Abstract
Cytosolic inflammasomes are supramolecular complexes that are formed in response to intracellular pathogens and danger signals. However, as to date, the detailed description of a homotypic caspase recruitment domain (CARD) interaction between NLRP1 and ASC has not been presented. We found the CARD–CARD interaction between purified NLRP1CARD and ASCCARD experimentally and the filamentous supramolecular complex formation in an in vitro proteins solution. Moreover, we determined a high-resolution crystal structure of the death domain fold of the human ASCCARD. Mutational and structural analysis revealed three conserved interfaces of the death domain superfamily (Type I, II, and III), which mediate the assembly of the NLRP1CARD/ASCCARD complex. In addition, we validated the role of the three major interfaces of CARDs in assembly and activation of NLRP1 inflammasome in vitro. Our findings suggest a Mosaic model of homotypic CARD interactions for the activation of NLRP1 inflammasome. The Mosaic model provides insights into the mechanisms of inflammasome assembly and signal transduction amplification.
Similar content being viewed by others
Introduction
Vertebrates have evolved an innate immune system as first line of defense against pathogen-derived molecules. The system includes intracellular pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs)1. Pattern recognition receptors (PRRs) recognize various microbial structures and activities that initiate the innate immune response of cells. PRRs consist of many protein families, including Toll-like receptors, NOD-like receptors (NLRs), RIG-I-like receptors, AIM2-like receptors, and C-type lectin receptors2,3. Formation of a multi-protein complex known as the inflammasome initiates a strong inflammatory response caused by the activation of the cysteine proteases caspase-1 and caspase-4/5/114. Inflammasomes are categorized as NLRP1, NLRP3, NLRC4, or AIM2 inflammasomes, depending on the type of sensor protein5. Malfunction of inflammasomes is associated with numerous autoimmune disorders, including vitiligo, Type II diabetes, and celiac disease6,7.
Inflammasomes are studied to better understand the cellular danger signal monitoring and signal transduction mechanisms8. NLRP1, a member of the nucleotide-binding-domain leucine-rich-repeat (NLR) superfamily9, was the first NLR discovered that forms inflammasome in the innate immune system10. NLRP1 senses danger signals and performs self-cleavage to release its C-terminal segment (UPA-CARD domains), whcich initiates downstream signal transduction pathways. Self- and homotypic interactions of ASC (apoptosis-associated speck-like protein containing a CARD) can mediate the assembly of a supramolecular complex, ultimately activating caspase-1 by an unknown mechanism11. The activated caspase-1 cleaves specific cytokines (interleukin-1β and -18) and Gasdermin D, which induces pro-inflammatory cell death known as pyroptosis12,13.
NLRP1 is a unique member of the NLRP family, because it has an extra FIIND and CARD following the Leucine-rich repeat domain in the C terminus. Furthermore, a PYD does not appear to be critical for the function because of the lack of a PYD as found in the N-terminal of murine homolog14. Unlike human NLRP1, there are three paralogs of Nlrp1(Nlrp1a,b,c) in mouse genome, and murine NLRP1A and NLRP1B inflammasome can be assembled by recruitment of caspase-1 either with ASC or without ASC15,16,17. Recently, a novel activation mechanism, called the functional degradation model, of the murine NLRP1B inflammasome was proposed18,19. In the model, the C-terminal function-to-find domain (FIIND) of NLRP1B self-cleaves to initiate the function of the NLRP1B inflammasome. The function of inflammasome is enabled by the degradation of the N terminus of NLRP1B by a lethal factor protease to expose a destabilizing N-degron. The inflammasome releases its C-terminal fragment that consists of a partial FIIND domain and an entire CARD domain, to recruit caspase-1 to the inflammasome for caspase activation. Interestingly, inhibitors of dipeptidyl peptidases DPP8 and DPP9 were also found to activate the NLRP1B inflammasome by an endogenous degradation pathway. It is unknown how DPP8/9-mediated signaling pathways regulate the auto-inhibited or activated state of NLRP1B20,21. The activation mechanism of human NLRP1, which consists of an extra N-terminal PYD domain different from murine NLRP1B, remains unknown18,22. Mutations of its PYD domain are related to several skin diseases, indicating an important function of PYD in the mechanism of human NLRP1 activation23. More investigations are needed to unravel the complicated regulation mechanisms of human NLRP1 in innate immunity.
NLRP1 is also an adapter protein, which along with ASC bind to themselves or each other by homotypic interactions among the death domain (DD) contained within them (e.g., PYD, CARD)24. The members of the DD superfamily engage in assembly of filaments by three different interaction types: Type I, II, and III. The binding interfaces do not overlap so that a protein with more than one type can interact with itself or other DD-containing proteins simultaneously. CARD–CARD interactions, such as pro-caspase-9/Apaf-1 and CED-4/CED-9 complexes, have been characterized by structural studies25,26. However, little information is available about CARD–CARD interactions involved in the assembly of NLRP1 inflammasome. Our understanding of the molecular details of the interaction between NLRP1CARD and ASCCARD is limited.
