Abstract
Imbalance in prefrontal cortical (PFC) pyramidal neuron excitation:inhibition is thought to underlie symptomologies shared across stress-related disorders and neuropsychiatric disease, including dysregulation of emotion and cognitive function. G protein-gated inwardly rectifying K+ (GIRK/Kir3) channels mediate excitability of medial PFC pyramidal neurons, however, the functional role of these channels in mPFC-dependent regulation of affect, cognition, and cortical dynamics is unknown. We used a viral-cre approach in male and female mice harboring a “floxed” version of the kcnj3 (Girk1) gene, to disrupt GIRK1-containing channel expression in pyramidal neurons within the prelimbic cortex (PrL). In males, loss of pyramidal GIRK1-dependent signaling differentially impacted measures of affect and impaired working memory and cognitive flexibility. Unexpectedly, ablation of PrL GIRK1-dependent signaling did not impact affect or cognition in female mice. Additional studies used a model of chronic unpredictable stress (CUS) to determine the impact on PrL GIRK-dependent signaling and cognitive function. CUS exposure in male mice produced deficits in cognition that paralleled a reduction in PrL pyramidal GIRK-dependent signaling akin to viral approaches whereas CUS exposure in female mice did not alter cognitive flexibility performance. Stress-induced behavioral deficits in male mice were rescued by systemic injection of a novel, GIRK1-selective agonist, ML297. In conclusion, GIRK1-dependent signaling in male mice, but not females, is critical for maintaining optimal PrL function and behavioral control. Disruption of this inhibition may underlie stress-related dysfunction of the PrL and represent a therapeutic target for treating stress-induced deficits in affect regulation and impaired cognition that reduce quality of life.
Similar content being viewed by others
Introduction
Dysfunction of the prefrontal cortex (PFC) is an underlying factor in both affect and cognition-related behavioral deficits that co-occur across neuropsychiatric disorders [1,2,3,4,5,6,7,8,9,10,11,12]. Similar symptomologies are observed in individuals with chronic psychosocial or self-perceived chronic stress [13,14,15,16]. Converging evidence indicates that prolonged exposure to environmental stressors can increase the risk and severity of these neuropsychiatric disorders, highlighting a need to identify neural substrates that contribute to optimal PFC function and behavioral control.
The prelimbic cortical subdivision (PrL) of the medial PFC (mPFC) is involved in top-down regulation of behavior related to anxiety, motivation, stress, and coordination of working memory and flexible decision-making (e.g., strategy shifting) [17,18,19,20,21,22,23,24,25,26]. Optimal function of the PrL relies on coordinated activity of principle output glutamatergic pyramidal neurons. This activity is critically dependent on a dynamic balance of cell excitation:inhibition mediated largely by intrinsic (physiological) membrane properties that influence the cellular response to synaptic input [27,28,29,30,31]. Accordingly, imbalances in pyramidal neuron excitation:inhibition are thought to contribute to numerous symptoms observed in neuropsychiatric disorders [4, 28, 32,33,34,35,36,37,38,39,40].
PrL pyramidal neuron excitability and spike firing is modulated by activity of G protein-gated inwardly rectifying K+ (GIRK/Kir3) channels which produce a slow hyperpolarizing current that acts as a neuronal “off switch” in both males and females [41,2d). These data indicate that reducing PrL pyramidal neuron GIRK1-dependent signaling in males reduces normal anxiety-like responses and escape-related strategies suggestive of an anxiolytic and pro-depressive phenotype [66, 67], without altering appetitive reward motivation.
