Abstract
Background and aims
Perinatal arterial ischemic stroke (PAIS) often has lifelong neurodevelopmental consequences. We aimed to review early predictors (<4 months of age) of long-term outcome.
Methods
We carried out a systematic literature search (PubMed and Embase), and included articles describing term-born infants with PAIS that underwent a diagnostic procedure within four months of age, and had any reported outcome parameter ≥12 months of age. Two independent reviewers included studies and performed risk of bias analysis.
Results
We included 41 articles reporting on 1395 infants, whereof 1255 (90%) infants underwent follow-up at a median of 4 years. A meta-analysis was performed for the development of cerebral palsy (n = 23 studies); the best predictor was the qualitative or quantitative assessment of the corticospinal tracts on MRI, followed by standardized motor assessments. For long-term cognitive functioning, bedside techniques including (a)EEG and NIRS might be valuable. Injury to the optic radiation on DTI correctly predicted visual field defects. No predictors could be identified for behavior, language, and post-neonatal epilepsy.
Conclusion
Corticospinal tract assessment on MRI and standardized motor assessments are best to predict cerebral palsy after PAIS. Future research should be focused on improving outcome prediction for non-motor outcomes.
Impact
-
We present a systematic review of early predictors for various long-term outcome categories after perinatal arterial ischemic stroke (PAIS), including a meta-analysis for the outcome unilateral spastic cerebral palsy.
-
Corticospinal tract assessment on MRI and standardized motor assessments are best to predict cerebral palsy after PAIS, while bedside techniques such as (a)EEG and NIRS might improve cognitive outcome prediction.
-
Future research should be focused on improving outcome prediction for non-motor outcomes.
Similar content being viewed by others
Introduction
Perinatal arterial ischemic stroke (PAIS) occurs in 1 in 3000–10,000 live births and often has lifelong consequences.1,2,3 Around one-third of infants with PAIS develop unilateral spastic cerebral palsy (USCP), while other consequences include cognitive deficits, language disorders, post-neonatal epilepsy, behavioral problems, and sensory deficits.4 Currently, there is no curative treatment available for PAIS, as therapy is limited to supportive care such as treating hypoglycemia or seizures. However, novel therapeutic strategies are currently being examined in several clinical trials: intranasal administration of mesenchymal stromal cells (#NCT03356821) and intravenously delivered darbepoetin (#NCT03171818) are being developed to promote neuroregeneration after PAIS.5,6,7 Besides these promising new therapeutics, early intervention strategies starting from four months of age, including constraint-induced movement therapy, hand-arm bimanual intensive therapy, and transcranial magnetic stimulation (TMS), have been developed to address the brain’s plasticity and reduce the severity of USCP.8,9,10,11
Predicting the long-term outcome after PAIS as early and accurately as possible is crucial in selecting infants that will benefit most from novel regenerative therapies and early intervention strategies. Moreover, precise outcome prediction will aid the clinician in adequately informing parents about the possible hurdles in their child’s development. In the first months of life, neonates suffering from PAIS often undergo multiple diagnostic procedures such as cranial ultrasound, electroencephalography (EEG), magnetic resonance imaging (MRI), and near-infrared spectroscopy (NIRS), as well as neurological examinations and/or (neuro)developmental and standardized motor assessments. These assessments can inform clinicians about the condition of the child during admission, and help them make decisions regarding short-term care (e.g. controlling seizures with anti-seizure medication). However, each performed diagnostic procedure might also provide valuable information about the long-term outcome. Currently, most research has focused on the prediction of USCP after perinatal stroke, even though other consequences including post-neonatal epilepsy, behavioral problems, or cognitive deficits might also be of great impact on the child’s and their families’ quality of life. The prediction of outcomes other than USCP is not standardized yet and still remains a big challenge.
The aim of this review is to systematically assess the long-term prognostic value of diagnostic procedures and early (neuro)developmental assessments performed in neonates with PAIS within four months of age. In this paper, we present a systematic review of the identified early predictors for outcome ≥12 months of age after PAIS, including a meta-analysis for USCP.
Methods
Search strategy
We performed this systematic review according to PRISMA guidelines (Supplementary Tables 5 and 6). The review was registered and the protocol published in Prospero (#CRD42020220508). We searched studies that included at least five infants to assess the long-term prognostic value of diagnostic procedures and early (neuro)developmental assessments performed in neonates with PAIS within four months of age. This work was supported by the European Society for Pediatric Research (ESPR). The funder had no role in the study design, data collection and analysis, preparation of the manuscript or decision to publish.
Inclusion and exclusion criteria
Articles were considered eligible if they described term neonates diagnosed with PAIS within 28 days after birth (also known as neonatal arterial ischemic stroke), and provided characteristics of any diagnostic method and/or assessment in combination with a reported outcome assessment. We searched for predictors collected within four months of age (since motor outcome starts to become apparent after this age), including brain imaging (ultrasonography and MRI), neurophysiology (including EEG, NIRS, evoked potentials, and TMS), and clinical and motor assessments (including the Hammersmith Infant Neurological Examination [HINE], the Alberta Infant Motor Scale [AIMS], the Hand Assessment for Infants [HAI], and the General Movements Assessment [GMA]). Furthermore, long-term outcome assessment ≥12 months of age by a standardized and validated assessment had to be reported, including motor outcome (USCP), cognition, language, behavior, vision, hearing, and (post-neonatal) epilepsy. Finally, we included articles written in English or Dutch, and published in a peer-reviewed journal. Conference papers, reviews, case reports with n < 5 patients, and articles without a full-text available were excluded.
