Abstract
Lymphatic metastasis is recognized as the leading manner of metastasis in bladder cancer (BLCa), but hematogenous metastasis accounts for a majority of cancer-associated deaths. The past two decades have witnessed tremendous attention in long non-coding RNAs (lncRNAs), which are a new hope for the development of targeted drug therapy for metastatic cancers; however, the underlying mechanism of lncRNAs involved in BLCa hematogenous metastasis remains to be elucidated. Here, we identified BLCa-associated transcript 3 (BLACAT3), a lncRNA, which was aberrantly upregulated in BLCa and corelated with poor prognosis of patients with muscle-invasive bladder cancer. Methodologically, m6A epitranscriptomic microarray, RNA sequencing and mass spectrometry (MS) were used to screen the key molecules of the regulatory axis. Functional assays, animal models and clinical samples were used to explore the roles of BLACAT3 in BLCa in vitro and in vivo. Mechanistically, m6A modification contributes to BLACAT3 upregulation by stabilizing RNA structure. BLACAT3 recruits YBX3 to shuttle into the nucleus, synergistically enhances NCF2 transcription, and promotes BLCa angiogenesis and hematogenous metastasis by activating downstream NF-κB signaling. Our findings will develop prognosis prediction tools for BLCa patients and discover novel therapeutic biological targets for metastatic BLCa.
Similar content being viewed by others
Introduction
Bladder cancer (BLCa) is the second most common genitourinary malignancy with approximately 549,000 new confirmed cases and 200,000 deaths worldwide each year [1]. Patients with non-muscle-invasive BLCa (NMIBC) undergo bladder-sparing surgeries and standardized intravesical therapy, yet there is a 30–45% chance of converting to muscle-invasive BLCa (MIBC) within 5 years, some even directly progressing to metastatic BLCa [2]. About 50% of MIBC patients may suffer local or distant recurrence after radical cystectomy (RC) [3]. As for metastatic BLCa, platinum-based chemotherapy is currently the first-line therapeutic, but the overall response rate is unfortunately less than 50% [4]. While alternative options such as radiotherapy and checkpoint inhibitors are available, both carry their own major drawbacks and new target drugs are still in clinical trial development [5,6,7,8,9]. Therefore, understanding the mechanism of BLCa metastasis is essential so that novel therapeutic targets can be developed to improve patient outcomes.
Previous studies have primarily focused on the mechanism of lymphangiogenesis and lymphatic metastasis in BLCa, because lymphatic metastasis is considered the leading metastatic manner in BLCa [10,11,12,13]. In more severe cases, BLCa cells metastasize to distant organs such as liver, lung, and bone through the hematogenous approach which is more associated with cancer-associated mortality [14,15,16,17] but remains poorly studied. Angiogenesis refers to the forming of new blood vessels, which can provide the necessary blood supply for invasive tumor growth and metastasis [18]. Accumulating evidence show reactive oxygen species (ROS) catalyzed by intracellular NADPH oxidase participate in angiogenesis, invasion and metastasis of BLCa by activating downstream signaling [19, 20]. NCF2/p67phox is one of the two important activating subunits of NADPH oxidase [21, 22], and plays an irreplaceable role in the biological function of NADPH oxidase. NCF2/p67phox expression has been reported to be upregulated in clear cell renal cell carcinoma (ccRCC) [23], urothelial carcinoma [20], malignant glioma [24, 25], hepatocellular carcinoma [26, 27] and colorectal carcinoma [28], and associated with poor prognosis. However, the regulatory mechanism of NCF2/p67phox in BLCa angiogenesis and metastasis remains unknown.
LncRNAs are a class of transcripts that contain 200–100,000 nucleotides and cannot be translated into peptides [29, 30]. LncRNAs have garnered significant attention over the past two decades as potential targets for metastatic BLCa drug therapy; however, the mechanism by which lncRNAs are involved in BLCa hematogenous metastasis remains to be elucidated. Using BLCa specimens and patient data, and with the help of m6A epitranscriptomic microarray technology and bioinformatics analysis based on public high-throughput data, we identified a potential target lncRNA, LINC01535–204, which we named BLCa-associated transcript 3 (BLACAT3). Our data showed BLACAT3 was highly expressed in BLCa tissues and positively correlated with poor prognosis in MIBC patients. Furthermore, we analyzed the regulatory mechanism of BLACAT3 on the malignant behavior of BLCa cells and the prognosis of the disease from the molecular, cellular, mammalian, and clinical levels. Using several m6A-associated assays, we demonstrated that m6A modification contributes to BLACAT3 upregulation by stabilizing RNA structure. Our data confirmed BLACAT3 regulates the expression of NCF2/p67phox by interacting with RNA binding protein (RBP) YBX3 to mediate BLCa angiogenesis and hematogenous metastasis. Overall, this study has provided strong insights and an experimental basis for the identification of novel therapeutic targets for metastatic BLCa.