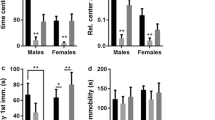
a Schematic targeting of infusion to the PrL in male mice. b Percent time in the open arm of the EPM was increased in cre+ male mice compared to cre- controls. c Total time (s) spent immobile during the FST was increased in cre+ compared to cre- mice. d Breakpoint during a PR test was similar in cre+ males versus cre- controls. e During the forced alternation T-maze paradigm, a main effect of virus was found, with cre+ male mice having reduced percent correct choice. f Comparison of trials across days showed that Cre+ male mice performed significantly worse than cre- during trials 2, 7, 9, and 11. g–n Attentional set-shifting paradigm. g There was no difference between cre- and cre+ male mice in the number of days to reach lever training criterion. h During the visual cue test, cre+ mice took significantly more trials to reach criterion compared to cre- mice. i During the ED Shift test, cre- and cre+ male mice took similar number of trials to reach criterion. j During the reversal test, cre- and cre+ mice took similar trials to reach criterion. k Cre+ and cre- male mice did not differ in trials to reach criterion for visual cue training (l) Using an alternative response-to-cue paradigm, where the visual cue test is the ED shift, Cre+ male mice took significantly more days to reach lever training criterion. m Cre+ mice required greater number of trials to reach criterion during a response test. n Cre+ mice also took more trials to reach criterion during the subsequent ED Shift visual cue test. *p < 0.05, **p < 0.01. Star in schematics denotes correct response, yellow circle denotes cue, gray rectangles denote left and right levers.
Working memory in PrL pyramidal neuron GIRK knockout male mice
The most consistently documented cognitive deficits in neuropsychiatric disease and stress pathologies includes impaired behavioral flexibility and working memory [68, 69], thus we examined whether loss of GIRK promotes similar impairments. Using a forced alternation T-maze paradigm to assess working memory, we found no effect of day (F(5,65) = 0.24, p = 0.95), or condition (virus) by day interaction (F(5,65) = 0.66, p = 0.65) on the percent of correct trials for each day. A main effect of condition was observed (F(1,13) = 22.46, p < 0.001), with cre+ male mice showing an overall reduction in percent correct choices compared to cre- controls (Fig. 2e). When averaging performance of each trial across days, there was a significant condition by trial interaction (F(11,143) = 2.10, p = 0.24; Fig. 2f), with post-hoc comparisons indicating that the cre+ male mice performed significantly worse on trials 2 (p = 0.004), 7 (p = 0.018), 9 (p = 0.034), 10 (p = 0.008), and 11 (p = 0.008). These data indicate that PrL pyramidal neuron GIRK1-dependent signaling in male mice plays a key role in information processing related to working memory.
Impact of PrL pyramidal neuron GIRK knockout on cognitive flexibility in male mice
To determine if PrL pyramidal GIRK channels play a role in complex forms of PFC-dependent cognition, we examined the impact of PrL GIRK1 suppression on cognitive flexibility. We used a modified operant-based ASST [61, 63] that is reliant on PrL function [24, 70]. This task resembles the Wisconsin Card Sorting Task in its sensitivity to distinct components of decision making such as suppression of irrelevant strategies, acquisition and generation of novel strategies, and maintenance of effective strategies. This is the first known study to investigate cognitive flexibility in GIRK1flox/flox mice, therefore we wanted to determine if GIRK1flox/flox mice exhibit a baseline phenotype. We first compared GIRK1flox/flox mice to C57BL/6 mice receiving sham surgery and found no difference in their performance on any measure in ASST (lever train: t(11) = −0.98, p = 0.35; VC trials: t(11) = −0.35, p = 0.74; ED trials: t(11) = −0.31, p = 0.76; REV trials: t(11) = −1.27, p = 0.23; data not shown), thus data were combined for further analyses. There was no difference in days to criterion for lever training between cre- and cre + (t(19) = −1.04, p = 0.31; Fig. 2g). Following lever training, comparison of performance in the visual cue test showed that cre+ male mice required significantly greater number of trials (t(19) = −2.52, p = 0.02; Fig. 2h) and errors (t(19) = −2.56, p = 0.019; Supplementary 1a) to reach criterion compared to cre- but did not differ on omissions (U = 45.00, p = 0.58; Supplementary Table 1). Further investigation of error type revealed no difference in initial errors (U = 51.00, p = 0.94) however the cre+ male mice had significantly more regressive errors compared to cre- control mice (t(19) = −2.87, p = 0.01; data not shown). During the ED Shift, cre- and cre+ mice required a similar number of trials (U = 28.50, p = 0.09; Fig. 2i), errors (t(19) = 0.95, p = 0.35; Supplementary 1b) and omissions to reach criterion (t(19) = 1.17, p = 0.26; Supplemental Table 1). Similarly, no difference was observed in trials (t(19) = −0.35, p = 0.73; Fig. 2j), errors (t(19) = −0.87, p = 0.40; Supplementary 1c) or omissions (t(19) = −1.42, p = 0.17; Supplementary Table 1) to criterion in a subsequent reversal learning test.
