Abstract
Heparanase (HPA) is the predominant enzyme that cleaves heparan sulfate and plays a critical role in a variety of pathophysiological processes. HPA activity has been traditionally correlated with tumor metastasis due to participation in the cleavage and remodeling of the extracellular matrix (ECM). Apart from its well-characterized catalytic properties, HPA was noticed to exert biological functions not rely on its enzymatic activity. This feature is supported by studies showing induction of signaling events, such as Src and AKT, by nonenzymatic HPA mutant. We provide evidence here that active HPA and inactive HPA mutant proteins enhance gastric cancer cell growth, possibly attributed to TFEB-mediated autophagy. Similarly, HPA gene silencing resulted in decreased gastric cancer cell proliferation and autophagy. Besides, TFEB inhibition reduced cell growth and autophagy induced by nonenzymatic HPA. Notably, HPA and TFEB were significantly elevated in gastric carcinomas compared with the adjacent gastric tissue. Moreover, the elevation of HPA gene expression and upregulation of TFEB levels have been associated with advanced clinical stage and poor prognosis of gastric cancer, providing strong clinical support for a connection between TFEB and HPA. Thus, neutralizing the nonenzymatic function of HPA and the related TFEB-driven autophagy may profoundly impact gastric cancer progression.
Similar content being viewed by others
Introduction
Mammalian heparanase (HPA) is the only endoglycosidase that degrades heparan sulfate, a vital element of the extracellular matrix (ECM) and tumor microenvironment [1, 2]. HPA activity is strongly implicated in the metastatic potential of tumor cells, a result of heparan sulfate degradation and remodeling of ECM barriers [3, 4]. Importantly, Epithelial–mesenchymal transition (EMT) is a key biological process for HPA-derived cells to acquire the ability of migration and invasion. Previous studies have reported that HPA modulates the activity of factors such as FGF-2 and TGF-β-induced EMT [5, 6]. Besides, EMT makers were also found higher in gastric signet ring cell adenocarcinoma with higher HPA expression [7]. Similarly, HPA activity contributes to angiogenesis, inflammation, and autoimmunity [8,9,10]. Evidence accumulating over the past two decades, indicates that highly expressed HPA exists in the majority of primary tumors, including carcinomas, sarcomas, and hematological malignancies, correlates with larger tumor size, higher microvessel density, and worse prognosis [11, 12]. Apart from its roles as an enzymatic element, heparanase also fulfils enzymatic-independent biological functions, including signal transduction [13, 14] and gene transcription [15, 16]. Activation of serine/threonine kinase (AKT) is regarded as a prominent nonenzymatic signaling event exerted by HPA [17], and its inhibition attenuates cell growth [18]. HPA can also promote cell proliferation by enhancing the phosphorylation of epidermal growth factor receptor, independent of its enzymatic activity [19, 20].
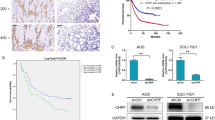
Autophagy is an intracellular catabolic process that degrades cytoplasmic macromolecules and subcellular organelles, essential for cell survival and homeostasis [21]. Autophagy is a dynamic recycling system, consisting of the formation of autophagosomes, fusion of autophagosome–lysosome, and the degradation of autolysosomes [22, 55,56]. To further analyzed the expression of HPA and TFEB in GC tissues, we used a GC tissue microarray containing 15 pairs of primary GC tissues and adjacent nontumor tissues. Immunohistochemistry was applied to detect HPA and TFEB in human specimens derived from fifteen paired patients with gastric cancer. As shown in Fig. 8a, b, expression of HPA and TFEB was markedly increased in primary GC tissues compared to their adjacent normal-looking tissues. We further analyzed TFEB and HPA in gastric tumors using the TCGA database. Expression of HPA was significantly increased in gastric cancer (Fig. 8c and Supplementary Data 1). Moreover, clinicopathological analysis revealed that the patients with poorly differentiated tumors (Fig. 8d and Supplementary Data 2) and advanced tumor stage (Fig. 8e and Supplementary Data 3) exhibited significantly higher HPA expression. We next applied the Kaplan–Meier analysis and explored the relationship between TFEB expression and the GC patient outcome. The data revealed that TFEB elevation is significantly correlated with poor prognosis of in patients wih GC (OS HR = 1.61, log-rank P = 8.5e-06; FP HP = 1.44, log-rank P = 0.0011; PPS HP = 1.68, log-rank P = 4.9e-06) (Fig. 8f–h). Taken together, these results indicate that increased expression of HPA and TFEB in gastric cancer is correlated with advanced tumor stage and poor prognosis.