We found that the CARD of NLRP1 (NLRP1CARD) and the CARD of ASC (ASCCARD) interact with each other in solution and cells. We investigated the function of the CARDs of each protein and found that NLRP1CARD binds with ASCCARD to form filament complexes using each of the three DD interaction types. Heteromeric and homomeric interactions play significant roles in inflammasome activation and danger signal transduction amplification in NLRP1. The assembly of NLRP1CARD and ASCCARD for signal transduction amplification was named as Mosaic model. The mutations of three asymmetric interactions between NLRP1CARD and ASCCARD led to the suppression of NLRP1 inflammasome activation. We propose a ASC-dependent activation model-the Mosaic model-that reveals new insights into the formation of NLRP1 inflammasome and highlights the importance of CARDs in the mechanism of NLRP1 signal transduction amplification.
Results
NLRP1 binds with ASC by homotypic CARD–CARD interaction
The self-cleavage of NLRP1 and release of the C-terminal fragment containing the partial FIIND and the entire CARD are necessary for NLRP1 activation. The C-terminal fragment subsequently recruits ASC to activate caspase-127. This function differs from NLRP3 and AIM2 inflammasomes whose N-terminal PYD domains recruit adapter ASC to form the fiber-like assembly28,29. We first wanted to investigate the role of CARD–CARD interactions in the human NLRP1 inflammasome.
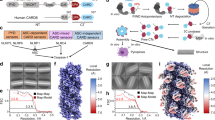
We evaluated the complex formation of NLRP1CARD and ASCCARD in an in vitro proteins solution. ASCCARD formed homotypic aggregates and precipitated from the solution. To overcome the precipitation problem, the maltose-binding protein which is shown to be a crystallization chaperone30 was linked to ASCCARD (MBP-ASCCARD). Most of the MBP-ASCCARD eluted as a single symmetrical peak at approximately 16 mL from the size-exclusion chromatographic column, indicating that the MBP-ASCCARD was homogenous and existed as a monomer in solution. After incubating MBP-ASCCARD with a threefold concentration of NLRP1CARD for 4 h, an MBP-ASCCARD/NLRP1CARD complex was observed by size-exclusion chromatography (Fig. 1a). The MBP-ASCCARD/NLRP1CARD complex eluted at ~9 mL, which corresponded to a molecular weight of ~400 kDa. By the gray scales analysis of the polyacrylamide gel electrophoresis (PAGE) gel, the molecular ratio between NLRP1CARD and ASCCARD is ~1 : 2 in the gel filtration assay (Supplementary Fig. S1a). The analysis of the negative-stain EM showed that the MBP-ASCCARD/NLRP1CARD complex formed extended filament structures (Fig. 1b). The MBP fusion tag did not appear to interfere with the interaction between NLRP1CARD and ASCCARD, because there was a long flexible linker between MBP and ASCCARD. The filaments formed from this complex had varying lengths but were uniform in the diameter (~10 nm), which is consistent with the cryogenic electron microscopy (cryo-EM) structure of ASCCARD and NLRC4CARD-only filaments31. We performed size-exclusion chromatography analysis at different concentrations of salt, to investigate the importance of charge complementary in the NLRP1CARD and ASCCARD interaction. The MBP-ASCCARD/NLRP1CARD complex showed salt dependence at 0.15, 0.5, and 1.0 M NaCl using gel filtration analysis. The MBP-ASCCARD/NLRP1CARD complex decreased and the monomer peaks increased with increasing NaCl concentration. This indicates that ionic interactions play an important role in NLRP1CARD and ASCCARD interactions (Fig. 1c, d). We conclude that charge complementary is essential in mediating NLRP1CARD/ASCCARD filament formation.