Past studies have shown that pharmacological inhibition of the PrL does not impact performance in an operant or maze-based visual cue discriminative learning task in rats [63, 71]. To ensure that impaired performance during the visual cue test in male GIRK1-knockout mice did not reflect deficits in general attention to a cue or visual acuity, we next trained a new cohort of mice on the visual cue test only (i.e., no lever training) with similar criterion to test sessions (i.e., 10 consecutive correct responses). During visual cue training, there were no differences in the trials (t(16) = 0.95, p = 0.36; Fig. 2k), errors (t(16) = 0.33, p = 0.75; Supplementary 2a), or omissions (U = 32.00, p = 0.48; Supplementary Table 2) to reach criterion between cre+ and cre- mice indicating that the cre+ male mice do not have deficits in the visual cue simply due to attentional deficits to the cue or due to visual acuity.
As mice were initially lever trained in a pseudorandom fashion to press either the left or the right lever when it was presented (i.e., only attend to levers), it is possible that the addition of a visual cue rule was acting as an attentional shift (i.e., ignore previous lever presentation order and attend to visual cue) which may have resulted in the unexpected deficits during the visual cue test in male cre+ mice. To address this, additional cohorts underwent lever training during which cre+ male mice took longer to obtain criterion compared to controls (t(8) = −3.06, p = 0.02; Fig. 2l). Notably, this significance appears largely driven by the lack of variability in days to reach criterion in cre- mice. After lever training, mice received a response test (lever opposite of bias is correct) and a subsequent visual cue test. During the response test, cre+ mice took significantly more trials (t(8) = −2.55, p = 0.03; Fig. 2m) and errors (t(8) = −3.78, p = 0.01; Supplementary 3b) and had similar omissions (t(8) = −2.05, p = 0.08; Supplementary Table 3). During the visual cue set-shift, cre+ mice also required a greater number of trials (t(8) = −2.67, p = 0.03; Fig. 2n) but did not significantly differ in errors (t(8) = −2.06, p = 0.07; Supplementary 3c) or omissions (t(8) = −1.06, p = 0.32; Supplementary Table 3) to reach criterion compared to cre- mice. These findings combined with a lack of difference to acquire a visual cue training indicate that loss of GIRK channel activity alters PrL function (i.e., cognitive flexibility) in a manner distinct from lesions.
Influence of PrL pyramidal neuron GIRK knockout on female performance in EPM, FST, and progressive ratio
Intrinsic sex differences in mPFC physiology and function may have translational significance regarding resilience or susceptibility to pathological disorders [72,73,74]. Past studies have identified sex-dependent differences in GIRK-dependent signaling in adolescence [79,80,81].