Immunohistochemical analysis of HPA (a) and TFEB (b) expression in gastric tissues. c Relative expression level of HPA in normal gastric tissue and gastric primary tumors from UALCAN database. d Relative expression of HPA in gastric cancer based on tumor grade from UALCAN database. e expression of HPA in gastric cancer based on lymph nodal metastasis status from UALCAN database. Kaplan–Meier analysis from TCGA database of the correlation between relative expression of TFEB and overall survival (OS) (f), first progression (FP) (g), or post-progression survival (h). *P < 0.05, **P < 0.01 versus the adjacent or normal group. STAD stomach adenocarcinoma.
Discussion
Mammalian cells express a single functional endoglycosidase (heparanase, HPA) which degrades heparan sulfate (HS). Cleavage of HS by HPA contributes to the disassembly of the ECM, thereby facilitating cancer metastasis and inflammation. Compelling evidence strongly implies that heparanase serve crucial roles in all aspects of the tumorigenic process, namely tumor initiation, growth, chemotherapy resistance, and metastasis [1,2,3,4,5,6,7,8,9,10,11,12,13], pointing to the potential of heparanase as an anti-cancer drug target and incentivizing the development of small molecular inhibitors of heparanase [14, 15]. A critical venue by which heparanase exerts multiple effects on its target tissues and cells is by modulating the bioavailability of HS-bound cytokines, chemokines, and growth factors, initiating the tissue microenvironment. Such architecture allows heparanase-mediated tumor-host crosstalk and promotes several basic cellular processes (i.e., exosome formation, autophagy, immune-inflammatory responses) that together coordinate tissue remodeling [16, 17]. Heparanase functions as an “activator” of HS proteoglycans and therefore is a pivotal player in creating a supportive environment for tumor cell proliferation and dissemination. In addition, it is required for various cellular processes from gene expression regulation to signal transduction and DNA damage signaling [3, 18]. In addition to its enzymatic activity, nonenzymatic functions of HPA have been reported and are well-documented [57, 58]. For example, HPA could augment cell proliferation, mobility, and angiogenesis through activation of β1 integrin, HIF-2α, Flk-1, and/or AKT signaling, independent of its enzymatic activity [17, 18, 59]. In addition, HPA has been documented to impact on the blood clotting system, independent of its catalytic function [60, 61]. Altogether, these and other results indicate that nonenzymatic activities of HPA play essential roles in a variety of pathological processes. Here, we demonstrate that, among other effects, overexpression of enzymatically inactive heparanase promotes GC cell proliferation and autophagy.