a Size-exclusion chromatograph of the MBP-ASCCARD/NLRP1CARD complex. ASCCARD was fused with an N-terminal His-tag and incubated with untagged NLRP1CARD, which was first purified by Ni-affinity chromatography. The complex eluted in the void position on a SuperdexTM 24 gel filtration column. b A negative-stain EM image of MBP-ASCCARD/NLRP1CARD complex. c Size-exclusion chromatograph of the MBP-ASCCARD/NLRP1CARD complex in different salt concentrations. d High salt significantly disrupted filament formation. e Y2H analysis of the NLRP1CARD and ASCCARD interaction. Yeast cells co-expressing GAL4 DNA-binding domain (BD)-ASC CARD fusion and GAL4 activation domain (AD)-NLRP1CARD fusion were grown on agar plates lacking leucine and tryptophan (-Leu/-Trp) for transformant growth and lacking histidine, leucine, and tryptophan (-His/-Leu/-Trp) for detecting CARD–CARD interaction. Three individual clones for each combination were plated. (–) denotes empty vector control. f Measurement of the protein–protein interaction of wild-type NLRP1CARD with ASCCARD by the M2H experiment. Luciferase activity in the HEK293T cells was normalized to Renilla and data were presented as the fold of negative control. Mean values ± SEM are representative of three independent experiments. g Structure-based sequence alignment of NLRP1CARD (NP_127497.1) and ASCCARD (NP_037390.2). Different colors are highlighted to show the interfacial residues involved in the three asymmetric interactions of death domain superfamily. The secondary structure of NLRP1CARD and ASCCARD are labeled on the top and bottom, respectively.
We examined the interaction between NLRP1CARD and ASCCARD in cells using yeast two-hybrid (Y2H) and mammalian two-hybrid (M2H) systems. A strong binding interaction was verified between wild-type full-length NLRP1 and ASC in the M2H system. However, there was no interaction between CARD-deletion mutants of NLRP1 and ASC, implying that NLRP1 recruits ASC through CARD domains (Supplementary Fig. S1b). Yeast cells expressing both NLRP1CARD and ASCCARD grew on SD/-Trp/-Leu/-His media, indicating that the NLRP1CARD and ASCCARD interact with each other directly (Fig. 1e). We observed that, in the M2H system, the interaction between NLRP1CARD and ASCCARD was significantly stronger than in the control groups (Fig. 1f). Immunoblotting experiments showed that NLRP1CARD and ASCCARD were expressed at similar levels in the M2H system (Supplementary Fig. S1c). We conclude that homotypic CARD–CARD interactions mediate NLRP1CARD/ASCCARD complex formation in solution and the recruitment of ASC by NLRP1 in cells.
Primary sequences of NLRP1CARD and ASCCARD were more conserved than other CARD proteins by sequence alignment of nine kinds of CARD proteins (Supplementary Fig. S2). According to charged residues and three-dimensional position in the NLRP1CARD and ASCCARD structure, the interfacial residues of the three major asymmetric interfaces and secondary structures in NLRP1CARD and ASCCARD showed a highly similarity, perhaps indicating a more compatible protein–protein interaction between NLRP1CARD and ASCCARD (Fig. 1g). The sequence and structural similarity between NLRP1CARD and ASCCARD may provide a foundation for NLRP1 and ASC to form heterotypic supramolecular complexes via their CARD domains during NLRP1 inflammasome activation.
AI of NLRP1CARD is important for binding with ASCCARD
X-ray crystallography of the CARD–CARD interactions between NLRP1CARD proteins found that NLRP1CARD crystals formed two kinds of lattice contactsFull size image
Identification of homotypic CARD interface of ASCCARD
The functions of DD proteins often require homotypic DD interactions24. It has been shown that filament assembly by members of the DD superfamily is mediated by the three major asymmetric interfaces35. Each of these three asymmetric interactions were observed in the cryo-EM ASCCARD filament structure31.
We aligned the three major asymmetric interfaces of the DD superfamily responsible for filament formation (Type I, II, and III) with the corresponding interfaces in the PIDD/RAIDD DD complex (Fig. 3d). We found that the Type I interaction mediated by the residues from helices 1 and 4 (Type Ia) of ASCCARD and residues from helix 2 and 3 (Type Ib) of a second ASCCARD is highly conserved. The Type II interaction formed by the residues in the loop between helix 4 and helix 5 (Type IIa) of ASCCARD and residues at the helix 5–helix 6 corner (Type IIb) of a second ASCCARD is less conserved. The Type III interaction formed by residues at helix 3 (Type IIIa) of one ASCCARD and residues at helix 3–helix 4 corner (Type IIIb) of a second ASCCARD is also less conserved.
The M2H system was employed to validate the functional relevance of interfacial residues for the self-interaction of ASCCARD determined by the structure analysis. ASCCARD demonstrated a strong interaction with itself when expressed in HEK293T cells (Fig. 3e). According to the conserved, charged residues, three-dimensional position in the MBP-ASCCARD structure, and the published data31,36, some residues were picked to test the self-interaction of ASCCARD. Mutations of the Type I residues R119D, D134K, and N128A/E130R considerably decreased ASCCARD self-interaction. Concomitant mutations of the Type IIa residues W169G/N170A and the Type IIb residues R125A/T127A abolished ASCCARD self-interaction. Mutations of the Type III residues R160E and D134A/E144A also decreased ASCCARD self-interaction. These in vitro mutagenesis studies show that Type I, II, and III interfaces are involved in the self-interaction of ASCCARD.