We find that PrL GIRK1 ablation produced a consistent reduction in working memory in male mice as assessed using a forced alternation model. Assessment of more complex processes using the ASST showed an impaired performance in cre+ during the visual cue test in males, while not altering performance during the ED shift. These findings were unexpected as PrL lesions have previously shown to impair performance in the ED shift, but insufficient to disrupt performance in a cue-based discriminative test [14, 34]. It is important to note, however, that reduction in GIRK1-signaling and disorganized mPFC activity, both of which we show is evident in GIRK1 knockout mice, is inherently different than PrL lesioning (and therefore lack of activity). Given that loss of GIRK channel activity did not alter the ability to acquire a visual cue-based learning task, it is unlikely that this deficit reflects general attention to a cue or reduced visual acuity [82]. Rather, loss of GIRK1 alters PrL function in a manner where the addition of a visual cue contingency not present during lever training is sufficient to act as an attentional shift. In support, experiments involving response-to-cue shifts showed that cre+ male mice also performed worse when the contingency was changed from lever training to response (attend to one lever only), and that these impairments persisted during a subsequent response-to-visual cue ED Shift. Cognitive processes associated with working memory are reliant, although not exclusively, on coordinated PrL activity [83,84,85,86] therefore it is likely that increased/disorganized firing following loss of PrL pyramidal neuron GIRK underlies the observed deficits in working memory and cognitive flexibility.
PrL PYR GIRK1 knockout and CUS exposure in females
Sex differences in susceptibility to anxiety, mood, and other neuropsychiatric disorders have been established in humans [87,88,89,90]. Therefore, the influence that mPFC physiology and function has on behavior in both males and females has significant translational value for identifying both susceptibility and treatment options. We replicated findings showing that GABAB-GIRK signaling is present in female PrL pyramidal neurons [106, 107] and alter mPFC plasticity in female rats [108]. Importantly, studies showing set-shift task deficits in females following stress exposure used a task that is not operant-based, but rather employs odor and digging medium as cues [17, 109], which may have different capacities to identify stress-induced cognitive changes.
Impact of CUS on GABAB-GIRK signaling and cognitive flexibility in males
Similar to cognitive inflexibility in humans [9, 110, 111], exposure to CUS impairs performance in the ED Shift test in male mice. Deficits in flexibility aligned with a reduction in GABAB-GIRK signaling, highlighting a role in CUS-induced PrL dysfunction. Unlike GIRK1 knockouts, deficits in flexibility following CUS were specific to the ED Shift, as has previously been shown following chronic stress in male rats [101, 112]. Similar to PrL lesion studies [18, 34], CUS exposure may impair ED Shift performance rather than producing deficits in the visual cue test as it likely has more global effects than just reducing PrL pyramidal neuron GIRK1-dependent signaling [113]. As converging lines of evidence show that exposure to unpredictable stressors negatively impacts cognitive control in humans and rodents [9, 110, 111, 114, 115], the ability of systemic ML297, a GIRK1-selective agonist, to rescue this deficit has significant impact on future therapeutics aimed at treating cognition-related deficits produced by stress. Collectively, we highlight the importance of GIRK-dependent signaling in male, but not female, PrL pyramidal neurons in the regulation of both affect and cognition and demonstrate that PrL GIRK channels in males are targets of stress.
There are several limitations to the study. First, it is important to note that the collection of female data for both GIRK and stress-related studies were performed at a later date, however, all groups of animals were run with proper controls in parallel. As direct comparison of sex may confer false positive or negative data, findings were presented separately. Thus, while our data provide a proper assessment of the impact of GIRK knockout and stress in females and males alone, without a direct statistical comparison conference of sex differences in the functional role GIRK channels play should be considered with caution. Second, although the locus of ML297 effects were not identified, systemic ML297 can rescue stress-induced cognitive deficits in males and therefore demonstrates therapeutic potential. It is possible that the rescue of deficits is due to augmentation of residual GIRK1 signaling in PrL pyramidal neurons, and that this is sufficient to restore optimal neuronal activity within this region. It is also possible that the effectiveness of ML297 reflects activation of GIRK1 and thus inhibition of other cortical structures that drive behavioral rigidity. While we demonstrate that loss of GIRK1 increases output of PrL pyramidal neurons, it is unknown how viral- and stress-related elevations in activity alter an often reciprocally and/or collaterally connected mPFC microcircuitry. It is also not clear whether behavioral deficits in males are disproportionally driven by reduced GIRK signaling and increased output of pyramidal neuron subpopulations based on the downstream target. These questions have important implications as both increased (disorganized) activity as well as mPFC hypoactivity have been highlighted in neuropsychiatric disease states.