Data extraction
We carried out a literature search in PubMed and Embase (search strategies are presented in supplementary table 2). There were no restrictions with regard to publication date, and studies published until July 8, 2022 were included. Two independent reviewers (LMB and AAEV) performed title and abstract screening based on the inclusion and exclusion criteria using the Rayyan application.12 In case of discrepancies, a consensus was reached by discussion. After full-text screening, we collected the data using a data extraction form. Data collection included; gestational age at birth (GA); sex; co-morbidities; the characteristics of the diagnostic method including the age of the infant at the time of the procedure; the type and outcome of the long-term outcome assessment; age at follow-up; and data for 2 × 2 tables, if available. Furthermore, two reviewers (LMB and AAEV) independently assessed the risk of bias for each selected article with the Checklist for Case Series developed by the Joanna Briggs Institute, which has previously been adapted by adding methodological items.13 The methodological quality of all included studies was rated by two reviewers independently (LMB and AAEV), and in case of discrepancies, a third reviewer was consulted and a consensus was reached (NEA). For this review, we categorized long-term outcomes in (1) motor outcome (USCP), (2) cognitive deficits, (3) language problems, (4) behavioral problems, (5) post-neonatal epilepsy, and (6) sensory deficits.
Statistical analysis
Continuous data is presented as means with standard deviations (SD) or medians with ranges, depending on the reported statistics in the original studies. For continuous long-term outcome data, we calculated receiver operating characteristic curves (ROC) and optimal cut-off values with MedCalc Software (version 19.0.7; Ostend, Belgium). For motor outcome, a meta-analysis was performed with the Meta4diag package in R Studio Desktop (version 1.4.1717, RStudio, Inc., Boston, MA).14 The Meta4Diag package uses a bivariate random effects approach with a binomial distribution with pooled logit sensitivity and logit specificity, it performs well in diagnostic test accuracy studies and is specifically useful for studies with smaller sample sizes.15 The diagnostic odds ratio (DOR = [true positives/false positives] / [false negatives/true negatives)] was calculated, as it is less dependent on the used threshold than sensitivity and specificity. Diagnostic predictors were included in a meta-analysis when reported in three or more studies. Sensitivity, specificity, and DORs of the meta-analysis are presented as mean[95% confidence interval; CI]. For the other outcome categories and the described predictors that were reported in only one or two studies, analyses were performed with Graphpad Prism (version 9, GraphPad Software, La Jolla, CA) and results were demonstrated in separate forest plots as mean values with confidence intervals (additional data is reported in Supplementary Tables 3 and 4). When zeros were present in 2×2 data of a study, a Haldane-Anscombe correction was performed to make calculations possible.
Results
Article search
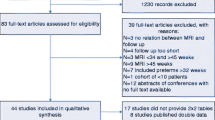
We searched PubMed and Embase for each diagnostic method separately and collected 2683 articles in total. After removal of duplicates, 2498 unique entries were assessed by title and abstract screening. One-hundred-nine articles met our inclusion and exclusion criteria. After full-text screening, we included 41 articles, whereof 30 were suitable for quantitative analysis, and data from 23 articles were included in the meta-analysis (Supplementary Fig. 1). Nineteen studies had a low risk of bias in all domains or a unclear risk of bias in one domain; 18 studies had a high risk of bias in one domain, and 4 studies had a high or unclear risk of bias in two or more domains (Supplementary Table 1).
Study characteristics
All studies together included 1395 infants with PAIS, whereof 1255 infants had a completed neurodevelopmental long-term follow-up (90%). Study sample size ranged from 6 to 187 infants with a mean ± SD of 34 ± 35 infants. Of 41 studies, 15 (37%) studies had a prospective design.
Table 1 summarizes the characteristics of all included studies. In total, the predictors of eight different diagnostic methods performed within four months of age were described: amplitude-integrated EEG (aEEG; n = 1), EEG (n = 9), GMA (n = 4), HAI (n = 1), MRI (n = 37, including 7 diffusion-weighted imaging [DWI], 4 diffusion-tensor imaging [DTI], and 1 magnetic resonance angiography [MRA] study), NIRS (n = 1), somatosensory-evoked potentials (SEP; n = 1), and TMS (n = 1). No studies were found for quantitative EEG, (doppler) ultrasonography, actigraphy, visual-evoked potentials, or the neurological assessments HINE and AIMS. About half of the articles described more than one diagnostic method (n = 21, 51%). The location of perinatal arterial ischemic stroke was clearly described in all studies: 25 studies included all PAIS subtypes including bilateral stroke, 7 studies only included unilateral PAIS, 5 studies only included MCA strokes, 1 study included all PAIS subtypes except posterior cerebellar artery (PCA) strokes, 1 study only included perforator stroke, and 1 study only included PCA strokes. Median age of the patients at follow up ranged from 1 to 11.9 years with a median follow up period of 4 years. Thirty-five (85%) studies had ≤15% loss to follow up. Table 2 summarizes all predictors of outcome that were described in the included studies.
Amplitude-integrated electroencephalography
Two-channel aEEG recordings were studied in one article.16 A correlation between time to recovery to a normal background pattern in the ipsilesional hemisphere and the development of adverse neurodevelopmental outcome was found. For every 24 h an abnormal background pattern was present, the odds ratio for a cognitive deficit at two years of age increased with an OR of 1.9. A cutoff of time to a normal background pattern of more than 45 h was predictive for later development of USCP (sens: 67%, spec: 88%, DOR: 16; Fig. 2), while time to normal sleep-wake cycling was not predictive of long-term outcome.