Results
BLACAT3 exhibits high-abundance m6A modification and elevated expression in MIBC tissues and is a potential prognostic indicator
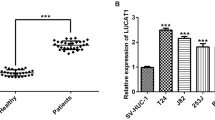
To identify novel potential vulnerabilities associated with BLCa progression, we screened the differential m6A modification and expression level profile of lncRNAs between three pairs of fresh tumor tissues and adjacent normal tissues by human m6A epitranscriptomic microarray (Fig. 1A, sFig. 1A, B, Supplementary Tables 7, 8). We then focused on 84 lncRNAs with co-upregulation of m6A modification and expression level (Fig. 1B). We further extracted the BLCa sequencing dataset from The Cancer Genome Atlas (TCGA) (Fig. 1C). Two lncRNA candidates, LINC01535-204 and ENST00000439898.1 (Fig. 1D, Supplementary Table 9), were identified from the intersection of the three datasets. M6A RNA immunoprecipitation (MeRIP) and quantitative real-time PCR (qRT-PCR) analyses verified that, the relative m6A modification of BLACAT3 in BLCa, compared with paired normal tissues, was significantly higher than that of ENST00000439898.1 (Fig. 1E). BLACAT3 is 815nt long and is located in Chromosome 19: 37,251,922-37,265,473 (sFig. 1C). GSEA based on microarray data suggested that BLACAT3 is closely involved in biological functions including cell division, protein binding, HIF-1 signaling pathway, and tumor metabolism (sFig. 1D-G). We also confirmed that BLACAT3 does not have the ability to encode a protein by Coding Potential Calculator 2 [31] (sFig. 1H, I).
A Flowchart of m6A epitranscriptomic microarray. B Differentially m6A modified lncRNAs (abs(log2FC) > 0.585, P < 0.05) and differentially expressed lncRNAs ((abs(log2FC) > 0.585) between paired BLCa and adjacent normal tissues (n = 3). C Volcano plot of differential lncRNA expression profile between 411 BLCa tissues and 19 normal bladder tissues based on TCGA data (abs(log2FC) > 0.585, P < 0.05). D Venn diagrams of microarray-derived differential lncRNA m6A modification and expression profiles intersected with lncRNA expression profiles from the TCGA dataset. E MeRIP and qRT-PCR were conducted to detect m6A modification levels of BLACAT3 and ENST00000439898.1 (n = 3). F qRT-PCR was used to detect BLACAT3 expression between paired BLCa and adjacent normal tissues (n = 104). G Comparison of BLACAT3 expression between earlier stage (n = 86) and advanced stage (n = 18). H Kaplan-Meier analysis of the relationship between BLACAT3 expression and OS of BLCa patients (Log-rank (Mantel-Cox) test, P < 0.001). 104 BLCa patients were divided into high and low expression groups based on the median value of relative BLACAT3 expression. I Univariate and multivariate regression analyses were conducted to screen the independent predictors associated with OS in BLCa patients. Hazard ratio (HR) and corresponding 95% confidence intervals (CI) are shown. J Nomogram was constructed to predict the prognosis of BLCa patients undergoing RC. Statistical significance was assessed with a two-tailed Student’s t test between two groups, *P < 0.05, **** P < 0.0001.
We detected the BLACAT3 expression in 104 pairs of BLCa and adjacent normal tissues using qRT-PCR. It was found that BLACAT3 expression in BLCa tissue was significantly higher than in normal tissues (Fig. 1F; sFig. 1J). Moreover, BLACAT3 expression was significantly higher in TNM stage III/IV BLCa compared to 0is/I/II BLCa (Fig. 1G). Additionally, MIBC patients with higher BLACAT3 expression had worse overall survival (OS) and disease-specific survival (DSS) (Fig. 1H; sFig. 1K). We used univariate and multivariate regression analyses to screen out three key variables including BLACAT3 expression, marital status, and gender (Fig. 1I) and established a prognostic risk prediction model for MIBC patients who underwent RC (Fig. 1J). Receiver operating characteristic curves and decision curve analysis demonstrated the high prediction accuracy and fitting degree of this model (sFig. 1L, M).
