Abstract
The p53 tumour suppressor has an important role in cancer cells. Here we show that p53 regulates expression of major histocompatibility complex I on the cell surface. We show that the tumour cell line HCT116, which lacks p53 exhibits significantly lower major histocompatibility complex I expression than its wild-type counterpart. Using a combination of chromatin immunoprecipitation sequencing and gene expression analysis, we demonstrate that p53 upregulates expression of endoplasmic reticulum aminopeptidase 1 by binding to its cognate response element in the ERAP1 gene. Silencing of p53 decreases endoplasmic reticulum aminopeptidase 1 protein levels and therefore major histocompatibility complex I expression. We further show that this mechanism operates in A549 cells infected with H1N1 influenza virus, in which H1N1 activates p53, leading to endoplasmic reticulum aminopeptidase 1 upregulation and a corresponding increase in major histocompatibility complex I expression. Our study suggests a previously unrecognized link between p53 function and the immunosurveillance of cancer and infection.
Similar content being viewed by others
Introduction
The tumour suppressor protein p53 regulates diverse cellular processes including apoptosis, cell cycle, senescence and metabolism1. It does so by sensing cellular and genotoxic stress signals and responds by activating or repressing gene targets that bear a p53-response element (p53RE) in their regulatory region. Recent evidence suggests that p53 participates in a broader range of cellular activities than previously thought2, including, perhaps, the immune response3,4.
We had observed that in p53 isogenic HCT116 cell lines, differing only in their p53 status, major histocompatibility complex (MHC) Class I expression at the cell surface was also disparate, and wondered whether this observation might strengthen the evidence between p53 and regulation of immune responses. Appropriate regulation of MHC expression is important for effective tumour surveillance and protection against viral infection5,6, but no interaction of p53 and the MHC I pathway had yet been convincingly shown. The MHC class I antigen presentation pathway comprises a series of complex steps, which integrate degradation and preparation of the peptides to be loaded for presentation with the assembly and expression of the MHC molecules themselves. Regulation of MHC I expression thus occurs at multiple levels and involves numerous components of the presentation pathway machinery7. One molecule that has received much interest is the endoplasmic reticulum aminopeptidase 1 (ERAP1), which is responsible for the final trimming of antigen precursors before loading into MHC class I proteins8. Defects in ERAP1 result in changes in MHC class I expression and impaired T-cell responses9, and ERAP1 allelic variants are associated with diseases including ankylosing spondylitis and hypertension10,11,12. Altered ERAP1 expression was also observed in some human cancer cell lines13,14 and has recently been linked to clinical outcome in cervical carcinoma15.
In this study, we asked how the p53 status of cells relates to their MHC I expression. Using two isogenic human colon carcinoma cell lines HCT116 (p53−/− and p53+/+), we show that p53 can affect MHC I levels via transcriptional regulation of ERAP1. We use expression profiling analysis to identify p53-regulated genes in these cells and validate ERAP1 as a direct target of p53. This mechanism is also active during viral infection, as in a human pulmonary epithelial cell line exposed to H1N1 influenza virus, p53 is activated and in turn increases the surface expression of MHC class I via ERAP1. Our data reveal an important new role for p53 in the immune response and define a mechanism that explains the relationship between p53 and MHC I in both transformed and virally infected cells.
Results
MHC class I levels are higher in p53+/+ than p53−/− cells
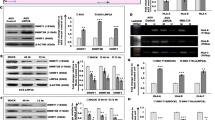
We used a pair of isogenic cell lines that differ in their p53 status (HCT116 p53+/+ and HCT116 p53−/−), and assessed the levels of MHC I on their cell surface. HCT116 (p53+/+) cells expressed substantially more surface MHC Class I molecules than p53−/− cells, evident by flow cytometry (Fig. 1a), and fluorescence microscopy (Fig. 1b). The same results were observed in two osteosarcoma cell lines that similarly differ in p53 expression, U2OS (p53+/+) and Saos-2 (p53−/−), with higher MHC class I expression in the U2OS cells, which possess wild-type p53 alleles (Fig. 1c,d). Thus, we hypothesized that the differential expression of MHC Class I observed might be linked to the differential p53 status of the cell lines tested. The effect of p53 expression was specific for MHC I, and did not affect MHC II levels (Fig. 1a,c).