Electroencephalography
Nine studies described EEG characteristics in relation to long-term outcome,17,18,19,20,21,22,23,24,25 of which five provided data for quantitative analyses.19,20,21,23,25 All studies except McBride and colleagues17 performed EEGs after the onset of presenting symptoms, mostly within the first week of life, and all studies recorded for at least 30 min. The following predictors were studied: occurrence of seizures (n = 64, 4 studies), an abnormal background pattern (n = 33, 3 studies), and focal hypersynchrony (n = 6, 1 study). For prediction of the development of USCP, the occurrence of an abnormal background pattern and seizures had a pooled sensitivity and specificity of 90 [95% CI: 64–94]% and 60 [25–88]% (DOR = 113 [1–737)], and 75 [47–93]% and 58 [28–83]% (DOR = 9 [0.5–47]), respectively (Fig. 1). In contrast, Nevalainen and colleagues described that the background pattern was always continuous in infants with PAIS without concomitant asphyxia (n = 22),23 and in the study population of Tekgul et al. all infants (n = 13) had a favorable neurodevelopmental outcome regardless of neonatal EEG background patterns.18 Consistent with aEEG observations, all seven PAIS infants in the cohort of McBride et al. showed preserved sleep-wake cycling.17 Neonatal EEG characteristics seem not to have any prognostic value for the development of other neurodevelopmental deficits, although sample sizes are small (Fig. 2). The study from Yi and colleagues shows a predictive effect of neonatal seizures on EEG for the development of post-neonatal epilepsy (n = 7; Fig. 2),21 but two larger studies (n = 63 and n = 55) could not identify any EEG predictor of post-neonatal epilepsy.22,24
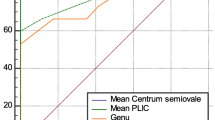
Black rectangles and black lines indicate means and confidence intervals, gray diamonds and gray lines indicate pooled means and confidence intervals of sensitivity, specificity, or diagnostic odds ratio (DOR), respectively. Dashed vertical lines indicate a sensitivity or specificity of 0.8, or a DOR of 25. TP true positive, FP false positive, TN true negative, FN false negative, EEG electroencephalography, GMA general movements assessments, FM fidgety movements, MRI magnetic resonance imaging, BG basal ganglia, PLIC posterior limb of the internal capsule, PM1 proximal M1 or main MCA stroke.
Rectangles/triangles and lines indicate means and confidence intervals, dashed vertical lines indicate a sensitivity or specificity of 0.8, or a DOR of 25. CP cerebral palsy, Cogn cognitive deficit, aEEG amplitude-integrated electroencephalography, BG background pattern, EEG electroencephalography, GMA general movements assessment, WM writhing movements, PTA post-term age, HAI hand assessment for infants, AI asymmetry index, NIRS near-infrared spectroscopy, rScO2 cerebral oxygenation saturation, SEP somatosensory-evoked potential.
Near-infrared spectroscopy
The use of NIRS in the first five days after the onset of PAIS symptoms in relation to long-term outcome was studied in one article.16 An asymmetry of the cerebral oxygen saturation (rScO2) between the hemispheres was found from day 3 onward after the start of symptoms, with higher rScO2 values in the ipsilesional hemisphere. The rScO2-asymmetry could predict later neurodevelopment; a rScO2-asymmetry above 18.6%, and 19.4% showed high specificity for both the development of USCP and a cognitive deficit at two years of age, respectively (Fig. 2).
Standardized motor assessments
Four studies reported the predictive value of the GMA for the development of USCP specifically for infants with PAIS.26,27,28,29 Abnormal fidgety movements (FM) observed between 9 and 18 weeks post-term age (PTA) had a pooled sensitivity and specificity of 84 [95% CI: 57–97]% and 80 [49–97]%, respectively (DOR: 112 [2–683], Fig. 1). Earlier GMA assessment (0–6 weeks post-term age) had high sensitivity but low specificity (Fig. 2). The General Movement Optimality Score (GMOS), a quantitative scoring method for GMA, showed that infants that would later develop USCP had significantly lower total scores and scores for the contralesional limbs at 0–5 weeks PTA.29 The HAI assessment was only reported by one study; an asymmetry between hand scores ≥23% at 3–5 months of age had an excellent predictive value for USCP at 1–3 years of age (Fig. 2).28
Somatosensory evoked potentials
SEP assessments were reported by one study, performed in the first week of life in 22 infants.23 After stimulation of the median nerve at the wrists, the absence of SEPs in the ipsilesional and/or contralesional hemisphere was found to be predictive for the later development of USCP (Fig. 2). Furthermore, the combination of an absent SEP and a continuous background pattern on EEG improved USCP prediction.
Transcranial magnetic stimulation
One study described TMS as a predictive tool for motor development in a cohort of 13 infants with PAIS.30 At term and at three months of age, TMS could not predict hand function at two years of age, although the predictive value increased when TMS was performed at later time points.
