X, Lan X, Han X, Durham F, et al. Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Front Neurol 2018;9:581.
Heinzel J, Langle G, Oberhauser V, Hausner T, Kolbenschlag J, Prahm C, et al. Use of the CatWalk gait analysis system to assess functional recovery in rodent models of peripheral nerve injury - a systematic review. J Neurosci Methods. 2020;345:108889.
Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci 2004;5:409–19.
Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 2006;26:7907–18.
Keep RF, Andjelkovic AV, **ang J, Stamatovic SM, Antonetti DA, Hua Y, et al. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J Cereb Blood Flow Metab 2018;38:1255–75.
Dummula K, Vinukonda G, Chu P, **ng Y, Hu F, Mailk S, et al. Bone morphogenetic protein inhibition promotes neurological recovery after intraventricular hemorrhage. J Neurosci 2011;31:12068–82.
Ballabh P, de Vries LS. White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies. Nat Rev Neurol 2021;17:199–214.
Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in develo** white matter. J Neurosci 2000;20:9235–41.
Pandya CD, Vekaria H, Joseph B, Slone SA, Gensel JC, Sullivan PG, et al. Hemoglobin induces oxidative stress and mitochondrial dysfunction in oligodendrocyte progenitor cells. Transl Res 2021;231:13–23.
Bao Z, Liu Y, Chen B, Miao Z, Tu Y, Li C, et al. Prokineticin-2 prevents neuronal cell deaths in a model of traumatic brain injury. Nat Commun 2021;12:4220.
Chen C, Chen J, Wang Y, Liu Z, Wu Y. Ferroptosis drives photoreceptor degeneration in mice with defects in all-trans-retinal clearance. J Biol Chem 2021;296:100187.
Ge H, Xue X, **an J, Yuan L, Wang L, Zou Y, et al. Ferrostatin-1 alleviates white matter injury via decreasing ferroptosis following spinal cord injury. Mol Neurobiol. 2022;59:161–176.
Jhelum P, Santos-Nogueira E, Teo W, Haumont A, Lenoel I, Stys PK, et al. Ferroptosis mediates cuprizone-induced loss of oligodendrocytes and demyelination. J Neurosci 2020;40:9327–41.
Hu CL, Nydes M, Shanley KL, Morales Pantoja IE, Howard TA, Bizzozero OA. Reduced expression of the ferroptosis inhibitor glutathione peroxidase-4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurochem 2019;148:426–39.
Weiland A, Wang Y, Wu W, Lan X, Han X, Li Q, et al. Ferroptosis and its role in diverse brain diseases. Mol Neurobiol 2019;56:4880–93.
Wu JR, Tuo QZ, Lei P. Ferroptosis, a recent defined form of critical cell death in neurological disorders. J Mol Neurosci 2018;66:197–206.
Nobuta H, Yang N, Ng YH, Marro SG, Sabeur K, Chavali M, et al. Oligodendrocyte death in Pelizaeus-Merzbacher disease is rescued by iron chelation. Cell Stem Cell 2019;25:531–41. e6
Conrad M, Kagan VE, Bayir H, Pagnussat GC, Head B, Traber MG, et al. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev 2018;32:602–19.
Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 2014;3:e02523.
Conrad M, Proneth B. Selenium: tracing another essential element of ferroptotic cell death. Cell Chem Biol 2020;27:409–19.
Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M, et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol 2018;9:1371.
Lu R, Jiang Y, Lai X, Liu S, Sun L, Zhou ZW A. Shortage of FTH induces ROS and sensitizes RAS-proficient neuroblastoma N2A cells to ferroptosis. Int J Mol Sci. 2021;22:8898.
Kang R, Zeng L, Zhu S, **e Y, Liu J, Wen Q, et al. Lipid peroxidation drives gasdermin d-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 2018;24:97–108. e4
Lovatt M, Adnan K, Kocaba V, Dirisamer M, Peh GSL, Mehta JS. Peroxiredoxin-1 regulates lipid peroxidation in corneal endothelial cells. Redox Biol 2020;30:101417.
Wang J, Fields J, Dore S. The development of an improved preclinical mouse model of intracerebral hemorrhage using double infusion of autologous whole blood. Brain Res 2008;1222:214–21.
Zhu W, Gao Y, Wan J, Lan X, Han X, Zhu S, et al. Changes in motor function, cognition, and emotion-related behavior after right hemispheric intracerebral hemorrhage in various brain regions of mouse. Brain Behav Immun 2018;69:568–81.
Unal Cevik I, Dalkara T. Intravenously administered propidium iodide labels necrotic cells in the intact mouse brain after injury. Cell Death Differ 2003;10:928–9.
Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, et al. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc 2007;2:1044–51.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60.
Neumann M, Wang Y, Kim S, Hong SM, Jeng L, Bilgen M, et al. Assessing gait impairment following experimental traumatic brain injury in mice. J Neurosci Methods. 2009;176:34–44.
Walter J, Kovalenko O, Younsi A, Grutza M, Unterberg A, Zweckberger K. The CatWalk XT® is a valid tool for objective assessment of motor function in the acute phase after controlled cortical impact in mice. Behav Brain Res. 2020;392:112680.
Acknowledgements
We thank **angrong Liu Ph.D. (Department of National Clinical Research Center for Neurological Diseases, Bei**g Tiantan Hospital, Capital medical university) for providing the instruments of Catwalk XT system. We thank Dr. Lailai Yan (Peking University, School of public health) for performing the ICP-MS experiment.
Funding
National Natural Science Foundation of China 81873790 (Q.L.). National Natural Science Foundation of China 32070735 (Q.L.). National Natural Science Foundation of China 81971037 (F.Y.). The Bei**g Natural Science Foundation Program and Scientific Research Key Program of Bei**g Municipal Commission of Education KZ202010025033 (Q.L.). The Bei**g Natural Science Foundation Program and Scientific Research Key Program of Bei**g Municipal Commission of Education KZ201910025026 (F.Y.). Support Project of High-level Teachers in Bei**g Municipal Universities in the Period of 13th Five–year Plan CIT&TCD201904092 (Q.L.). The Ministry of Science and Technology of China 2019YFA0707103 (Z.Z.). The Ministry of Science and Technology of China 2020AAA0105601 (Z.Z.). National Natural Science Foundation of China 31730039 (Z.Z.).
Author information
Authors and Affiliations
Contributions
Conceptualization: DS, QL Data curation: DS, WW, JL, ZX, KG, CW, XW, FY, QL Formal analysis: DS, WW, TL, CZ, KG, LH, ZL, CW, ZZ, FY, QL Methodology: DS, WW, ZZ, QL Investigation: DS, WW, TL, CZ, KG, LH, ZL, CW, FY, QL Visualization: DS, ZZ, QL Funding acquisition: ZZ, FY, QL Project administration: PW, YW, YL, FY, QL Supervision: FY, QL Writing—original draft: DS, QL Writing—review & editing: DS, YL, CZ, FY, QL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Bertrand Joseph.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shen, D., Wu, W., Liu, J. et al. Ferroptosis in oligodendrocyte progenitor cells mediates white matter injury after hemorrhagic stroke. Cell Death Dis 13, 259 (2022). https://doi.org/10.1038/s41419-022-04712-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-04712-0
- Springer Nature Limited