Funding and disclosure
These studies were supported by funding from the Brain and Behavior Research Foundation (#26299; MH), Marquette University Regular Research Grant (MH), the Charles E Kubly Mental Health Research Foundation at Marquette University (MH), and NIH grants DA034696 and AA027544 (KW). The authors have no biomedical financial interests or potential conflicts of interest.
References
Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Hormones Behav. 2005;48:1–10.
Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J Nerv Ment Dis. 1998;186:661–9.
Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41.
Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15.
Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L. Cognitive impairment in major depression. Eur J Pharm. 2010;626:83–6.
Remijnse PL, van den Heuvel OA, Nielen MM, Vriend C, Hendriks GJ, Hoogendijk WJ, et al. Cognitive inflexibility in obsessive-compulsive disorder and major depression is associated with distinct neural correlates. PLoS One. 2013;8:e59600.
Scult MA, Knodt AR, Swartz JR, Brigidi BD, Hariri AR. Thinking and feeling: individual differences in habitual emotion regulation and stress-related mood are associated with prefrontal executive control. Clin Psychol Sci. 2017;5:150–57.
Dajani DR, Uddin LQ. Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends Neurosci. 2015;38:571–8.
Schwabe L, Wolf OT. Stress prompts habit behavior in humans. J Neurosci. 2009;29:7191–8.
Zeng H, Lee TM, Waters JH, So KF, Sham PC, Schottenfeld RS, et al. Impulsivity, cognitive function, and their relationship in heroin-dependent individuals. J Clin Exp Neuropsychol. 2013;35:897–905.
Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:193–218.
Negron-Oyarzo I, Aboitiz F, Fuentealba P. Impaired functional connectivity in the prefrontal cortex: a mechanism for chronic stress-induced neuropsychiatric disorders. Neural Plast. 2016;2016:7539065.
Gold JI, Treadwell M, Weissman L, Vichinsky E. An expanded Transactional Stress and Co** Model for siblings of children with sickle cell disease: family functioning and sibling co**, self-efficacy and perceived social support. Child Care Health Dev. 2008;34:491–502.
Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–83.
Strauss GP, Duke LA, Ross SA, Allen DN. Posttraumatic stress disorder and negative symptoms of schizophrenia. Schizophr Bull. 2011;37:603–10.
Strauss K, Vicari S, Valeri G, D’Elia L, Arima S, Fava L. Parent inclusion in Early Intensive Behavioral Intervention: the influence of parental stress, parent treatment fidelity and parent-mediated generalization of behavior targets on child outcomes. Res Dev Disabil. 2012;33:688–703.
Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4.
Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–30.
Boettiger CA, D’Esposito M. Frontal networks for learning and executing arbitrary stimulus-response associations. J Neurosci. 2005;25:2723–32.
Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem. 2011;96:553–63.
Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress 2004;7:131–43.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69.
McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, et al. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol Psychiatry. 2016;80:754–64.
Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84.
Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC. Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci. 2014;8:230.
Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav brain Res. 2008;190:85–96.
Bissonette GB, Roesch MR. Neural correlates of rules and conflict in medial prefrontal cortex during decision and feedback epochs. Front Behav Neurosci. 2015;9:266.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011;477:171–8.
Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron 2011;72:231–43.
Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009;459:698–702.
Kim H, Ahrlund-Richter S, Wang X, Deisseroth K, Carlen M. Prefrontal parvalbumin neurons in control of attention. Cell 2016;164:208–18.
Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38:950–7.
Covington HE 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–90.
Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–41.
Kleschevnikov AM, Belichenko PV, Faizi M, Jacobs LF, Htun K, Shamloo M, et al. Deficits in cognition and synaptic plasticity in a mouse model of Down syndrome ameliorated by GABAB receptor antagonists. J Neurosci. 2012;32:9217–27.
Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 2008;1:6.