MRI
Thirty-two studies reported on predictors from conventional imaging (T1, T2 and/or T1IR), consisting of 21 studies that described injured brain structures (some studies scored in combination with DWI),4,19,20,21,25,26,29,31,32,33,34,35,36,37,38,39,40,41,42,43,44 17 studies described the arterial classification of the stroke,4,16,20,22,23,24,28,29,31,33,37,39,40,45,46,47,48 and 6 studies described the size of the stroke lesion in relation to long-term outcome.32,35,42,43,49,50
Injured brain structures
Involvement of the posterior limb of the internal capsule (PLIC) and basal ganglia (BG) on neonatal MRI both were good predictors of USCP (DOR: 30 [95% CI: 3–128] and 62 [4–310], respectively), and both combined were the second best predictor of USCP in our meta-analysis (pooled sensitivity and specificity: 88 [72–97]% and 95 [87–99]%, DOR: 453 [26–2452]; Fig. 1). At three months of age, involvement of the PLIC and/or cerebral peduncle on MRI were good predictors for USCP development (Fig. 3). A recent study that used a voxel-based lesion-symptom map** (VLSM) approach, found a correlation between a lesion in the left external capsule and impaired gross motor functioning, after correction for the size of the stroke.41 Regarding cognitive functioning, multiple studies described that children that suffered PAIS have mildly lower cognitive scores than average, with differences becoming more apparent from approximately 8 years onwards.32,33,35 Isolated injury of the BG or the thalamus were not predictive for cognitive outcomes (Fig. 3), but combined BG and thalamus involvement was predictive for later cognitive deficits in two studies, together reporting on nearly 200 children.4,33 A VSLM study found a correlation of stroke lesions in the frontal lobe (left inferior and middle frontal gyri) with cognitive impairment at two years. They also showed a correlation between lesions in the left supramarginal gyrus with the need of speech therapy at two years, with a possible sex-dependent effect of males performing worse after damage in this area.41 Another VSLM study found similar results: lesions in the supramarginal gyrus (and the posterior periventricular white matter, fusiform gyrus, and inferior temporal gyrus) correlated with a sensory processing deficit.43
Rectangles/triangles and lines indicate means and confidence intervals, dashed vertical lines indicate a sensitivity or specificity of 0.8, or a DOR of 25. CP cerebral palsy, Cogn cognitive deficit, FSIQ full scale IQ, VIQ verbal IQ, PIQ performance IQ, VCI verbal comprehension index, PRI perceptual reasoning index, WMI working memory index, PSI processing speed index, VF visual field, PSOM pediatric stroke outcome measure, WD Wallerian degeneration, PLIC posterior limb of the internal capsule, BG basal ganglia, OR optic radiation, PVS primary visual cortex, PT posterior trunk MCA, PCA posterior cerebellar artery, MCA middle cerebral artery, pedASPECTS pediatric modification of Alberta Stroke Program Early Computed Tomography Score, CST corticospinal tract, AI asymmetry index, RD radial diffusity, FA fractional anisotropy, AD axial diffusity, MD mean diffusity.
Wagenaar and colleagues described that combined involvement of the basal ganglia and thalamus was also related to the development of behavioral problems, post-neonatal epilepsy, and visual field (VF) defects.4 Furthermore, Hajek et al. showed that a combination of cortical and subcortical lesions was related to lower scores of inhibitory control.32 Regarding VF defects, involvement of the optic radiation on T1 and/or T2 imaging had high specificity but markedly lower sensitivity on scans made in the neonatal period or at 3 months of age (Fig. 3).
Diffusion-weighted imaging
The scoring of injured brain structures using DWI only was reported in seven studies.36,37,42,46,51,53,53 DWI imaging was mostly performed within the first week of life. Involvement of the middle cerebral peduncle on DWI was the best predictor of USCP in our meta-analysis with a pooled sensitivity and specificity of 90 [95% CI: 73–98]% and 94 [84–99]% (DOR: 695 [24–3805]; Fig. 1). Both qualitative and quantitative measurements of injury to the corticospinal tracts on DWI (e.g. PLIC, cerebral peduncle, and/or medullary pyramid) were good predictors for USCP prediction (Fig. 3).53
Arterial distribution
PM1 (or main) MCA stroke had a low to moderate sensitivity (72 [95% CI: 54–86]%) but a high specificity (97 [93–99]%) for the prediction of USCP (DOR: 132 [20–506]; Fig. 1), a cognitive deficit, epilepsy, and VF defects (Fig. 3). In the study of Wagenaar et al., all children with a main MCA had an adverse neurodevelopmental outcome in at least one domain.4 Perforator and posterior cerebral artery (PCA) strokes were generally associated with a favorable neurodevelopmental outcome, except when additional brain injury was present (Fig. 3).31,45 Bilateral stroke was associated with USCP and tri-/quadriplegia.48 Post-neonatal epilepsy was not associated to the stroke’s side,24 but a main MCA, a bilateral stroke, or a stroke involving multiple territories seem to increase the chance of epilepsy at later age.4,22 Strokes covering the full PCA territory were associated with VF defects (Fig. 3).
Lesion size
Lesion size was described in various ways: by volume segmentation of the stroke lesion volume,32,42,43 by the scoring methods pedASPECT and modASPECT,42,50 or by describing the involvement of cerebral lobes.35 A stroke volume of ≥3.3% of the total brain volume predicted the development of USCP (Fig. 3).42 The predictive value of lesion size in relation to cognitive outcome is unclear, as one study did not find a strong predictive value (n = 26, Fig. 3),35 but another described a correlation between stroke volume and cognitive deficits (n = 36).32 Giudice and colleagues did not find a relation between stroke volume and sensory processing deficits.43 Wusthoff et al. described that stroke size, calculated by the modASPECT score, was significantly associated with the development of later seizures.50
Diffusion tensor imaging
Four articles reported on DTI characteristics in relation to long-term outcome at different timeframes: neonatal,34 around three months of age,44,53 and at both of these timepoints.54 Neonatal DTI measurements in the CST did show high predictive value for USCP in one study,34 but not in another.54 However, at around three months of age, asymmetry of fractional anisotropy (FA) values in the CST, showed a sensitivity and specificity of ±95% for the development of USCP (Fig. 3).53 FA values of the corpus callosum, the anterior limb of the internal capsule, posterior thalamic radiation, and optic radiation also correlated with development of USCP, and FA values of the corpus callosum correlated to cognitive outcome.54 DTI measurements of the optic radiation at three months of age were predictive of develo** VF defects in two studies (Fig. 3).44,54
Magnetic resonance angiography
One study described MRA characteristics in 30 infants with a unilateral MCA in relation to long-term motor outcome.55 A visible arterial occlusion on MRA had low sensitivity but high specificity for the development of USCP, while the detection of any vascular abnormality on MRA (arterial occlusion, thrombus, or increased blood flow) showed fair sensitivity but low specificity (Fig. 3).