M6A modification mediates BLACAT3 upregulation by enhancing RNA stability, and promotes malignant behaviors of BLCa cells
MeRIP and qRT-PCR analyses verified BLACAT3 m6A modification and expression level were consistently higher than those of SV-HUC-1 in most BLCa cell lines, with the most significant differences in T24, 5637, and UM-UC-3 (Fig. 2A, sFig. 2A). Dot blot assay showed that total m6A modification levels of BLACAT3 in the three cell lines were higher than in SV-HUC-1 (sFig. 2B). SRAMP prediction server [32] identified 6 potential m6A modification motifs in the BLACAT3 sequence (Fig. 2B). We then focused on the GGACU motif, which exhibits the highest prediction confidence. Single-base map** of m6A [33] showed that the m6A modification ratios of the GGACU motif in UM-UC-3, 5637 and T24 cell lines were 10.63%, 30.41% and 56.63%, respectively (Fig. 2C). Therefore, we verified the high m6A modification ratio of the BLACAT3 sequence in BLCa cells.
A MeRIP and qRT-PCR were used to detect the m6A modification levels of BLACAT3 in different BLCa cell lines (RT4, UM-UC-3, 5637, T24, J82 and SW780) and the normal urothelial cell line (SV-HUC-1). B The SRAMP prediction server (https://www.cuilab.cn/sramp) was used to predict potential m6A modification motifs in the BLACAT3 sequence. C Single-base map** of m6A against adenine in the “GGACU” motif was performed to detect the m6A abundance of BLACAT3 in several BLCa cell lines (UM-UC-3, 5637 and T24). D, E WB were performed to detect the protein level of ALKBH5 in paired MIBC and adjacent normal tissues (n = 12). F, G WB were performed to verify the knockdown efficiency of ALKBH5 in 5637 cells and the overexpression efficiency of ALKBH5 in T24 cells. H Anti-m6A dot blot assay detected the effect of ALKBH5 knockdown or overexpression on the overall m6A modification level of 5637 or T24 cells. I MeRIP and qRT-PCR detected the effect of ALKBH5 knockdown on BLACAT3 m6A enrichment in 5637 cells. J QRT-PCR quantified ALKBH5 knockdown efficiency and BLACAT3 expression in 5637 cells. K QRT-PCR examined the effect of ALKBH5 knockdown on the half-life of BLACAT3 in 5637 cells pretreated with actinomycin-D (5 μg/mL). L MeRIP and qRT-PCR assays were conducted to detect the effect of ALKBH5 overexpression on BLACAT3 m6A enrichment in T24 cells. M QRT-PCR assay was performed to detect the overexpression efficiency of ALKBH5 and BLACAT3 expression in T24 cells. N QRT-PCR assay was performed to examine the effect of ALKBH5 overexpression on the half-life of BLACAT3 in T24 cells pretreated with actinomycin-D (5 μg/mL). ns no significance. Statistical significance was assessed using two-tailed Student’s t test between two groups, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
METTL3, METTL14, WTAP, FTO and ALKBH5 are five well-known m6A modification related enzymes, of which the first three are methyltransferases and the last two are demethylases [34]. Using small interference RNAs (siRNAs) (sFig. 2C), we found that silencing ALKBH5 most significantly upregulated the m6A modification level of BLACAT3 (sFig. 2D). Western blots (WB) demonstrated that ALKBH5 protein level in BLCa tissue was significantly lower than that of matched normal tissue (Fig. 2D, E), and lower in BLCa cell lines compared to normal urothelial cell line (sFig. 2E).
To investigate the effect of m6A modification on BLACAT3 expression, we overexpressed or silenced ALKBH5 in 5637 and T24 cells respectively (Fig. 2F, G). Dot blot assay showed that the overall m6A abundance of BLCa cells was significantly up- or down-regulated after ALKBH5 knockdown or overexpression (Fig. 2H), respectively. MeRIP and qRT-PCR analyses demonstrated that ALKBH5 knockdown significantly upregulated both m6A modification and expression of BLACAT3 in 5637 cells (Fig. 2I, J). Actinomycin D is an inhibitor of RNA transcription and is commonly used to explore RNA half-life [35, 36]. We used qRT-PCR to quantify residual BLACAT3 at different time points and found that silencing ALKBH5 could significantly reduce the degradation rate of BLACAT3 at 3 and 6 h after the addition of actinomycin D (Fig. 2K). ALKBH5 overexpression in T24 cells could significantly downregulate m6A modification levels and BLACAT3 expression (Fig. 2L, M), and significantly shorten the RNA half-life of BLACAT3 (Fig. 2N).