Dichter GS, Felder JN, Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Soc Cogn Affect Neurosci. 2009;4:215–26.
Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol psychiatry. 2007;12:158–66.
Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry. 2016.
Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y, et al. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry. 2012;2:e142.
Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Lujan R, et al. Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron. 2013;80:159–70.
Marron Fernandez de Velasco E, Hearing M, **a Z, Victoria NC, Lujan R, Wickman K. Sex differences in GABA(B)R-GIRK signaling in layer 5/6 pyramidal neurons of the mouse prelimbic cortex. Neuropharmacology 2015;95:353–60.
Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ. Ethanol dependence abolishes monoamine and GIRK (Kir3) channel inhibition of orbitofrontal cortex excitability. Neuropsychopharmacology. 2017;42:1800–12.
Victoria NC, Marron Fernandez de Velasco E, Ostrovskaya O, Metzger S, **a Z, Kotecki L, et al. G Protein-gated K(+) channel ablation in forebrain pyramidal neurons selectively impairs fear learning. Biol Psychiatry. 2016;80:796–806.
Luján R, Aguado C. Localization and targeting of GIRK channels in mammalian central neurons. Int Rev Neurobiol. 2015;123:161–200.
Glaaser IW, Slesinger PA. Structural insights into GIRK channel function. Int Rev Neurobiol. 2015;123:117–60.
Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–62.
Fatemi SH, Folsom TD, Thuras PD. Deficits in GABA(B) receptor system in schizophrenia and mood disorders: a postmortem study. Schizophr Res. 2011;128:37–43.
Yamada K, Iwayama Y, Toyota T, Ohnishi T, Ohba H, Maekawa M, et al. Association study of the KCNJ3 gene as a susceptibility candidate for schizophrenia in the Chinese population. Hum Genet. 2012;131:443–51.
Cooper A, Grigoryan G, Guy-David L, Tsoory MM, Chen A, Reuveny E. Trisomy of the G protein-coupled K+ channel gene, Kcnj6, affects reward mechanisms, cognitive functions, and synaptic plasticity in mice. Proc Natl Acad Sci USA. 2012;109:2642–7.
Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Herve D, et al. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat Med. 2016;22:254–61.
Pravetoni M, Wickman K. Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes Brain Behav. 2008;7:523–31.
Wydeven N, Marron Fernandez de Velasco E, Du Y, Benneyworth MA, Hearing MC, Fischer RA, et al. Mechanisms underlying the activation of G-protein-gated inwardly rectifying K+ (GIRK) channels by the novel anxiolytic drug, ML297. Proc Natl Acad Sci USA. 2014;111:10755–60.
Yao G, Chen XN, Flores-Sarnat L, Barlow GM, Palka G, Moeschler JB, et al. Deletion of chromosome 21 disturbs human brain morphogenesis. Genet Med. 2006;8:1–7.
Valetto A, Orsini A, Bertini V, Toschi B, Bonuccelli A, Simi F, et al. Molecular cytogenetic characterization of an interstitial deletion of chromosome 21 (21q22.13q22.3) in a patient with dysmorphic features, intellectual disability and severe generalized epilepsy. Eur J Med Genet. 2012;55:362–6.
Lazary J, Juhasz G, Anderson IM, Jacob CP, Nguyen TT, Lesch KP, et al. Epistatic interaction of CREB1 and KCNJ6 on rumination and negative emotionality. Eur Neuropsychopharmacol. 2011;21:63–70.
Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci USA. 1997;94:923–7.
Kotecki L, Hearing M, McCall NM, Marron Fernandez de Velasco E, Pravetoni M, Arora D, et al. GIRK channels modulate opioid-induced motor activity in a cell type- and subunit-dependent manner. J Neurosci. 2015;35:7131–42.
Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, et al. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci USA. 2016;113:757–62.
Anderson EM, Gomez D, Caccamise A, McPhail D, Hearing M. Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiol Stress. 2019;10:100152.
