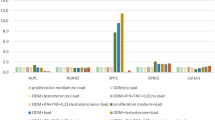
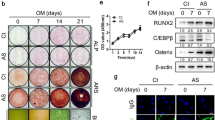
Abstract
The mechanism of pathological osteogenesis in Ankylosing spondylitis (AS) is largely unknown. Our previous studies demonstrated that the imbalance between BMP-2 and Noggin secretion induces abnormal osteogenic differentiation of marrow-derived mesenchymal stem cells (MSCs) from AS patients in a two-dimensional culture environment. In this study, HA/β-TCP scaffolds were further used as a three-dimensional (3D) biomimetic culture system to mimic the bone microenvironment in vivo to determine the abnormal osteogenic differentiation of AS-MSCs. We demonstrated that when cultured in HA/β-TCP scaffolds, AS-MSCs had a stronger osteogenic differentiation capacity than that of MSCs from healthy donors (HD-MSCs) in vitro and in vivo. This dysfunction resulted from BMP2 overexpression in AS-MSCs, which excessively activated the Smad1/5/8 and ERK signalling pathways and finally led to enhanced osteogenic differentiation. Both the signalling pathway inhibitors and siRNAs inhibiting BMP2 expression could rectify the enhanced osteogenic differentiation of AS-MSCs. Furthermore, BMP2 expression in ossifying entheses was significantly higher in AS patients. In summary, our study demonstrated that AS-MSCs possess enhanced osteogenic differentiation in HA/β-TCP scaffolds as a 3D biomimetic microenvironment because of BMP2 overexpression, but not Noggin. These results provide insights into the mechanism of pathological osteogenesis, which can aid in the development of niche-targeting medications for AS.
Similar content being viewed by others
Introduction
Ankylosing spondylitis (AS) is a common autoimmune disease that affects the axial skeleton, and one of its critical pathogenic features is new bone formation at local sites of entheses1, which is confined to the periosteal bone compartment, outside the cortical bone lining. In recent years, many researchers have investigated the mechanism of pathological osteogenesis in AS2,3. However, the concrete details of pathological osteogenesis are still controversial, impeding the development of a specific medication for AS. Furthermore, the lack of a specific therapeutic target for pathological osteogenesis results in a high disability rate in AS patients4.
Mesenchymal stem cells (MSCs) are one of the most important kinds of multipotential stem cells and possess strong immunoregulatory and trilineage differentiation abilities5. Many studies have determined that MSCs are the major origin of osteoblasts6. Nevertheless, dysfunction of the osteogenic differentiation ability of MSCs contributes to bone metabolism disorders in rheumatic diseases7. For example, although mesenchymal progenitors increased, decreased osteoblast differentiation is progressive with disease development in Interleukin-1 receptor antagonist knock-out mice, which spontaneously develop RA-like disease8. The activated NF-kB pathway in MSCs from Systemic lupus erythematosus (SLE) patients inhibits osteoblastic differentiation through BMP/Smad signaling pathway, which may participate in the pathology of osteoporosis in SLE patients9. Several histopathologic studies in AS patients prove that endochondral new bone formation during bone erosion involves a series of events from chondrocyte apoptosis to colonization of preosteoblasts, differentiation of the preosteoblasts to osteolasts and bone matrix secretion10. MSCs as progenitor cells may play an important role in pathophysiological processes.