(a) Flow cytometric analysis of MHC class I (HLA-ABC, W6/32 labelling) and MHC class II (HLA-DR, L243 labelling) expression on HCT116 (p53+/+) (red) and HCT116 (p53−/−) (blue) cells. Cells incubated with fluorescence-labelled secondary antibodies alone served as background controls (Grey). (b) Immunofluorescence images of MHC class I expression shown by W6/32 labelling of HCT116 (p53+/+) and HCT116 (p53−/−) cells. DAPI and Brightfield images confirm the relatively equal number of cells captured. Scale bar, 50 μm. (c) Flow cytometric analysis of MHC class I (W6/32 labelling) and MHC class II (L243 labelling) expression in U2OS (p53+/+) (magenta) and Saos-2 (p53−/−) (cyan) cells. (d) Immunofluorescence images of MHC class I expression shown by W6/32 labelling of U2OS (p53+/+) and Saos-2 (p53−/−) cells. Scale bar, 50 μm.
ERAP1 is a potential p53-target gene
To understand how p53 expression and MHC I levels might be linked, we asked whether the expression of any other molecules in the MHC I presentation pathway were also affected by p53 expression. We transfected HCT116 (p53−/−) with constructs encoding either wild-type p53, or one of six mutant p53 sequences, then subjected the cells to microarray analysis of global gene expression. The dominant-negative effects of the six mutant p53 constructs used were previously reported (IARC p53 database) and again confirmed by a correlation analysis (R2) of our customized luciferase assays using 16 dinucleotide core combinationsSupplementary Table S3.
Western blot
Cells were lysed with 1% Nonidet P-40 cell lysis buffer supplemented with the complete protease inhibitor cocktail (Roche) for 30 min at 4 °C and the protein concentration determined by Bradford Protein Assay (Bio-rad). Approximately 20 μg of cell lysate was separated by 7.5–12.5% SDS–PAGE, and transferred to Hybond PVDF membranes (GE Healthcare). Antibodies against p53 (DO-1, sc-126, 1:3,000), p-p53 (Ser15, sc-101762, 1:100), p21 (C-19, sc-397, 1:250), NS1 (NS1-23-1, sc-130568, 1:100), HA (F-7, sc-7392, 1:100) (Santa Cruz Biotechnology), ERAP1 (ab124669, 1:2,000) and ERAP2 (ab69037,1:3,000) (Abcam) were used for protein detection. Equal loading of protein samples was verified with an antibody to β-actin (1:10,000, Millipore). Immunoreactive signals were visualized with enhanced chemiluminescence reagents (GE Healthcare). Full-length images of immunoblots are shown in Supplementary Fig. S8.
Flow cytometry
Cells were harvested after different treatments by trypsinization using 0.05% Trypsin-EDTA (Life Technologies) and washed with PBS. Cells were resuspended in labelling buffer (PBS supplemented with 10% FBS and 1% NaN3) to a final concentration of 1 × 106 per ml and stained with W6/32 monoclonal antibody (1:10, ATCC: HB-95) or L243 monoclonal antibody (1:10, ATCC: HB-55) at 4 °C for 1 h, followed by donkey-anti-mouse-AF488 secondary antibody (1:400, A21202, Life Technologies) at 4 °C for 30 min. Cells were washed after each incubation and resuspended in 1 ml of labelling buffer for flow cytometric analysis using a LSRII 4-laser flow cytometer (Becton Dickinson). The results were analysed and MFI calculated by FlowJo.