Discussion
In this review, we found that corticospinal tract assessment on MRI and standardized motor assessments are best to predict unilateral spastic cerebral palsy after PAIS. Bedside techniques including aEEG and NIRS might be able to improve the prediction of cognitive outcomes. Injury to the optic radiation on DTI predicted the development of VF defects.
Motor outcome
During the last two decades, more studies have focused on accurate prediction of USCP after PAIS, with MRI becoming the golden standard. In this systematic review, involvement of the corticospinal tracts (including the PLIC and cerebral peduncle), either visible as pre-Wallerian degeneration on neonatal T1, T2 and/or DWI sequences, or as asymmetry at a later time point, visualized with conventional MRI or DTI, were good to excellent predictors for the development of USCP at later age. The vast majority of infants suffering from a large middle cerebral artery (MCA) stroke experience at least one adverse outcome, and USCP will develop in nearly all of these infants.4 However, for infants suffering from smaller stroke subtypes other predictors of USCP are needed. The (prolonged) presence of an abnormal background pattern on (a)EEG, and an asymmetry of the cerebral oxygen saturation measured with NIRS also seem of prognostic value, even though the reported number of patients was relatively small. Combining techniques might further improve outcome prediction, as the combination of absent SEPs and a continuous background pattern on EEG predicted USCP accurately. Standardized motor assessments performed in the first months of life, including the GMA and the HAI, seem excellent predictors for later USCP development. Although these assessments were not extensively studied in the PAIS population, our findings are in line with larger studies investigating a population at high-risk for USCP caused by various etiologies.56,57 No studies of the Hammersmith Infant Neurological Examination (HINE) were identified in the PAIS population, however one could expect that the predictive performance for PAIS is similar to the high-risk for USCP population and therefore useful in predicting outcome.58,59 Concluding, the combination of MRI, bedside neurophysiology, and standardized motor assessments will be able to provide accurate prediction of the development of USCP in all PAIS infants within the first months of life.
Non-motor outcome
Most of the literature is focused on long-term motor outcome after PAIS, although other outcome domains might be equally or even more important for a good quality of life. Cognitive development can be influenced by multiple factors, including genetics and environmental factors, making accurate prediction of later cognitive functioning a challenge.60,61 We showed that combined injury to the basal ganglia and thalami on MRI is predictive of the development of a cognitive deficit, although this might partly be due to the fact that this type of injury represents larger-sized strokes. Furthermore, aEEG and NIRS findings might aid to improve future cognitive outcome prediction, although validation in other patient cohorts is needed. Other innovative analyzing techniques, such as voxel-based lesion symptom map** (VBLM), might provide new insights regarding the effect of lesion location on cognitive outcome. A recent VBLM study described an association between frontal lesions and cognitive impairment at two years of age, and both VBLM studies included in this review found a correlation between lesions in the left supramarginal gyrus (a region known to be important in language processing) with either the need for speech therapy or a sensory processing deficit.41,43 Although validation is needed, a possible role of sex in language outcomes was found, possibly caused by females having a more symmetrical distribution of language functioning, and therefore being able to switch language function to the (non-damaged) hemisphere more easily.41 Since IQ scores are generally mildly to moderately impaired after PAIS, adaptive functioning and behavioral control might be more important for adequate functioning in an academic or working environment. Lo and colleagues reported that children with neonatal and childhood strokes had impaired adaptive and social functioning without IQ or behavioral deficits.62 In our review, we did not find any predictors of behavioral problems besides the presence of a main MCA stroke, and that infants with combined cortical and subcortical lesions showed poorer inhibitory control.32
Accurate identification of patients at high risk of post-neonatal epilepsy is valuable to select those that should be closely monitored with regularly performed EEGs, since it was reported that children with post-neonatal seizures after PAIS had lower hippocampal volumes, and these reduced hippocampal volumes corresponded to poorer memory functioning.63 The development of epilepsy seems to be related not to a specific location of the lesion, but to the extensiveness of the stroke.50 This is in accordance with the predictive value of a main MCA stroke, and the presence of bilateral or multiple strokes for the development of post-neonatal epilepsy, as described in this review. Neonatal seizures on EEG seem not predictive of post-neonatal epilepsy: although there seems to be a predictive effect in one study (n = 7; Fig. 2),21 two studies with larger populations (n = 63 and n = 55) do not show such an effect.22,24 These findings differ from studies performed in children with cerebral palsy caused by a wide range of etiologies, where neonatal seizures were predictive of later epilepsy.64,65
Limitations
This systematic review has several limitations. Firstly, there was high variability in classifying and reporting methods of predictors and outcomes, making it difficult to group outcomes, and introducing heterogeneity in the meta-analysis. Furthermore, information about (neurological) comorbidities was frequently not reported, and could therefore not be taken into account. Secondly, some studies described patients that underwent a diagnostic procedure beyond four months of age. We excluded these individual subjects, however this was not possible for all studies. Therefore, some included infants underwent a standardized motor assessment at four and a half months of age.28 Thirdly, multiple studies described the long-term outcome for a population at risk for USCP, including multiple etiologies. We were not able to extract the data of PAIS patients from all of these papers, since they did not all report the etiology or PAIS patients separately. Furthermore, the number of available studies on non-motor outcomes and their duration of follow-up was limited, making it highly likely that these outcomes did not develop yet and were therefore underreported. Finally, most studies used qualitative MRI analysis instead of quantitative analysis which is more subjective and therefore less well reproducible.