We also explored the effect of m6A modification on the malignant behaviors of BLCa cells. CCK-8 assay showed that up- or down-regulation of m6A modification could promote or inhibit the BLCa cell proliferation (sFig. 3A, B), respectively. Human umbilical vein endothelial cell (HUVEC) tube formation and transwell migration assays demonstrated that up- or down-regulation of m6A modification could respectively enhance or inhibit the angiogenesis and migration of BLCa cells (sFig. 3C–H).
BLACAT3 Upregulation promotes proliferation of BLCa cell both in vitro and in vivo
To explore the effect of BLACAT3 on the malignant behaviors of BLCa cells, we constructed the stable T24 strain with BLACAT3 knockdown and 5637 strain with BLACAT3 overexpression for establishing the subcutaneous xenograft model (sFig. 4A, B, sFig. 5A, B). Both cell stains also stably expressed the luciferase gene. On the 28th day after subcutaneous injection of BLCa cells, in vivo imaging showed that BLACAT3 knockdown and overexpression respectively inhibited and promoted tumor growth (Fig. 3A, B, F, G). We plotted tumor growth curves of subcutaneous tumors (Fig. 3C, H). At the endpoint, gross samples of the subcutaneous tumors were isolated and photographed (sFigs. 4Cs, 5C). Tumor weights were recorded (sFigs. 4D, 5D), and the tumor pathology was confirmed by haematoxylin and eosin (H&E) staining (sFigs. 4E, 5E). Ki67 and CD31 are commonly used to evaluate proliferation and tumor angiogenesis [60]. Increasing evidences have shown that YBX3 is aberrantly upregulated in hepatocellular carcinoma [40], pancreatic cancer [61], colon cancer [62] and lung cancer [63]. Using TCGA data analysis we demonstrated no significant difference in the mRNA level of YBX3 between BLCa and normal bladder tissues, and there was no correlation with OS of BLCa patients with YBX3 mRNA expression. However, several studies have confirmed that YBX3 is highly expressed at the protein level and promotes BLCa cell invasion [64, 65]. Unexpectedly, we found that silencing or overexpression of BLACAT3 in BLCa cells had no effect on the protein level of YBX3. Regulation of transcription initiation is performed specifically in the nucleus and is the most direct mode of regulation of gene expression [66]. It is reported that the aberrant nuclear retention of YBX3 facilitates the proliferation of cancer cells [63]. Here, although BLACAT3 has no effect on the overall expression level of YBX3, whether it affects its spatial distribution needs further exploration. Saying this, our WB assay based on nucleocytoplasmic separation and IF staining based on the subcutaneous tumor tissue confirmed that the upregulated BLACAT3 can promote the distribution of YBX3 into the nucleus.
Dual luciferase reporter gene and ChIP assays displayed that YBX3 can bind directly to NCF2 promoter. In addition, BLACAT3 knockdown can decrease the binding of YBX3 to NCF2 promoter. WB analysis further confirmed the cooperative regulation of BLACAT3/YBX3 on NCF2. It has been reported that lncRNA and TF act together on the regulation of target gene promoters [67, 68]. We have shown that BLACAT3 induces YBX3 entry into the nucleus. We speculate that BLACAT3 and YBX3 may combine with the NCF2 promoter in the form of a complex to promote transcription and ultimately upregulate NCF2/p67phox expression. EMT (Epithelial-mesenchymal transition) and NF-κB signaling pathways attracted our attention through GSEA analysis of transcriptomic sequencing data. The role of EMT in various malignant behaviors such as tumorigenesis, metastasis and drug resistance has been widely studied, so we detected the most well-known epithelial marker E-Cadherin and mesenchymal markers N-Cadherin and Vimentin [69]. Our data showed that BLACAT3 can positively promote EMT progression, and that BLACAT3 and YBX3 have a synergistic regulatory effect on EMT, which is consistent with the trend of RNA-seq-based GSEA analysis results, and further verifies the role of BLACAT3 on BLCa metastasis. The role of NF-κB/VEGF signaling pathway in tumor angiogenesis and metastasis has been reported in colon cancer [70], gastric cancer [71], hepatocellular carcinoma [46]. In addition, the development of new therapeutics targeting RNA m6A modification including enzyme inhibitors has also made progress [76], but there is still a long way to go before clinical application.
Taken together, our data showed that m6A modification contributes to BLACAT3 upregulation by stabilizing RNA structure. We have found that BLACAT3 recruits YBX3 to shuttle into the nucleus, which synergistically enhances NCF2 transcription, and promotes BLCa angiogenesis and hematogenous metastasis by activating downstream NF-κB signaling. Our novel findings will help to develop prognostic prediction tools for BLCa patients and discover new therapeutic biological targets for metastatic BLCa.