Fluorescence microscopy
Isogenic HCT116 cells, U2OS and Saos-2 cells underwent different treatments were replated into chamber slides (Becton Dickinson) for overnight culture. Cells were fixed in para-formaldehyde (4% in PBS) at room temperature for 10 min, permeabilized in Triton-X100 (0.1% in PBS) for 3 min and blocked in bovine serum albumin (1% in PBS) for 1 h before incubating with antibodies specific for MHC class I (mouse monoclonal W6/32, 1:10 dilution) then donkey-anti-mouse-AF488 secondary antibody (1:400 dilution, A21202, Life Technologies). Cells were washed with PBS containing 0.1% Tween-20 after each incubation and finally mounted with ProLong Gold antifade reagent with DAPI (Life Technologies). The fluorescence signals were observed and captured using an Axiovert 200 inverted fluorescence microscope (Carl Zeiss). A549 cells infected with H1N1 PR8 or mock control were also seeded, fixed, permeabilized and co-labelled with different combinations of antibodies including mouse monoclonal W6/32 (1:10), goat polyclonal anti-p53 (C-19, sc1311, 1:250 dilution, Santa Cruz Biotechnology), rabbit polyclonal anti-p-p53 (Ser15) (sc-101762, 1:250 dilution, Santa Cruz Biotechnology), rabbit monoclonal anti-ERAP1 (ab124669, 1: 500 dilution, Abcam), and mouse monoclonal anti-NS1 (sc-130568, 1:200 dilution, Santa Cruz Biotechnology) followed by secondary antibodies at 1:400 dilution including donkey-anti-mouse-AF488 (A21202), donkey-anti-goat-AF555 (A21432), goat-anti-rabbit-AF488 (A11034) and goat-anti-rabbit-AF546 (A11035) (Life Technologies).
Luciferase assay
All luciferase constructs based on LASP1 promoter region used to study the function of p53 mutants and the luciferase construct containing the p21 promoter regions used as positive control have been described previously16. To validate the functionality of the proposed response elements of ERAP1, the identified RE sequences were repeated 2–3 times and annealed to be directly cloned between Sac I and Xho I restriction site upstream of the minimal SV40 promoter in pGL3-Promoter vector (Promega). Annealing primers are listed in Supplementary Table S4. Different promoter luciferase reporter constructs (2 μg ml−1) were co-transfected with pCMV-p53, pCMV-p53-mutant or pcDNA3.1 control plasmid (200 ng ml−1), together with 20 ng ml−1 of Renilla control vector into HCT116 (p53−/−) cells seeded in 96-well plates. The luciferase activity was measured 30 h post transfection by the Dual-Luciferase Reporter Assay System (Promega). For all luciferase assays, the normalized luciferase activity of each construct (Firefly/Renilla ratio) in the pCMV-p53 or pCMV-p53-mutant co-transfected cells was then compared with that of the pcDNA3.1 control co-transfected cells.
ProLabel Protein–DNA binding assay
To measure the binding affinity of p53 to the p53-target RE sequence, we performed ProLabel Protein–DNA Binding Assay (Clontech) according to manufacturer’s protocol. The p53WT or different p53 mutants lacking TAD (residue 75-393) was cloned between the EcoR I and BamH I restriction sites of pProLabel-C vector using universal primer set p53-ProLabel-F: 5′-AAAGAATTCTCCTGCACCAGCAGCTCCTACAC-3′ (forward) and p53-ProLabel-R: 5′-AAAGGATCCTCAGTCTGAGTCAGGCCCTTCTGT-3′ (reverse). The probe sequences used were wild-type p53RE from p21 (Biotin; 5′-GAACATGTCCCAACATGTTG-3′) and ERAP1 (Biotin; 5′-TGTCATGTGTcagGCACATGTTA-3′), as well as mutant p53RE from p21 (Biotin; 5′-GAAAATATCCCAAAATATTG-3′) and ERAP1 (Biotin; 5′-TGTAATATGTcagGCAAATATTA-3′).
Whole-genome Exon Array analysis
p53WT, the 6 p53 mutant expression vectors and the pcDNA3.1 control plasmid were separately transfected into HCT116 (p53−/−) cells. Total RNA was extracted 24 h post transfection. Total RNA (1 μg) was used as initial material for GeneChip Human Exon 1.0 ST Arrays (Affymetrix) according to manufacturer’s instructions. After washing and staining of hybridized arrays with an Affymetrix Fluidics Station, they were scanned on Gene Array Scanner 2500 (Affymetrix) to capture the raw probe signal intensities in CEL files. Background subtraction, quantile normalization and summarizing probe sets from Affymetrix expression microarrays raw CEL files were done with Affymetrix Power Tools (APT) software. The resulting probeset signals were annotated with Affymetrix annotation library HuEx-1_0-st-v2.na30.hg19.probeset.csv.