Recently, our research, as well as that of other researchers, demonstrated that MSCs from AS patients (AS-MSCs) outperformed MSCs from healthy donors (HD-MSCs) in osteogenic differentiation in two-dimensional (2D) culture, which could be an important mechanism of pathological osteogenesis in AS11, Taurog, J. D., Chhabra, A. & Colbert, R. A. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 375, 1303 (2016). Lories, R. The balance of tissue repair and remodeling in chronic arthritis. Nat Rev Rheumatol. 7, 700–707 (2011). Neerinckx, B. & Lories, R. Mechanisms, impact and prevention of pathological bone regeneration in spondyloarthritis. Curr Opin Rheumatol. 29, 287–292 (2017). Sieper, J. & Poddubnyy, D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 12, 282–295 (2016). Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 8, 726–736 (2008). Grayson, W. L. et al. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 11, 140–150 (2015). Cipriani, P. et al. Mesenchymal stromal cells and rheumatic diseases: New tools from pathogenesis to regenerative therapies. Cytotherapy. 17, 832–849 (2015). Mohanty, S. T. et al. Alterations in the self-renewal and differentiation ability of bone marrow mesenchymal stem cells in a mouse model of rheumatoid arthritis. Arthritis Res Ther. 12, R149 (2010). Tang, Y. et al. Activated nf-kappab in bone marrow mesenchymal stem cells from systemic lupus erythematosus patients inhibits osteogenic differentiation through downregulating smad signaling. Stem Cells Dev. 22, 668–678 (2013). Miceli-Richard, C. Enthesitis: The clue to the pathogenesis of spondyloarthritis? Joint Bone Spine. 82, 402–405 (2015). Layh-Schmitt, G. et al. Generation and differentiation of induced pluripotent stem cells reveal ankylosing spondylitis risk gene expression in bone progenitors. Clin Rheumatol. 36, 143–154 (2017). **e, Z. et al. Imbalance between bone morphogenetic protein 2 and noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol. 68, 430–440 (2016). Knight, E. & Przyborski, S. Advances in 3d cell culture technologies enabling tissue-like structures to be created in vitro. J Anat. 227, 746–756 (2015). Bellucci, D., Sola, A. & Cannillo, V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: State of the art and current applications. J Biomed Mater Res A. 104, 1030–1056 (2016). Bassi, G. et al. Effects of a ceramic biomaterial on immune modulatory properties and differentiation potential of human mesenchymal stromal cells of different origin. Tissue Eng Part A. 21, 767–781 (2015). Mebarki, M. et al. Enhanced human bone marrow mesenchymal stromal cell adhesion on scaffolds promotes cell survival and bone formation. Acta Biomater. 59, 94–107 (2017). Braun, J. & Sieper, J. Ankylosing spondylitis. Lancet. 369, 1379–1390 (2007). Wang, J. et al. Effect of phase composition on protein adsorption and osteoinduction of porous calcium phosphate ceramics in mice. J Biomed Mater Res A. 102, 4234–4243 (2014). **e, Z. et al. Mcp1 triggers monocyte dysfunctions during abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. J Mol Med. 95, 143–154 (2017). Nakahama, K. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 67, 4001–4009 (2010). Walsh, N. C. & Gravallese, E. M. Bone remodeling in rheumatic disease: A question of balance. Immunol Rev. 233, 301–312 (2010). Lories, R. J., Luyten, F. P. & de Vlam, K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther. 11, 221 (2009). Caparbo, V. F., Saad, C. G. S., Moraes, J. C., de Brum-Fernandes, A. J., Pereira, R. M. R. Monocytes from male patients with ankylosing spondylitis display decreased osteoclastogenesis and decreased rankl/opg ratio. Osteoporos Int. 29, 2565–2573 (2018). Lalwani, G. et al. Three-dimensional carbon nanotube scaffolds for long-term maintenance and expansion of human mesenchymal stem cells. J Biomed Mater Res A. 105, 1927–1939 (2017). Ravi, M., Paramesh, V., Kaviya, S. R., Anuradha, E. & Solomon, F. D. 3d cell culture systems: Advantages and applications. J Cell Physiol. 