Autophagy is a major cellular degradation and recycling process, critical for maintaining cellular homeostasis [62, 63]. Autophagy is a dynamic process that sequesters misfolded and/or potentially dangerous proteins and damaged organelles in double-membranes vesicles called autophagosomes, that are ultimately degraded within lysosomes [64, 65]. The process of autophagy is schematically subdivided into three critical steps: autophagosome formation and maturation, autophagosome–lysosome fusion, and auto-lysosomal acidification [66]. Briefly, the activation of ULK1 and BECN1-VPS34 complexes leads to the formation of autophagosomes [65, 67] that are then fused with lysosomes to produce autolysosomes, followed by intraluminal acidification and subsequent activation of lysosomal hydrolases to mediate autophagic cargo degradation [68, 69]. Given its major role in cellular metabolism, autophagy is connected with numerous disease states [24, 41], yet the role of autophagy in cancer is disputable and context-dependent [24, 26, 48, 70,71,72]. It has previously been shown that HPA promotes the growth of head and neck carcinoma by enhancing autophagy [32]. In the present study, we found that both active or enzymatically inactive HPA increase autophagosome formation and the expression of related genes in gastric cancer cells. We show that nonenzymatic HPA-induced LC3-II protein expression and cell viability were attenuated by 3MA and CQ, compounds that inhibit autophagosome formation and disrupt autophagosome–lysosome fusion, respectively. We next assessed the biogenesis and formation of lysosomes to further ascertain the role of autophagy in nonenzymatic HPA-induced GC cell proliferation. We observed that both wild-type and mutant HPA increased the expression of autophagy- and lysosomal-related genes as well as the level of the lysosomal membrane protein LAMP2. Fluorescence imaging showed that the formation of autophagosomes, autolysosomes, and lysosomes was increased after transfection with native or nonenzymatic HPA, indicating that nonenzymatic HPA may also trigger cell autophagy by inducing lysosomal biogenesis and formation of autolysosomes in gastric cancer cells.
The MiT/TFE transcription factors are composed of four members: TFEB, TFEC, and TFE3 and MITF. Among them, TFEB and TFE3 are master regulators of lysosomal and autophagosome biogenesis, which bind to a conserved 10-base palindromic sequences, named Coordinated Lysosomal Expression and Regulation (CELEAR) site that promotes the transcription of several lysosomal and autophagy genes [50, 73,74,75]. A variety of stimuli are related to TFEB and TFE3 activities, among which nutritional deprivation is well-characterized [52, 76, 77]. In the presence of nutrients, TFEB and TFE3 have been shown to reside in the cell cytoplasm, but are rapidly translocated to the nucleus in the absence of nutrients [76, 78]. Subsequently, molecules involved in nutrient sensing and cellular growth have been shown to regulate TFEB and TFE3 activity [43, 79, 80]. The most studied regulatory mechanism of TFEB and TFE3 subcellular localization is involved in modulating the phosphorylation status of multiple serine residues [52]. In particular, mTOR kinase was shown to phosphorylate TFEB and TFE3 and act as a regulatory in TFEB and TFE3 subcellular localization [51, 52]. Several serine residues in TFEB and TFE3 are phosphorylated by mTOR, but S211 in TFEB and S321 in TFE3 are particularly relevant [81,82,83,84]. In the presence of adequate nutrition, mTOR phosphorylates these residues to create a cytoplasmic chaperone 14-3-3 binding site. Interaction with 14-3-3 leads to the accumulation of TFEB/TFE3 in the cytoplasm. In contrast, when nutrition is insufficient, inactivation of mTORC1 dephosphorylates S211 and S321 and prevents binding to 14-3-3 leading to rapid TFEB and TFE3 nucleus translocation occurred. The mechanism underlying autophagy induction by HPA is not clear. Shteingauz et al reported that HPA-induced autophagy is mediated by mTOR signaling because HPA overexpression is associated with decreased mTOR activity, whereas HPA inhibition leads to increased mTOR activity and substrate phosphorylation [32]. Here, we report that the mRNA and protein levels of TFEB, but not TFE3, were significantly changed after transfection with either wild-type or nonenzymatic HPA. Moreover, active and mutant HPA promoted nuclear translocation of TFEB but had no significant effect on TFE3 subcellular localization. These data suggest that TFEB- but not TFE3-mediated autophagy is predominant involved in HPA-induced autophagy in GC cells, independent of HPA enzymatic activity. Additionally, upregulation of nonenzymatic HPA decreased phosphorylation of TFEB S211, and abolished the interaction between TFEB and 14-3-3 protein, further indicating that nonenzymatic HPA induces TFEB-mediated autophagy in TFEB dephosphorylation-dependent manner.