ChIP-seq and data analysis
ChIP using fresh PBMCs extracted from a healthy donor and then treated with 5-FU (50 μg ml−1) for 24 h was carried out as described by Wei et al.32 Approximately 10 ng of ChIP or input DNA was used for library construction and single-end 36 bp sequencing with Illumina Genome Analyser II performed by Genotypic Technology (India) according to standard protocol. Raw illumina ChIP-seq fastq files were analysed at Galaxy ( http://main.g2.bx.psu.edu) and Galaxy/Cistrome ( http://cistrome.org). Briefly, single-end ChIP-seq reads were mapped to the human reference genome assembly HG19 using an ultrafast and memory-efficient short read aligner Bowtie33 taking into account a maximum number of two mismatches permitted in the seed of 28 nt in length. ChIP-seq peak calling from mapped reads via MACS34 empirically models the sequenced ChIP fragments (tag size of 36 nt) with a dynamic Poisson distribution to effectively capture local biases in the genome sequence, and uses it to improve the spatial resolution of predicted binding sites that satisfied P-value cutoff of 0.00001. R script was used for annotation.
Statistical analyses
All experiments were repeated at least three times with similar results achieved. Representative images or data are shown. Differences between averages and percentages between control and tests were statistically analysed using paired two-tailed Student’s t-tests. P-values less than 0.05 were considered statistically significant.
Additional information
Accession codes: Microarray data have been deposited in the GEO repository under accession code GSE49046. ChIP-seq data have been deposited in NCBI SRA under accession code SRP027583.
How to cite this article: Wang, B. et al. p53 increases MHC class I expression by upregulating the endoplasmic reticulum aminopeptidase ERAP1. Nat. Commun. 4:2359 doi: 10.1038/ncomms3359 (2013).
Accession codes
References
Vousden, K. H. & Prives, C. Blinded by the light: the growing complexity of p53. Cell 137, 413–431 (2009).
Menendez, D., Inga, A. & Resnick, M. A. The expanding universe of p53 targets. Nat. Rev. Cancer 9, 724–737 (2009).
Mori, T. et al. Identification of the interferon regulatory factor 5 gene (IRF-5) as a direct target for p53. Oncogene 21, 2914–2918 (2002).
Taura, M. et al. p53 regulates Toll-like receptor 3 expression and function in human epithelial cell lines. Mol. Cell Biol. 28, 6557–6567 (2008).
Hewitt, E. W. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology 110, 163–169 (2003).
Lampen, M. H. & van Hall, T. Strategies to counteract MHC-I defects in tumors. Curr. Opin. Immunol. 23, 293–298 (2011).
Neefjes, J., Jongsma, M. L., Paul, P. & Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836 (2011).
York, I. A. et al. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8-9 residues. Nat. Immunol. 3, 1177–1184 (2002).
Yan, J. et al. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J. Exp. Med. 203, 647–659 (2006).
Haroon, N. & Inman, R. D. Endoplasmic reticulum aminopeptidases: Biology and pathogenic potential. Nat. Rev. Rheumatol. 6, 461–467 (2010).
Maksymowych, W. P. et al. Association of a specific ERAP1/ARTS1 haplotype with disease susceptibility in ankylosing spondylitis. Arthritis. Rheum. 60, 1317–1323 (2009).
Yamamoto, N. et al. Identification of 33 polymorphisms in the adipocyte-derived leucine aminopeptidase (ALAP) gene and possible association with hypertension. Hum. Mutat. 19, 251–257 (2002).
Fruci, D. et al. Expression of endoplasmic reticulum aminopeptidases in EBV-B cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines. J. Immunol. 176, 4869–4879 (2006).
Fruci, D. et al. Altered expression of endoplasmic reticulum aminopeptidases ERAP1 and ERAP2 in transformed non-lymphoid human tissues. J. Cell Physiol. 216, 742–749 (2008).
Mehta, A. M. et al. Single nucleotide polymorphisms in antigen processing machinery component ERAP1 significantly associate with clinical outcome in cervical carcinoma. Genes. Chromosomes. Cancer 48, 410–418 (2009).
Wang, B., **ao, Z. & Ren, E. C. Redefining the p53 response element. Proc. Natl Acad. Sci. USA 106, 14373–14378 (2009).