230, 16–26 (2015). Baker, B. M. & Chen, C. S. Deconstructing the third dimension: How 3d culture microenvironments alter cellular cues. J Cell Sci. 125, 3015–3024 (2012). Lemos, D. R. & Duffield, J. S. Tissue-resident mesenchymal stromal cells: Implications for tissue-specific antifibrotic therapies. Sci Transl Med. 10, pii: eaan5174 (2018). Lories, R. J. & Schett, G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum Dis Clin North Am. 38, 555–567 (2012). Maksymowych, W. P., Elewaut, D. & Schett, G. Motion for debate: The development of ankylosis in ankylosing spondylitis is largely dependent on inflammation. Arthritis Rheum. 64, 1713–1719 (2012). Ceylan, H. et al. Bone-like mineral nucleating peptide nanofibers induce differentiation of human mesenchymal stem cells into mature osteoblasts. Biomacromolecules. 15, 2407–2418 (2014). Minardi, S. et al. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials. 62, 128–137 (2015). Gamie, Z. et al. Stem cells combined with bone graft substitutes in skeletal tissue engineering. Expert Opin Biol Ther. 12, 713–729 (2012). Chen, Q., Shou, P. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 23, 1128–1139 (2016). Braem, K., Luyten, F. P. & Lories, R. J. Blocking p38 signalling inhibits chondrogenesis in vitro but not ankylosis in a model of ankylosing spondylitis in vivo. Ann Rheum Dis. 71, 722–728 (2012). Corr, M. Wnt signaling in ankylosing spondylitis. Clin Rheumatol. 33, 759–762 (2014). Salazar, V. S., Gamer, L. W. & Rosen, V. Bmp signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 12, 203–221 (2016). Rosen, V. Bmp2 signaling in bone development and repair. Cytokine Growth Factor Rev. 20, 475–480 (2009). Zara, J. N. et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 17, 1389–1399 (2011). Lories, R. J., Derese, I. & Luyten, F. P. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 115, 1571–1579 (2005). Krause, C., Guzman, A. & Knaus, P. Noggin. Int J Biochem Cell Biol. 43, 478–481 (2011). Baker, B. A. et al. Ontology analysis of global gene expression differences of human bone marrow stromal cells cultured on 3d scaffolds or 2d films. Biomaterials. 35, 6716–6726 (2014).References
Acknowledgements
This study was financially supported by the Engineering Technology Research Center for Comprehensive Diagnosis and Treatment of Ankylosing Spondylitis of Guangdong Province (2015B090903059), the Science and Technology Project of Guangdong Province (2015B020228001, 2017A030310554) and the Science and Technology Project of Guangzhou City (201704020045).
Author information
Authors and Affiliations
Contributions
P.W., Y.W. and H.S. designed the experiments. G.Z., Z.X., J.L., M.L. and S.C. conceived and performed the experiments. S.T., W.L. and G.Y. conceived the experiments and analysed the data. Y.L., S.W., X.W. and H.S. carried out the experiments. All authors were involved in writing the manuscript and approved the submitted and published versions.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by D. Aberdam
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, G., **e, Z., Wang, P. et al. Enhanced osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis: a study based on a three-dimensional biomimetic environment. Cell Death Dis 10, 350 (2019). https://doi.org/10.1038/s41419-019-1586-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-019-1586-1
- Springer Nature Limited
This article is cited by
-
ILK inhibition reduces osteophyte formation through suppression of osteogenesis in BMSCs via Akt/GSK-3β/β-catenin pathway
Molecular Biology Reports (2024)
-
Sequence of Events in the Pathogenesis of Axial Spondyloarthritis: A Current Review—2023 SPARTAN Meeting Proceedings
Current Rheumatology Reports (2024)
-
A novel Anti-ROS osteoblast-specific delivery system for ankylosing spondylitis treatment via suppression of both inflammation and pathological new bone formation
Journal of Nanobiotechnology (2023)
-
MYC promotes fibroblast osteogenesis by regulating ALP and BMP2 to participate in ectopic ossification of ankylosing spondylitis
Arthritis Research & Therapy (2023)
-
Biology and therapeutic potential of mesenchymal stem cell extracellular vesicles in axial spondyloarthritis
Communications Biology (2023)