Aberrant activation of TFEB is closely related to tumor oncogenesis and development [51, 85, 86]. Cells rely on effective lysosomal function, and increasing evidence indicates that cancer cells may utilize TFEB-dependent transcriptional activation of lysosomal degradation pathways to maintain survival. Overexpression of TFEB was reported to drive pancreatic ductal adenocarcinoma by inducing autophagy [48, 87]. TFEB gene silencing results in a significant decrease in the hyperproliferative phenotype and progression of pancreatic carcinoma to advanced stages [31, 54]. Here, we report that TFEB gene silencing significantly attenuated hyperproliferation induced by nonenzymatic HPA via decreased autophagy and lysosome biogenesis. Moreover, upregulation of HPA in GC is significantly linked to advanced tumor stage, while increased TFEB expression in GC tissues is correlated with poor prognosis. These results further indicate that HPA induces GC progression through TFEB-mediated autophagy, independent of HPA enzymatic activity (Graphical abstract), offering a new strategy for the design of nonenzymatic HPA targeting drugs.
However, our results are mainly derived from gastric cancer cell lines, and it is necessary to repeatedly verify the regulatory relationship between HPA and TFEB in animal in vivo experiments. We will make further improvements in follow-up research.
Change history
18 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41389-023-00487-x
References
Freeman C, Browne AM, Parish CR. Evidence that platelet and tumour heparanases are similar enzymes. Biochem J. 1990;342:361–8.
Vlodavsky I, Friedmann Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest. 2001;108:341–7.
Nakajima M, Irimura T, Nicolson GL. Heparanases and tumor metastasis. J Cell Biochem. 1988;36:157–67.
Bashkin P, Razin E, Eldor A, Vlodavsky I. Degranulating mast cells secrete an endoglycosidase that degrades heparan sulfate in subendothelial extracellular matrix. Blood. 1990;75:2204–12.
Valentina M, Gianluigi Z, Maria FS, Giovanni G, Antonio L, Maurizio O. Heparanase is a key player in renal fibrosis by regulating TGF-β expression and activity. Biochim Biophys Acta. 2014;1843:2122–8.
Valentina M, Giovanni G, Elena T, Anna MB, Alessandra G, Angela D’A, et al. Heparanase and syndecan-1 interplay orchestrates fibroblast growth factor-2-induced epithelial-mesenchymal transition in renal tubular cells. J Biol Chem. 2012;287:1478–88.
Shahid S, Caroline F, Simon D, Shahsoltan M, Genevieve C, Cynthia P, et al. The close relationship between heparanase and epithelial mesenchymal transition in gastric signet-ring cell adenocarcinoma. Oncotarget. 2018;9:33778–87.
Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–73.
Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–43.
Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, et al. Heparanase: from basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat. 2016;29:54–75.
Zhang GL, Gutter-Kapon L, Ilan N, Batool T, Singh K, Digre A, et al. Significance of host heparanase in promoting tumor growth and metastasis. Matrix Biol. 2020;93:25–42.
Bhattacharya U, Gutter-Kapon L, Kan T, Boyango I, Barash U, Yang SM, et al. Heparanase and chemotherapy synergize to drive macrophage activation and enhance tumor growth. Cancer Res. 2020;80:57–68.
Zetser A, Bashenko Y, Edovitsky E, Levy-Adam F, Vlodavsky I, Ilan N. Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and Src ac tivation. Cancer Res. 2006;66:1455–63.
Koganti R, Suryawanshi R, Shukla D. Heparanase, cell signaling, and viral infections. Cell Mol Life Sci. 2020;77:5059–77.