Future prospects
To improve outcome prediction after PAIS, future studies should focus on non-motor outcomes, including adaptive, social, behavioral, and emotional functioning. Furthermore, combining the results of various techniques and assessments can improve outcome prediction, as was shown in a high risk for USCP population.58,66 Regarding PAIS, one study reported a significant correlation between a lower hand assessment scores of the HAI and more severe CST asymmetry on MRI.52 Advanced analysis techniques such as machine learning are currently being developed to optimize this process, with the benefit that an early diagnosis and prognosis can be established even when there is no expertise available.67 Quantitative (semi-)automatic analyzing techniques, such as brain volume segmentations and stroke lesion map** in MRI, can stimulate the transition from qualitative to quantitative analyses.68 Other measures of cerebral organization and functioning, including functional MRI, interhemispheric functional connectivity,69 and the role of cerebellar functioning,70 are all areas that might improve the understanding of brain development after PAIS.
Recommendations
Based on this systematic review, we recommend to perform a (structural and diffusion-weighted) MRI within a week after onset of PAIS symptoms to improve outcome prediction. We have demonstrated that on average 75–84% of infants with asymmetry in the descending CST on MRI develop future motor problems. Furthermore, a follow-up assessment at three to four months of age, ideally including a DTI scan, and the performance of standardized motor assessments such as the GMA and/or HAI are currently the best aids for the clinician to predict motor outcome after PAIS. At 3-4 months, CST asymmetry on DTI is related to 100% adverse motor development, and abnormal clinical assessments are related to adverse motor development in 63–100% of cases. Regarding non-motor outcomes, prediction is less precise but children with large (bilateral or main MCA) strokes are at risk for adverse outcomes in multiple domains and should therefore be closely monitored. Also, there might be a role for bedside techniques including (a)EEG and NIRS in the prediction of long-term cognitive outcomes. Injury to the optic radiation on DTI correctly predicted later visual field defects. The findings of this systematic review highlight the importance of improving outcome prediction for non-motor outcome domains after perinatal stroke.
Data availability
Template data collection forms can be found in the protocol on Prospero. Data extracted from included studies and data used for analysis can be made available upon request with the corresponding author.
References
Sorg, A. L. et al. Incidence estimates of perinatal arterial ischemic stroke in preterm- and term-born infants: a national capture-recapture calculation corrected surveillance study. Neonatology 118, 727–733 (2021).
Gale, C. et al. Neonatal brain injuries in England: Population-based incidence derived from routinely recorded clinical data held in the National Neonatal Research Database. Arch. Dis. Child. Fetal Neonatal Ed. 103, F301–F306 (2018).
Ferriero, D. M. et al. Management of stroke in neonates and children: A scientific statement from the American Heart Association/American stroke association. Stroke 50, E51–E96 (2019).
Wagenaar, N. et al. Neurodevelopment after perinatal arterial ischemic stroke. Pediatrics 142, e20174164 (2018).
Benders, M. J. et al. Feasibility and safety of erythropoietin for neuroprotection after perinatal arterial ischemic stroke. J. Pediatr. 164, 481–482 (2014).
Baak, L. M. et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): a first-in-human, open-label intervention study. Lancet Neurol. 21, 528–536 (2022).
Wagenaar, N. et al. Promoting neuroregeneration after perinatal arterial ischemic stroke: neurotrophic factors and mesenchymal stem Brain Activity and Cerebral Oxygenation After Perinatal Arterial Ischemic Stroke Are Associated With Neurodevelopment. Pediatr. Res. 83, 372–384 (2018).
Hoare, B. J. et al. Constraint-induced movement therapy in children with unilateral cerebral palsy. Cochrane Database Syst. Rev. 2019, CD004149 (2019).
Charles, J. & Gordon, A. M. Development of hand-arm bimanual intensive training (HABIT) for improving bimanual coordination in children with hemiplegic cerebral palsy. Dev. Med. Child Neurol. 48, 931–936 (2006).
Kirton, A. et al. Brain stimulation and constraint for perinatal stroke hemiparesis: The PLASTIC CHAMPS Trial. Neurology 86, 1659–1667 (2016).
Kirton, A. et al. Transcranial direct current stimulation for children with perinatal stroke and hemiparesis. Neurology 88, 259–267 (2017).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5, 1–10 (2016).
Moola, S. et al. Chapter 7: Systematic reviews of etiology and risk. in Joanna Briggs Institute Reviewer’s Manual. (eds. Aromataris, E. & Munn, Z.) (The Joanna Briggs Institute, 2017).
Guo, J. & Riebler, A. Meta4diag: Bayesian bivariate meta-analysis of diagnostic test studies for routine practice. J. Stat. Softw. 83, 1–27 (2018).
Hamza, T. H., Reitsma, J. B. & Stijnen, T. Meta-analysis of diagnostic studies: A comparison of random intercept, normal-normal, and binomial-normal bivariate summary ROC approaches. Med. Decis. Mak. 28, 639–649 (2008).