Materials and methods
Clinical specimens and patient information
Clinical samples and patient information were obtained from two sources for this study. First, publicly available transcriptome sequencing data and corresponding patient clinical data from TCGA. Second, paired tumor and adjacent normal tissues from 107 patients with MIBC who underwent RC in Shanghai Tenth People’s Hospital. Among them, 3 pairs of tumor and adjacent normal tissues were collected from May to September 2020 (Supplementary Table 1). The other 104 pairs of tumor and adjacent normal tissues were collected from January 2012 to December 2017, and information on the demographic and pathological characteristics of patients and clinical samples is provided in Supplementary Table 2. All recruited patients had complete clinicopathological and follow-up data. The study was approved by the Ethics Committee of Shanghai Tenth People’s Hospital (Ethics approval number: SHSY-IEC-4.1/19-210/01). All enrolled patients had signed informed consent forms. All research activities comply with Declaration of Helsinki ethical guidelines.
Statistics
Under R version 4.0.2 environment, multiple regression analysis and nomogram construction were carried out by rms package, forest chart was drawn by forestplot package, and the ROC curve was produced by pROC package. The other statistical analyses were conducted by SPSS 24.0 and GraphPad Prism 8. The two-tailed Student t test was used to compare parameters between two groups of normally distributed data, and the analysis of variance was used to compare multiple groups. The Kaplan-Meier survival analysis method was used to calculate the OS and DSS, and the Log-rank (Mantel-Cox) test was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 were set as statistically significant, and P > 0.05 means no significance.
Data availability
The human m6A epitranscriptomic microarray and RNA sequencing data are available in Gene Expression Omnibus: GSE228952, GSE229172. The computer code used in this study is available from the authors if requested by the readers. More details on materials and methods are available in the supplementary information file.
References
Compérat E, Amin MB, Cathomas R, Choudhury A, De Santis M, Kamat A, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet (Lond, Engl). 2022;400:1712–21.
Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–53.
Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. 2021;79:82–104.
Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström PU, Choi W, et al. Bladder cancer. Lancet (Lond, Engl). 2016;388:2796–810.
Raby SEM, Hoskin P, Choudhury A. The role of palliative radiotherapy in bladder cancer: a narrative review. Ann Palliat Med. 2020;9:4294–9.
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl J Med. 2020;383:1218–30.
Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl J Med. 2019;381:338–48.
Tagawa ST, Balar AV, Petrylak DP, Kalebasty AR, Loriot Y, Fléchon A, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39:2474–85.
Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo H, et al. Open-label, multicenter, phase II study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin Cancer Res. 2021;27:43–51.
He W, Zhong G, Jiang N, Wang B, Fan X, Chen C, et al. Long noncoding RNA BLACAT2 promotes bladder cancer-associated lymphangiogenesis and lymphatic metastasis. J Clin Investig. 2018;128:861–75.
Chen C, He W, Huang J, Wang B, Li H, Cai Q, et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun. 2018;9:3826.
Chen C, Luo Y, He W, Zhao Y, Kong Y, Liu H, et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin Investig. 2020;130:404–21.
Chen C, Zheng H, Luo Y, Kong Y, An M, Li Y, et al. SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J Clin Investig. 2021;131:e146431.
Wallmeroth A, Wagner U, Moch H, Gasser TC, Sauter G, Mihatsch MJ. Patterns of metastasis in muscle-invasive bladder cancer (pT2-4): An autopsy study on 367 patients. Urologia Internationalis. 1999;62:69–75.
Clatterbuck RE, Sampath P, Olivi A. Transitional cell carcinoma presenting as a solitary brain lesion: a case report and review of the world literature. J Neuro-Oncol. 1998;39:91–4.
Zhu LK, Li ZJ, Wang ZB, Chen JT, Zhang HJ, Zhao XW, et al. A rare case of bladder cancer that metastasized to brain, heart, and lung lymph nodes benefited from immunotherapy. World J Surg Oncol. 2022;20:402.
Zhong J, Chen Y, Wang LJ. Emerging molecular basis of hematogenous metastasis in gastric cancer. World J Gastroenterol. 2016;22:2434–40.
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8.
Shimada K, Fujii T, Tsujikawa K, Anai S, Fujimoto K, Konishi N. ALKBH3 contributes to survival and angiogenesis of human urothelial carcinoma cells through NADPH oxidase and tweak/Fn14/VEGF signals. Clin Cancer Res. 2012;18:5247–55.