Zhu, K. et al. p53 induces TAP1 and enhances the transport of MHC class I peptides. Oncogene 18, 7740–7747 (1999).
el-Deiry, W. S., Kern, S. E., Pietenpol, J. A., Kinzler, K. W. & Vogelstein, B. Definition of a consensus binding site for p53. Nat. Genet. 1, 45–49 (1992).
Turpin, E. et al. Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication. J. Virol. 79, 8802–8811 (2005).
Mukhopadhyay, T. & Roth, J. A. Induction of apoptosis in human lung cancer cells after wild-type p53 activation by methoxyestradiol. Oncogene 14, 379–384 (1997).
Randall, R. E. & Goodbourn, S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89, 1–47 (2008).
Falk, K. & Rotzschke, O. The final cut: how ERAP1 trims MHC ligands to size. Nat. Immunol. 3, 1121–1122 (2002).
Saveanu, L. et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat. Immunol. 6, 689–697 (2005).
Correale, P. et al. Treatment of colon and breast carcinoma cells with 5-fluorouracil enhances expression of carcinoembryonic antigen and susceptibility to HLA-A(*)02.01 restricted, CEA-peptide-specific cytotoxic T cells in vitro. Int. J. Cancer 104, 437–445 (2003).
Lubben, N. B. et al. HIV-1 Nef-induced down-regulation of MHC class I requires AP-1 and clathrin but not PACS-1 and is impeded by AP-2. Mol. Biol. Cell 18, 3351–3365 (2007).
Chen, Y., Cheng, M. & Tian, Z. Hepatitis B virus down-regulates expressions of MHC class I molecules on hepatoplastoma cell line. Cell Mol. Immunol. 3, 373–378 (2006).
Kim, S. et al. Human cytomegalovirus microRNA miR-US4-1 inhibits CD8(+) T cell responses by targeting the aminopeptidase ERAP1. Nat. Immunol. 12, 984–991 (2011).
Achdout, H., Manaster, I. & Mandelboim, O. Influenza virus infection augments NK cell inhibition through reorganization of major histocompatibility complex class I proteins. J. Virol. 82, 8030–8037 (2008).
Bajgelman, M. C. & Strauss, B. E. The DU145 human prostate carcinoma cell line harbors a temperature-sensitive allele of p53. Prostate 66, 1455–1462 (2006).
Firat, E. et al. The role of endoplasmic reticulum-associated aminopeptidase 1 in immunity to infection and in cross-presentation. J. Immunol. 178, 2241–2248 (2007).
Hammer, G. E., Gonzalez, F., James, E., Nolla, H. & Shastri, N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat. Immunol. 8, 101–108 (2007).
Wei, C. L. et al. A global map of p53 transcription-factor binding sites in the human genome. Cell 124, 207–219 (2006).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Acknowledgements
This work is funded by the Agency for Science, Technology and Research (A*STAR), Singapore. We thank Dr. Stanley Ng for help in analysing microarray and ChIP-seq data, Dr Bert Vogelstein (Johns Hopkins University) for providing p53+/+ and p53−/− HCT116 cells, pCMV-p53 and pCMV-p53-R175H vectors, Dr. Qiang Yu (Genome Institute of Singapore) for providing U2OS and Saos-2 cell lines, Dr. Vincent Chow (National University of Singapore) for H1N1 PR8 influenza virus, and Ms Wei ** Tien, Ms Yun Rui Ng for technical assistance. We also thank Dr. Lucy Robinson and Dr. Neil McCarthy of Insight Editing London for their critical review and manuscript editing.
Author information
Authors and Affiliations
Contributions
B.W. and E.C.R. designed research. B.W., D.N. and L.L. performed research. B.W., D.N. and E.C.R. Analysed data. B.W. did bioinformatics analysis and statistical analyses. B.W. and E.C.R. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1-S8 and Supplementary Tables S1-S4 (PDF 2093 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, B., Niu, D., Lai, L. et al. p53 increases MHC class I expression by upregulating the endoplasmic reticulum aminopeptidase ERAP1. Nat Commun 4, 2359 (2013). https://doi.org/10.1038/ncomms3359
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms3359
- Springer Nature Limited