He YQ, Sutcliffe EL, Bunting KL, Li J, Goodall KJ, Poon IK, et al. The endoglycosidase heparanase enters the nucleus of T lymphocytes and modulates H3 methylation at actively transcribed genes via the interplay with key chromatin modifying enzymes. Transcription. 2012;3:130–45.
Nadir Y, Brenner B, Zetser A, Ilan N, Shafat I, Zcharia E, et al. Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J Thromb Haemost. 2006;4:2443–51.
Gingis-Velitski S, Zetser A, Flugelman MY, Vlodavsky I, Ilan N. Heparanase induces endothelial cell migration via protein kinase B/Akt activation. J Biol Chem. 2004;279:23536–41.
Riaz A, Ilan N, Vlodavsky I, Li JP, Johansson S. Characterization of heparanase-induced phosphatidylinositol 3-kinase-AKT activation and its integrin dependence. J Biol Chem. 2013;288:12366–75.
Cohen-Kaplan V, Doweck I, Naroditsky I, Vlodavsky I, Ilan N. Heparanase augments epidermal growth factor receptor phosphorylation: correlation with head and neck tumor progression. Cancer Res. 2008;68:10077–85.
Cohen-Kaplan V, Jrbashyan J, Yanir Y, Naroditsky I, Ben-Izhak O, Ilan N, et al. Heparanase induces signal transducer and activator of transcription (STAT) protein phosphorylation: preclinical and clinical significance in head and neck cancer. J Biol Chem. 2021;287:6668–78.
Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42.
Tian X, Teng J, Chen J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy. 2021;17:2680–8.
**a Y, Liu N, **e X, Bi G, Ba H, Li L, et al. The macrophage-specific V-ATPase subunit ATP6V0D2 restricts inflammasome activation and bacterial infection by facilitating autophagosome-lysosome fusion. Autophagy. 2019;15:960–75.
Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25:1037–43.
Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42.
White E, Mehnert JM, Chan CS. Autophagy, metabolism, and cancer. Clin Cancer Res. 2015;21:5037–46.
Kumar S, Jain A, Choi SW, da Silva GPD, Allers L, Mudd MH, et al. Mammalian Atg8 proteins and the autophagy factor IRGM control mTOR and TFEB at a regulatory node critical for responses to pathogens. Nat Cell Biol. 2020;22:973–85.
Guo H, Pu M, Tai Y, Chen Y, Lu H, Qiao J, et al. Nuclear miR-30b-5p suppresses TFEB-mediated lysosomal biogenesis and autophagy. Cell Death Differ. 2021;28:320–36.
Li Y, Hodge J, Liu Q, Wang J, Wang Y, Evans TD, et al. TFEB is a master regulator of tumor-associated macrophages in breast cancer. J Immunother Cancer. 2020;8:e000543.
**ang H, Zhang J, Lin C, Zhang L, Liu B, Ouyang L. Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm Sin B. 2020;10:569–81.
He R, Wang M, Zhao C, Shen M, Yu Y, He L, et al. TFEB-driven autophagy potentiates TGF-β induced migration in pancreatic cancer cells. J Exp Clin Cancer Res. 2019;38:340.
Shteingauz A, Boyango I, Naroditsky I, Hammond E, Gruber M, Doweck I, et al. Heparanase enhances tumor growth and chemoresistance by promoting autophagy. Cancer Res. 2015;75:3946–57.
Dredge K, Hammond E, Handley P, Gonda TJ, Smith MT, Vincent C, et al. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. Br J Cancer. 2011;104:635–42.
Yang M, Pi H, Li M, Xu S, Zhang L, **e J, et al. From the cover: autophagy induction contributes to cadmium toxicity in mesenchymal stem cells via AMPK/FOXO3a/BECN1 signaling. Toxicol Sci. 2016;154:101–14.
Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–37.