Wagenaar, N. et al. Brain activity and cerebral oxygenation after perinatal arterial ischemic stroke are associated with neurodevelopment. Stroke 50, 2668–2676 (2019).
McBride, M. C., Laroia, N. & Guillet, R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 55, 506–513 (2000).
Tekgul, H. et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics 117, 1270–1280 (2006).
Schulzke, S., Weber, P., Luetschg, J. & Fahnenstich, H. Incidence and diagnosis of unilateral arterial cerebral infarction in newborn infants. J. Perinat. Med. 33, 170–175 (2005).
Lee, H. J. et al. Clinical presentations and neurodevelopmental outcomes of perinatal stroke in preterm and term neonates: a case series. J. Korean Med. Sci. 25, 888–894 (2010).
Yi, Y. Y. et al. Clinical outcomes of cerebral infarctions in neonates. Pediatr. Neurol. 45, 368–372 (2011).
Suppiej, A. et al. Pediatric epilepsy following neonatal seizures symptomatic of stroke. Brain Dev. 38, 27–31 (2016).
Nevalainen, P. et al. Bedside neurophysiological tests can identify neonates with stroke leading to cerebral palsy. Clin. Neurophysiol. 130, 759–766 (2019).
Wanigasinghe, J. et al. Epilepsy in hemiplegic cerebral palsy due to perinatal arterial ischaemic stroke. Dev. Med. Child Neurol. 52, 1021–1027 (2010).
Mercuri, E. et al. Early prognostic indicators of outcome in infants with neonatal cerebral infarction: a clinical, electroencephalogram, and magnetic resonance imaging study. Pediatrics 103, 39–46 (1999).
Guzzetta, A. et al. General movements detect early signs of hemiplegia in term infants with neonatal cerebral infarction. Neuropediatrics 34, 61–66 (2003).
Guzzetta, A. et al. Hand movements at 3 months predict later hemiplegia in term infants with neonatal cerebral infarction. Dev. Med. Child Neurol. 52, 767–772 (2010).
Pascal, A. et al. Motor outcome after perinatal stroke and early prediction of unilateral spastic cerebral palsy. Eur. J. Paediatr. Neurol. 29, 54–61 (2020).
Yin, H. et al. A pilot study of the General Movement Optimality Score detects early signs of motor disorder in neonates with arterial ischemic stroke. Early Hum. Dev. 163, 105484 (2021).
Eyre, J. A. et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann. Neurol. 62, 493–503 (2007).
Ecury-Goossen, G. M. et al. Neurodevelopmental outcome after neonatal perforator stroke. Dev. Med. Child Neurol. 58, 49–56 (2016).
Hajek, C. A. et al. Cognitive outcomes following arterial ischemic stroke in infants and children. J. Child Neurol. 29, 887–894 (2014).
van Buuren, L. M. et al. Cognitive outcome in childhood after unilateral perinatal brain injury. Dev. Med. Child Neurol. 55, 934–940 (2013).
Roze, E. et al. Tractography of the corticospinal tracts in infants with focal perinatal injury: comparison with normal controls and to motor development. Neuroradiology 54, 507–516 (2012).
Westmacott, R., Macgregor, D., Askalan, R. & Deveber, G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke 40, 2012–2019 (2009).
Kirton, A., Shroff, M., Visvanathan, T. & deVeber, G. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke 38, 974–980 (2007).
De Vries, L. S., Van Der Grond, J., Van Haastert, I. C. & Groenendaal, F. Prediction of outcome in new-born infants with arterial ischaemic stroke using diffusion-weighted magnetic resonance imaging. Neuropediatrics 36, 12–20 (2005).
Boardman, J. P. et al. Magnetic resonance image correlates of hemiparesis after neonatal and childhood middle cerebral artery stroke. Pediatrics 115, 321–326 (2005).
Mercuri, E. et al. Neonatal cerebral infarction and neuromotor outcome at school age. Pediatrics 113, 95–100 (2004).
Mercuri, E. et al. Neonatal cerebral infarction and visual function at school age. Arch. Dis. Child. Fetal Neonatal Ed. 88, F487–F491 (2003).
Núñez, C. et al. Neonatal arterial stroke location is associated with outcome at 2 years: A voxel-based lesion-symptom map** study. Arch. Dis. Child. Fetal Neonatal Ed. 107, F45–F50 (2022).
Wiedemann, A. et al. Impact of stroke volume on motor outcome in neonatal arterial ischemic stroke. Eur. J. Paediatr. Neurol. 25, 97–105 (2020).
Giudice, C., Rogers, E. E., Johnson, B. C., Glass, H. C. & Shapiro, K. A. Neuroanatomical correlates of sensory deficits in children with neonatal arterial ischemic stroke. Dev. Med. Child Neurol. 61, 667–671 (2019).
Koenraads, Y. et al. Prediction of visual field defects in newborn infants with perinatal arterial ischemic stroke using early MRI and DTI-based tractography of the optic radiation. Eur. J. Paediatr. Neurol. 20, 309–318 (2016).
van der Aa, N. E. et al. Neonatal posterior cerebral artery stroke: clinical presentation, MRI findings, and outcome. Dev. Med. Child Neurol. 55, 283–290 (2013).
Husson, B. et al. Motor outcomes after neonatal arterial ischemic stroke related to early MRI data in a prospective study. Pediatrics 126, 912–918 (2010).
Ricci, D. et al. Cognitive outcome at early school age in term-born children with perinatally acquired middle cerebral artery territory infarction. Stroke 39, 403–410 (2008).