Anderson EM, Demis S, D’Acquisto H, Engelhardt A, Hearing M. The role of parvalbumin interneuron GIRK signaling in the regulation of affect and cognition in male and female mice. Front Behav Neurosci. 2021;15:1–16.
Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12.
Brady AM, Floresco SB. Operant procedures for assessing behavioral flexibility in rats. J Visual Exp. 2015;96:e52387.
Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11.
Marron Fernandez de Velasco E, Carlblom N, **a Z, Wickman K. Suppression of inhibitory G protein signaling in forebrain pyramidal neurons triggers plasticity of glutamatergic neurotransmission in the nucleus accumbens core. Neuropharmacology. 2017;117:33–40.
Kara NZ, Stukalin Y, Einat H. Revisiting the validity of the mouse forced swim test: systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci Biobehav Rev. 2018;84:1–11.
Castagne V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Pharmacol. 2011;55:8:1–8.
Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68.
Etkin A, Gyurak A, O’Hara R. A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues Clin Neurosci. 2013;15:419–29.
Bissonette GB, Roesch MR. Neurophysiology of rule switching in the corticostriatal circuit. Neuroscience. 2017;345:64–76.
Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–94.
Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol. 2013;23:1165–81.
Ishii-Takahashi A, Takizawa R, Nishimura Y, Kawakubo Y, Kuwabara H, Matsubayashi J, et al. Prefrontal activation during inhibitory control measured by near-infrared spectroscopy for differentiating between autism spectrum disorders and attention deficit hyperactivity disorder in adults. NeuroImage Clin. 2014;4:53–63.
Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron 2014;83:1002–18.
Page CE, Coutellier L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: Evidence for over-inhibition. Neurosci Biobehav Rev. 2019;105:39–51.
Llamosas N, Bruzos-Cidon C, Rodriguez JJ, Ugedo L, Torrecilla M. Deletion of GIRK2 subunit of GIRK channels alters the 5-HT1A receptor-mediated signaling and results in a depression-resistant behavior. Int J Neuropsychopharmacol. 2015;18:pyv051.
Maaswinkel H, Gispen WH, Spruijt BM. Effects of an electrolytic lesion of the prelimbic area on anxiety-related and cognitive tasks in the rat. Behav Brain Res. 1996;79:51–9.
Stern CA, Do Monte FH, Gazarini L, Carobrez AP, Bertoglio LJ. Activity in prelimbic cortex is required for adjusting the anxiety response level during the elevated plus-maze retest. Neuroscience 2010;170:214–22.
de Kloet ER, Molendijk ML. Co** with the forced swim stressor: towards understanding an adaptive mechanism. Neural plasticity. 2016;2016:6503162.
De Pablo JM, Parra A, Segovia S, Guillamón A. Learned immobility explains the behavior of rats in the forced swimming test. Physiol Behav. 1989;46:229–37.
Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–34.
Prusky GT, West PW, Douglas RM. Reduced visual acuity impairs place but not cued learning in the Morris water task. Behav Brain Res. 2000;116:135–40.
Gilmartin MR, Miyawaki H, Helmstetter FJ, Diba K. Prefrontal activity links nonoverlap** events in memory. J Neurosci. 2013;33:10910–4.
Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron 2012;76:1057–70.
Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19:133–46.
Yang ST, Shi Y, Wang Q, Peng JY, Li BM. Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol brain. 2014;7:61.
Hodes GE, Epperson CN. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry. 2019;86:421–32.
McEwen BS. Hormones and behavior and the integration of brain-body science. Hormones Behav. 2020;119:104619.
Jaggar M, Rea K, Spichak S, Dinan TG, Cryan JF. You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan. Front Neuroendocrinol. 2020;56:100815.
Pinares-Garcia P, Stratikopoulos M, Zagato A, Loke H, Lee J. Sex: a significant risk factor for neurodevelopmental and neurodegenerative disorders. Brain Sci. 2018;8:1–27.