Barash U, Cohen-Kaplan V, Arvatz G, Gingis-Velitski S, Levy-Adam F, Nativ O, et al. A novel human heparanase splice variant, T5, endowed with protumorigenic characteristics. FASEB J. 2010;24:1239–48.
Zetser A, Bashenko Y, Miao HQ, Vlodavsky I, Ilan N. Heparanase affects adhesive and tumorigenic potential of human glioma cells. Cancer Res. 2003;63:7733–41.
Poillet-Perez L, White E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 2019;33:610–9.
Schaaf MB, Keulers TG, Vooijs MA, Rouschop KM. LC3/GABARAP family proteins: autophagy-(un)related functions. FASEB J. 2016;30:3961–78.
Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy. 2014;10:2208–22.
Kim KH, Lee MS. Autophagy-a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10:322–37.
Ceccariglia S, Cargnoni A, Silini AR, Parolini O. Autophagy: a potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy. 2020;16:28–37.
Pi H, Li M, Zou L, Yang M, Deng P, Fan T, et al. AKT inhibition-mediated dephosphorylation of TFE3 promotes overactive autophagy independent of MTORC1 in cadmium-exposed bone mesenchymal stem cells. Autophagy. 2019;15:565–82.
Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–66.
Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–42.
Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–60.
Wang H, Wang N, Xu D, Ma Q, Chen Y, Xu S, et al. Oxidation of multiple MiT/TFE transcription factors links oxidative stress to transcriptional control of autophagy and lysosome biogenesis. Autophagy. 2020;16:1683–96.
Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524:361–5.
Purushothaman A, Hurst DR, Pisano C, Mizumoto S, Sugahara K, Sanderson RD. Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT) activity to promote expression of genes that drive an aggressive tumor phenotype. J Biol Chem. 2011;286:30377–83.
Wang F, Wang Y, Zhang D, Puthanveetil P, Johnson JD, Rodrigues B. Fatty acid-induced nuclear translocation of heparanase uncouples glucose metabolism in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:406–14.
Raben N, Puertollano R. TFEB and TFE3: linking lysosomes to cellular adaptation to stress. Annu Rev Cell Dev Biol. 2016;32:255–78.
Puertollano R, Ferguson SM, Brugarolas J, Ballabio A. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J. 2018;37:e98804.
Saftig P, Puertollano R. How lysosomes sense, integrate, and cope with stress. Trends Biochem Sci. 2021;46:97–112.
Di Malta C, Siciliano D, Calcagni A, Monfregola J, Punzi S, Pastore N, et al. Transcriptional activation of RagD GTPase controls mTORC1 and promotes cancer growth. Science. 2017;356:1188–92.
Jiao W, Chen Y, Song H, Li D, Mei H, Yang F, et al. HPSE enhancer RNA promotes cancer progression through driving chromatin loo** and regulating hnRNPU/p300/EGR1/HPSE axis. Oncogene. 2018;37:2728–45.
Boyango I, Barash U, Naroditsky I, Li JP, Hammond E, Ilan N, et al. Heparanase cooperates with Ras to drive breast and skin tumorigenesis. Cancer Res. 2014;74:4504–14.
Wei RR, Sun DN, Yang H, Yan J, Zhang X, Zheng XL, et al. CTC clusters induced by heparanase enhance breast cancer metastasis. Acta Pharm Sin. 2018;39:1326–37.
Levy-Adam F, Feld S, Suss-Toby E, Vlodavsky I, Ilan N. Heparanase facilitates cell adhesion and spreading by clustering of cell surface heparan sulfate proteoglycans. PLoS ONE. 2008;3:e2319.
Hu X, Zhang L, ** J, Zhu W, Xu Y, Wu Y, et al. Heparanase released from mesenchymal stem cells activates integrin beta1/HIF-2alpha/Flk-1 signaling and promotes endothelial cell migration and angiogenesis. Stem Cells. 2015;33:1850–62.