Huang HS, Liu ZM, Chen PC, Tseng HY, Yeh BW. TG-interacting factor-induced superoxide production from NADPH oxidase contributes to the migration/invasion of urothelial carcinoma. Free Radic Biol Med. 2012;53:769–78.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Ogboo BC, Grabovyy UV, Maini A, Scouten S, van der Vliet A, Mattevi A, et al. Architecture of the NADPH oxidase family of enzymes. Redox Biol. 2022;52:102298.
Tan W, Hildebrandt MA, Pu X, Huang M, Lin J, Matin SF, et al. Role of inflammatory related gene expression in clear cell renal cell carcinoma development and clinical outcomes. J Urol. 2011;186:2071–7.
Ji H, Liu Z, Wang F, Sun H, Wang N, Liu Y, et al. Novel macrophage-related gene prognostic index for glioblastoma associated with M2 macrophages and T cell dysfunction. Front Immunol. 2022;13:941556.
Ji H, Zhao H, ** J, Liu Z, Gao X, Wang F, et al. Novel immune-related gene-based signature characterizing an inflamed microenvironment predicts prognosis and radiotherapy efficacy in glioblastoma. Front Genet. 2021;12:736187.
Huang N, Zhang J, Kuang S, Li Z, Zhao H, Wu J, et al. Role of NCF2 as a potential prognostic factor and immune infiltration indicator in hepatocellular carcinoma. Cancer Med. 2023;12:8991–9004.
Huang C, Jiang X, Huang Y, Zhao L, Li P, Liu F. Identifying dendritic cell-related genes through a co-expression network to construct a 12-gene risk-scoring model for predicting hepatocellular carcinoma prognosis. Front Mol Biosci. 2021;8:636991.
Osama A, Sabry D, Hassany SM, Abdelmoneim SS, Sabry A. SIRT-1expression is associated with expression of NANOG in patients with colorectal adenocarcinoma. Cancer Biomark. 2016;17:155–63.
Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018;172:393–407.
Furuno M, Pang KC, Ninomiya N, Fukuda S, Frith MC, Bult C, et al. Clusters of internally primed transcripts reveal novel long noncoding RNAs. PLoS Genet. 2006;2:e37.
Kang YJ, Yang DC, Kong L, Hou M, Meng YQ, Wei L, et al. CPC2: a fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017;45:W12–w6.
Zhou Y, Zeng P, Li YH, Zhang Z, Cui Q. SRAMP: prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016;44:e91.
Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution map** of m6A and m6Am throughout the transcriptome. Nat methods. 2015;12:767–72.
Meyer KD, Jaffrey SR. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol. 2014;15:313–26.
Zuo X, Chen Z, Gao W, Zhang Y, Wang J, Wang J, et al. M6A-mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J Hematol Oncol. 2020;13:5.
Qian X, Yang J, Qiu Q, Li X, Jiang C, Li J, et al. LCAT3, a novel m6A-regulated long non-coding RNA, plays an oncogenic role in lung cancer via binding with FUBP1 to activate c-MYC. J Hematol Oncol. 2021;14:112.
Seki T, Yang Y, Sun X, Lim S, **e S, Guo Z, et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature. 2022;608:421–8.
Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220:e202009045.
Nie M, Balda MS, Matter K. Stress- and Rho-activated ZO-1-associated nucleic acid binding protein binding to p21 mRNA mediates stabilization, translation, and cell survival. Proc Natl Acad Sci USA. 2012;109:10897–902.
Yasen M, Ka**o K, Kano S, Tobita H, Yamamoto J, Uchiumi T, et al. The up-regulation of Y-box binding proteins (DNA binding protein A and Y-box binding protein-1) as prognostic markers of hepatocellular carcinoma. Clin Cancer Res. 2005;11:7354–61.
Wang GR, Zheng Y, Che XM, Wang XY, Zhao JH, Wu KJ, et al. Upregulation of human DNA binding protein A (dbpA) in gastric cancer cells. Acta Pharmacol Sin. 2009;30:1436–42.
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74.
Huang H, Weng H, Chen J. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37:270–88.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6.
Li ZX, Zheng ZQ, Yang PY, Lin L, Zhou GQ, Lv JW, et al. WTAP-mediated m(6)A modification of lncRNA DIAPH1-AS1 enhances its stability to facilitate nasopharyngeal carcinoma growth and metastasis. Cell Death Differ. 2022;29:1137–51.
Mao W, Wang K, Zhang W, Chen S, **e J, Zheng Z, et al. Transfection with Plasmid-Encoding lncRNA-SLERCC nanoparticle-mediated delivery suppressed tumor progression in renal cell carcinoma. J Exp Clin Cancer Res. 2022;41:252.