Anderson EM, Engelhardt A, Demis S, Porath E, Hearing MC. Remifentanil self-administration in mice promotes sex-specific prefrontal cortex dysfunction underlying deficits in cognitive flexibility. Neuropsychopharmacology. 2021;0:1–12.
Spring S, Lerch JP, Henkelman RM. Sexual dimorphism revealed in the structure of the mouse brain using three-dimensional magnetic resonance imaging. NeuroImage. 2007;35:1424–33.
Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–73.
Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Hormones Behav. 2003;43:48–59.
Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol Behav. 2009;97:21–9.
Luine V, Gomez J, Beck K, Bowman R. Sex differences in chronic stress effects on cognition in rodents. Pharmacol, Biochem, Behav. 2017;152:13–19.
McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–54.
Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–89.
Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem. 2003;79:32–40.
Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55.
Moench KM, Breach MR, Wellman CL. Prior stress followed by a novel stress challenge results in sex-specific deficits in behavioral flexibility and changes in gene expression in rat medial prefrontal cortex. Hormones Behav. 2020;117:104615.
Moench KM, Wellman CL. Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress. Neuroscience. 2017;357:145–59.
Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, et al. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry. 2014;19:588–98.
Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207.
Yohn CN, Ashamalla SA, Bokka L, Gergues MM, Garino A, Samuels BA. Social instability is an effective chronic stress paradigm for both male and female mice. Neuropharmacology. 2019;160:107780.
Snyder K, Barry M, Plona Z, Ho A, Zhang XY, Valentino RJ. The impact of social stress during adolescence or adulthood and co** strategy on cognitive function of female rats. Behav Brain Res. 2015;286:175–83.
Hyer MM, Shaw GA, Goswamee P, Dyer SK, Burns CM, Soriano E, et al. Chronic adolescent stress causes sustained impairment of cognitive flexibility and hippocampal synaptic strength in female rats. Neurobiol stress. 2021;14:100303.
Urban KR, Valentino RJ. Age- and sex-dependent impact of repeated social stress on intrinsic and synaptic excitability of the rat prefrontal cortex. Cereb Cortex. 2017;27:244–53.
Heisler JM, Morales J, Donegan JJ, Jett JD, Redus L, O’Connor JC. The attentional set shifting task: a measure of cognitive flexibility in mice. J Visual Exp. 2015;96:51944–50.
Winstanley CA, Bachtell RK, Theobald DE, Laali S, Green TA, Kumar A, et al. Increased impulsivity during withdrawal from cocaine self-administration: role for DeltaFosB in the orbitofrontal cortex. Cereb Cortex. 2009;19:435–44.
Winstanley CA, Green TA, Theobald DE, Renthal W, LaPlant Q, DiLeone RJ, et al. DeltaFosB induction in orbitofrontal cortex potentiates locomotor sensitization despite attenuating the cognitive dysfunction caused by cocaine. Pharm Biochem Behav. 2009;93:278–84.
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4.
Wellman CL, Bollinger JL, Moench KM. Effects of stress on the structure and function of the medial prefrontal cortex: insights from animal models. Int Rev Neurobiol. 2020;150:129–53.
Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–46.
Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharm. 2002;13:169–88.
Author information
Authors and Affiliations
Contributions
All authors have contributed to this work in a meaningful way. EMA and MCH designed the work, acquired, analyzed, and interpreted the work, and wrote the manuscript. SL designed the work and acquired, analyzed, and interpreted the work. BW, AE, SD, KO, acquired, analyzed, and interpreted the work. EH and KW designed and interpreted the work. All authors helped with drafting and revision of the work and have given approval for this work to be published.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Anderson, E.M., Loke, S., Wrucke, B. et al. Suppression of pyramidal neuron G protein-gated inwardly rectifying K+ channel signaling impairs prelimbic cortical function and underlies stress-induced deficits in cognitive flexibility in male, but not female, mice. Neuropsychopharmacol. 46, 2158–2169 (2021). https://doi.org/10.1038/s41386-021-01063-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01063-w
- Springer Nature Switzerland AG