Nadir Y, Brenner B. Heparanase procoagulant activity in cancer progression. Thromb Res. 2016;140:S44–48.
Crispel Y, Ghanem S, Attias J, Kogan I, Brenner B, Nadir Y. Involvement of the heparanase procoagulant domain in bleeding and wound healing. J Thromb Haemost. 2017;15:1463–72.
Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383:1564–76.
Galluzzi L, Green DR. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177:1682–99.
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41.
Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76.
Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511.
Kraya AA, Piao S, Xu X, Zhang G, Herlyn M, Gimotty P, et al. Identification of secreted proteins that reflect autophagy dynamics within tumor cells. Autophagy. 2015;11:60–74.
Tsuboyama K, Koyama-Honda I, Sakamaki Y, Koike M, Morishita H, Mizushima N. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science. 2016;354:1036–41.
Wang Z, Miao G, Xue X, Guo X, Yuan C, Wang Z, et al. The Vici syndrome protein EPG5 is a Rab7 effector that determines the fusion specificity of autophagosomes with late endosomes/lysosomes. Mol Cell. 2016;63:781–95.
Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800.
Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300.
Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4:905–13.
Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–7.
Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–33.
Yang C, Zhu Z, Tong BC, Iyaswamy A, **e WJ, Zhu Y, et al. A stress response p38 MAP kinase inhibitor SB202190 promoted TFEB/TFE3-dependent autophagy and lysosomal biogenesis independent of p38. Redox Biol. 2020;32:101445.
Nnah IC, Wang B, Saqcena C, Weber GF, Bonder EM, Bagley D, et al. TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy. Autophagy. 2019;15:151–64.
Cinque L, De Leonibus C, Iavazzo M, Krahmer N, Intartaglia D, Salierno FG, et al. MiT/TFE factors control ER-phagy via transcriptional regulation of FAM134B. EMBO J. 2020;39:e105696.
López-Hernández T, Puchkov D, Krause E, Maritzen T, Haucke V. Endocytic regulation of cellular ion homeostasis controls lysosome biogenesis. Nat Cell Biol. 2020;22:815–27.
Peña-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TA, Zou L, et al. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 2011;30:3242–58.
Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17:288–99.
Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2021;31:1095–108.
Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–14.
Vega-Rubin-de-Celis S, Peña-Llopis S, Konda M, Brugarolas J. Multistep regulation of TFEB by MTORC1. Autophagy. 2017;13:464–72.
Martina JA, Diab HI, Lishu L, Jeong AL, Patange S, Raben N, et al. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal. 2014;7:ra9.
Haq R, Fisher DE. Biology and clinical relevance of the micropthalmia family of transcription factors in human cancer. J Clin Oncol. 2011;29:3474–82.
Kauffman EC, Ricketts CJ, Rais-Bahrami S, Yang Y, Merino MJ, Bottaro DP, et al. Molecular genetics and cellular features of TFE3 and TFEB fusion kidney cancers. Nat Rev Urol. 2014;11:465–75.
Alderton GK. Autophagy: Surviving stress in pancreatic cancer. Nat Rev Cancer. 2015;15:513.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81802477). The study was also generously supported by a research grant awarded to SY and IV by the ISF-NSFC joint research program [grant No. 2572/16 (Israel side) and No. 81661148050 (China side)].
Author information
Authors and Affiliations
Contributions
MY and BT were responsible for conducting the search, writing the manuscript, and designing the search protocol. SW and LT were responsible for completing experiment and analyzing data. DW contributed to assisting complete the HTRF assay and analyzing the data. IV was responsible for language polish and providing feedback on the manuscript. SY contributed to arbitrating potentially eligible studies and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, M., Tang, B., Wang, S. et al. Non-enzymatic heparanase enhances gastric tumor proliferation via TFEB-dependent autophagy. Oncogenesis 11, 49 (2022). https://doi.org/10.1038/s41389-022-00424-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41389-022-00424-4
- Springer Nature Limited