Shi H, **e J, Wang K, Li W, Yin L, Wang G, et al. LINC01451 drives epithelial-mesenchymal transition and progression in bladder cancer cells via LIN28/TGF-β/Smad pathway. Cell Signal. 2021;81:109932.
Mehus AA, Bergum N, Knutson P, Shrestha S, Kalonick M, Zhou X, et al. Chronic arsenic exposure upregulates the expression of basal transcriptional factors and increases invasiveness of the non-muscle invasive papillary bladder cancer Line RT4. Int J Mol Sci. 2022;23:12313.
** H, Ying X, Que B, Wang X, Chao Y, Zhang H, et al. N(6)-methyladenosine modification of ITGA6 mRNA promotes the development and progression of bladder cancer. EBioMedicine. 2019;47:195–207.
Yang F, ** H, Que B, Chao Y, Zhang H, Ying X, et al. Dynamic m(6)A mRNA methylation reveals the role of METTL3-m(6)A-CDCP1 signaling axis in chemical carcinogenesis. Oncogene. 2019;38:4755–72.
Yu H, Yang X, Tang J, Si S, Zhou Z, Lu J, et al. ALKBH5 inhibited cell proliferation and sensitized bladder cancer cells to cisplatin by m6A-CK2α-mediated glycolysis. Mol Ther Nucleic Acids. 2021;23:27–41.
Li F, Zhang H, Wang W, Yang P, Huang Y, Zhang J, et al. T cell receptor β-chain-targeting chimeric antigen receptor T cells against T cell malignancies. Nat Commun. 2022;13:4334.
Chalbatani GM, Momeni SA, Mohammadi Hadloo MH, Karimi Z, Hadizadeh M, Jalali SA, et al. Comprehensive analysis of ceRNA networks to determine genes related to prognosis, overall survival, and immune infiltration in clear cell renal carcinoma. Comput Biol Med. 2022;141:105043.
Chen Y, He F, Wang R, Yao M, Li Y, Guo D, et al. NCF1/2/4 are prognostic biomarkers related to the immune infiltration of kidney renal clear cell carcinoma. BioMed Res Int. 2021;2021:5954036.
Xu L, Lu Z, Yu S, Li G, Chen Y. Quantitative global proteome and phosphorylome analyses reveal potential biomarkers in kidney cancer. Oncol Rep. 2021;46:237.
Muthuswamy R, Corman JM, Dahl K, Chatta GS, Kalinski P. Functional reprogramming of human prostate cancer to promote local attraction of effector CD8(+) T cells. Prostate. 2016;76:1095–105.
Bucher-Johannessen C, Page CM, Haugen TB, Wojewodzic MW, Fosså SD, Grotmol T, et al. Cisplatin treatment of testicular cancer patients introduces long-term changes in the epigenome. Clin Epigenetics. 2019;11:179.
**e J, Ni J, Shi H, Wang K, Ma X, Li W, et al. LncRNA SNHG3 enhances BMI1 mRNA stability by binding and regulating c-MYC: Implications for the carcinogenic role of SNHG3 in bladder cancer. Cancer Med. 2023;12:5718–35.
Cabili MN, Dunagin MC, McClanahan PD, Biaesch A, Padovan-Merhar O, Regev A, et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16:20.
Dupasquier S, Delmarcelle AS, Marbaix E, Cosyns JP, Courtoy PJ, Pierreux CE. Validation of housekee** gene and impact on normalized gene expression in clear cell renal cell carcinoma: critical reassessment of YBX3/ZONAB/CSDA expression. BMC Mol Biol. 2014;15:9.
Nakatsura T, Senju S, Yamada K, Jotsuka T, Ogawa M, Nishimura Y. Gene cloning of immunogenic antigens overexpressed in pancreatic cancer. Biochem Biophys Res Commun. 2001;281:936–44.
Buchert M, Papin M, Bonnans C, Darido C, Raye WS, Garambois V, et al. Symplekin promotes tumorigenicity by up-regulating claudin-2 expression. Proc Natl Acad Sci USA. 2010;107:2628–33.
Ikari A, Watanabe R, Sato T, Taga S, Shimobaba S, Yamaguchi M, et al. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochimica et biophysica acta. 2014;1843:2079–88.
Chen B, Bu R, Xu X. Expression of tight junction proteins is altered in bladder cancer. Anal Cell Pathol (Amst). 2020;2020:6341256.
Xu X, You K, Wu B. Zonula occludens-1 associated nucleic acid binding protein plays an invasion-promoting role in bladder cancer. Neoplasma 2019;66:405–19.