Critical ASCCARD surface sites for interaction with NLRP1CARD
We used the Y2H system to map critical surface sites mediating NLRP1CARD and ASCCARD interaction to understand the molecular interaction in NLRP1 inflammasome assembly. Sequence alignment of ASCCARD from different species was performed to identify the conserved amino acids that may participate in the interactions between NLRP1CARD and ASCCARD (Fig. 3f). Most of the conserved residues are either hydrophobic amino acids involved in the packing of hydrophobic cores or charged interfacial amino acids interacting with other proteins. Some conserved charged residues (N128, E130, D143, E144, Q147, R150, D175, and Q179) were identified as possibly involved in the interaction between NLRP1CARD and ASCCARD. Site-directed mutagenesis on the Y2H construct of ASCCARD showed that only the double mutants of D143A/E144A and N128A/E130R grew on SD/-Trp/-Leu/ plates instead of SD/-Trp/-Leu/-His plates, demonstrating the roles of D143, E144, N128, and E130 in the interaction between NLRP1CARD and ASCCARD (Fig. 3g). Mutations of these four conserved charged residues abrogated the interaction between NLRP1CARD and ASCCARD. We tested the interaction between NLRP1CARD and CASP9CARD, a non-related CARD domain, to confirm the specificity of the CARD–CARD interactions between NLRP1CARD and ASCCARD. NLRP1CARD and human-derived CASP9CARD did not interact with each other in the Y2H system, demonstrating the specificity of CARD–CARD interactions between NLRP1CARD and ASCCARD.
Size-exclusion chromatography was used to test the effects of the mutants MBP-ASCCARD D143A/E144A (MBP-ASCCARD-DE) and MBP-ASCCARD N128A/E130R (MBP-ASCCARD-NE) on the MBP-ASCCARD/NLRP1CARD complex formation in solution. MBP-ASCCARD-DE and MBP-ASCCARD-NE failed to form supramolecular complexes with NLRP1CARD (Fig. 3h, i). These findings showed that D143, E144, N128, and E130 on the ASCCARD play an important role in mediating the interaction between NLRP1CARD and ASCCARD.
The CARD–CARD interactions between NLRP1 and ASC utilize three asymmetric interaction types of death-fold superfamily
As with the prior reports, some members of the DD superfamily utilize three distinct interface types to assemble macromolecular complex structure11,31,37.The major interacting amino acids at the AI interface of NLRP1CARD are part of the three asymmetric interaction types. R1427 is at the Type Ia interface of NLRP1CARD and the residues R1392 and R1422 are at Type IIIb interface of NLRP1CARD. The N128 and E130 residues at the Type Ib interface on ASCCARD and the residues D143 and E144 at the Type IIIa interface on ASCCARD play an important role in mediating the interaction between NLRP1CARD with ASCCARD42,43, which are similar to the NLRP1CARD/ASCCARD interactions in our study. Previously, the truncation mutants of NLRP1 indirectly proved that NLRP1 interacts with ASC via homotypic CARD–CARD interactions in cells23,44. Our results provided direct evidence of the binding between purified NLRP1CARD and ASCCARD proteins in an in vitro proteins solution.
We focused on the molecular details of human NLRP1 inflammasome assembly and presented evidence for the mechanism of NLRP1CARD and ASCCARD interactions in the formation of archetypical supramolecular filament during inflammasome activation. We confirmed that the interaction of NLRP1CARD and ASCCARD is primarily composed of ionic interactions. We showed that the asymmetric CARD–CARD interfaces in the NLRP1CARD crystals mediate the interaction between NLRP1CARD and ASCCARD through charge complementarity. We generated a high-resolution crystal structure of human ASCCARD and discovered that CARD structures are highly conserved with a six-helical bundle with a Greek key topology. Structure-directed mutagenesis on ASCCARD demonstrated that the three interaction types of the DD superfamily play critical roles in ASCCARD oligomerization. Finally, we showed that the primary structure sequence, the secondary structure, and the three-dimensional structure of NLRP1CARD and ASCCARD are similar. The high similarity suggests the possibility that the NLRP1CARD can assemble with ASCCARD and form the signal transduction complexes, i.e., filamentous structures, using the three asymmetric interfaces of DD superfamily. Mutagenesis and functional studies of NLRP1CARD and ASCCARD of the three asymmetric interfaces of the DD superfamily further supported that the archetypical supramolecular filament of NLRP1CARD co-assembles with ASCCARD to serve as an inflammasome signal amplification platform in NLRP1 inflammasome. In summary, our study presents a clear picture of a mosaic model of ASC-dependent signal amplification in human NLRP1 inflammasome activation.