Golomb, M. R., Garg, B. P., Saha, C., Azzouz, F. & Williams, L. S. Cerebral palsy after perinatal arterial ischemic stroke. J. Child Neurol. 23, 279–286 (2008).
Mackay, M. T. et al. Pediatric ASPECTS predicts outcomes following acute symptomatic neonatal arterial stroke. Neurology 94, e1259–e1270 (2020).
Wusthoff, C. J. et al. Risk of later seizure after perinatal arterial ischemic stroke: a prospective cohort study. Pediatrics 127, e1550–e1557 (2011).
Srivastava, R. et al. Diffusion imaging of cerebral diaschisis in neonatal arterial ischemic stroke. Pediatr. Neurol. 100, 49–54 (2019).
van der Aa, N. E. et al. Neonatal neuroimaging predicts recruitment of contralesional corticospinal tracts following perinatal brain injury. Dev. Med. Child Neurol. 55, 707–712 (2013).
Van Der Aa, N. E. et al. Does diffusion tensor imaging-based tractography at 3 months of age contribute to the prediction of motor outcome after perinatal arterial ischemic stroke? Stroke 42, 3410–3414 (2011).
van der Aa, N. E. et al. Quantification of white matter injury following neonatal stroke with serial DTI. Pediatr. Res. 73, 756–762 (2013).
Husson, B. et al. MR angiography findings in infants with neonatal arterial ischemic stroke in the middle cerebral artery territory: A prospective study using circle of Willis MR angiography. Eur. J. Radiol. 85, 1329–1335 (2016).
Ryll, U. C. et al. Predictive validity of the Hand Assessment for Infants in infants at risk of unilateral cerebral palsy. Dev. Med. Child Neurol. 63, 436–443 (2020).
Einspieler, C. et al. Cerebral palsy: Early markers of clinical phenotype and functional outcome. J. Clin. Med. 8, 1616 (2019).
Novak, I. et al. Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatr. 171, 897–907 (2017).
Haataja, L. et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J. Pediatr. 135, 153–161 (1999).
Tong, S., Baghurst, P., Vimpani, G. & McMichael, A. Socioeconomic Position, Maternal IQ, Home Environment, and Cognitive Development. J. Pediatr. 151, 284–288 (2007).
Mollon, J. et al. Genetic influence on cognitive development between childhood and adulthood. Mol. Psychiatry 26, 656–665 (2021).
Lo, W. et al. Social competence following neonatal and childhood stroke. Int. J. Stroke 9, 1037–1044 (2014).
Gold, J. J. & Trauner, D. A. Hippocampal volume and memory performance in children with perinatal stroke. Pediatr. Neurol. 50, 18–25 (2014).
Oskoui, M. & Shevell, M. I. Profile of pediatric hemiparesis. J. Child Neurol. 20, 471–476 (2005).
Carlsson, M., Hagberg, G. & Olsson, I. Clinical and aetiological aspects of epilepsy in children with cerebral palsy. Dev. Med. Child Neurol. 45, 371–376 (2003).
Morgan, C. et al. The pooled diagnostic accuracy of neuroimaging, general movements, and neurological examination for diagnosing cerebral palsy early in high-risk infants: A case control study. J. Clin. Med. 8, 1879 (2019).
Cérébrale, F. P. ENSEMBLE—European Newborn Study: Early Markers for a Better LifE. https://fondationparalysiecerebrale.org/en/ensemble-project.
Makropoulos, A., Robinson, E. C., Schuh, A. & Wright, R. The Develo** Human Connectome Project: a minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 173, 88–112 (2019).
Dick, A. S., Raja Beharelle, A., Solodkin, A. & Small, S. L. Interhemispheric functional connectivity following prenatal or perinatal brain injury predicts receptive language outcome. J. Neurosci. 33, 5612–5625 (2013).
Craig, B. T. et al. Crossed cerebellar atrophy in perinatal stroke. Stroke 50, 175–177 (2019).
Hellstrom-Westas, L., Resen, I. & Svenningsen, N. W. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch. Dis. Child 72, 34–38 (1995).
Einspieler, C. & Prechtl, H. F. R. Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 11, 61–67 (2005).
Govaert, P. Sonographic stroke templates. Semin. Fetal Neonatal. Med. 14, 284–298 (2009).
Kirton, A., Deveber, G., Pontigon, A.-M., Macgregor, D. & Shroff, M. Presumed perinatal ischemic stroke: vascular classification predicts outcomes. Ann. Neurol. 63, 436–443 (2008).
de Vries, L. S. et al. Infarcts in the vascular distribution of the middle cerebral artery in preterm and fullterm infants. Neuropediatrics 28, 88–96 (1997).
Funding
This work was supported by the European Society for Pediatric Research (ESPR). The funder had no role in the study design, data collection and analysis, preparation of the manuscript or decision to publish. The authors have no further conflicts of interest to declare.
Author information
Authors and Affiliations
Contributions
L.M.B. designed the protocol, performed the article search, and the statistical analyses, and wrote the original draft. L.M.B. and A.A.E.V. selected articles, extracted data and performed risk of bias analysis, with NEA as a third reviewer in case of discrepancies. NEA, F.G., J.D., C.H.A.N., M.J.N.L.B., and N.W. aided in design, conceptualization, and supervision. All authors reviewed and edited the paper and confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baak, L.M., van der Aa, N.E., Verhagen, A.A.E. et al. Early predictors of neurodevelopment after perinatal arterial ischemic stroke: a systematic review and meta-analysis. Pediatr Res 94, 20–33 (2023). https://doi.org/10.1038/s41390-022-02433-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02433-w
- Springer Nature America, Inc.