Andersson R, Sandelin A. Determinants of enhancer and promoter activities of regulatory elements. Nat Rev Genet. 2020;21:71–87.
Wang H, Li J, Wang S, Lu X, Zhang G, Zhuang Y, et al. Contribution of structural accessibility to the cooperative relationship of TF-lncRNA in myopia. Brief Bioinforma. 2021;22:bbab082.
Liu B, Ye B, Yang L, Zhu X, Huang G, Zhu P, et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol. 2017;18:499–508.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84.
Bakshi HA, Quinn GA, Nasef MM, Mishra V, Aljabali AAA, El-Tanani M, et al. Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-kB/VEGF Pathways. Cells 2022;11:1502.
Sokolova O, Naumann M. NF-κB signaling in gastric cancer. Toxins 2017;9:119.
Zhu T, Peng X, Cheng Z, Gong X, **ng D, Cheng W, et al. COMMD3 expression affects angiogenesis through the HIF1α/VEGF/NF-κB signaling pathway in hepatocellular carcinoma in vitro and in vivo. Oxid Med Cell Longev. 2022;2022:1655502.
Karashima T, Sweeney P, Kamat A, Huang S, Kim SJ, Bar-Eli M, et al. Nuclear factor-kappaB mediates angiogenesis and metastasis of human bladder cancer through the regulation of interleukin-8. Clin Cancer Res. 2003;9:2786–97.
Singh AV, Franke AA, Blackburn GL, Zhou JR. Soy phytochemicals prevent orthotopic growth and metastasis of bladder cancer in mice by alterations of cancer cell proliferation and apoptosis and tumor angiogenesis. Cancer Res. 2006;66:1851–8.
Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The limitless future of RNA therapeutics. Front Bioeng Biotechnol. 2021;9:628137.
Garbo S, Zwergel C, Battistelli C. m6A RNA methylation and beyond—The epigenetic machinery and potential treatment options. Drug Discov Today. 2021;26:2559–74.
Acknowledgements
We wish to thank the following individuals for their assistance during the experiments conducting and manuscript writing: Dr. Wei Li, Mr. Liang Xu, Dr. Rongbin Zheng, Dr. Tao Xu, Dr. Qikai Sun, Dr. Yiao Tan and Mr. Shuhan Liu. We acknowledge CSC (China Scholarship Council) for funding **bo **e to study at University of Toronto and St. Michael’s Hospital for one year, during which the main body of the manuscript was completed. We also acknowledge Home for Researchers (https://www.home-for-researchers.com/) for assistance in drawing up the schematic figures.
Funding
This work was supported by grants from the Sailing Program of Shanghai Science and Technology Commission (22YF1425000); Rui** Hospital Youth Incubation Project (KY20211600); National Natural Science Foundation of China (82203698, 31670772 and 32070646); Natural Science Foundation of Jiangsu Province (BK20230360).
Author information
Authors and Affiliations
Contributions
JX, WM, and BP designed this study. JX, KW and JN executed the experiments. HZ and JX executed bioinformatics and statistics analyses. HZ and RW collected clinical samples and patient data. JX and XM wrote the original draft. CJK, VP, BH and EGC reviewed and edited the manuscript. BP, DZ and LY provided funding and resources for this study. BP supervised and is responsible for this project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The ethics review of biomedical research involving humans was approved by the Ethics Committee of Shanghai Tenth People’s Hospital (SHSY-IEC-4.1/19-210/01). All animal studies conducted in this project were reviewed and approved by Tongji University Laboratory Animal Welfare Ethics Review Working Group Committee (TJBA01920101).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
**e, J., Zhang, H., Wang, K. et al. M6A-mediated-upregulation of lncRNA BLACAT3 promotes bladder cancer angiogenesis and hematogenous metastasis through YBX3 nuclear shuttling and enhancing NCF2 transcription. Oncogene 42, 2956–2970 (2023). https://doi.org/10.1038/s41388-023-02814-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02814-3
- Springer Nature Limited
This article is cited by
-
The m6A modification mediated-lncRNA POU6F2-AS1 reprograms fatty acid metabolism and facilitates the growth of colorectal cancer via upregulation of FASN
Molecular Cancer (2024)
-
Exosome-derived lncRNA A1BG-AS1 attenuates the progression of prostate cancer depending on ZC3H13-mediated m6A modification
Cell Division (2024)
-
FTO-mediated LINC01134 stabilization to promote chemoresistance through miR-140-3p/WNT5A/WNT pathway in PDAC
Cell Death & Disease (2023)