Abstract
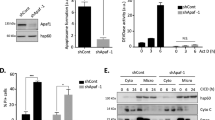
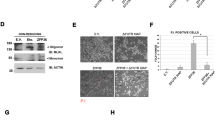
Here, we report the identification of GIDE, a mitochondrially located E3 ubiquitin ligase. GIDE contains a C-terminal RING finger domain, which is mostly conserved with those of the IAP family members and is required for GIDE's E3 ligase activity. Overexpression of GIDE induces apoptosis via a pathway involving activation of caspases, since caspase inhibitors, XIAP and an inactive mutant of caspase-9 block GIDE-induced apoptosis. GIDE also activates JNK, and blockage of JNK activation inhibits GIDE-induced release of cytochrome c and Smac as well as apoptosis, suggesting that JNK activation precedes release of cytochrome c and Smac and is required for GIDE-induced apoptosis. These pro-apoptotic properties of GIDE require its E3 ligase activity. When somewhat over- or underexpressed, GIDE slows or accelerates cell growth, respectively. These pro-apoptotic or growth inhibition effects of GIDE may account for its absence in tumor cells.
Similar content being viewed by others
Introduction
Apoptosis is required for normal development in all metazoans. Abnormal regulation of apoptosis leads to various diseases, such as cancer and autoimmune and neurodegenerative diseases. Apoptosis is mediated by activation of caspases, which cleave various cellular substrates and lead to characteristic apoptotic morphological changes 1, 2, 3. Caspases are synthesized as cytoplasmic zymogen precursors and are activated in a hierarchical pattern in which downstream caspases are activated by upstream caspases 1, 2, 3. The upstream caspases are activated through distinct mechanisms. For example, it has been proposed that the death receptor associated caspase-8 is activated by so-called “induced proximity” 4. In most cases, however, activation of the initial caspases involves mitochondria. Upon stimulation by various death signals, the outer membrane of the mitochondrion is permeabilized, resulting in the release of molecules, including cytochrome c, Smac/DIABLO, Omi/HtrA and GSPT1/eRF3, to the cytosol. Released cytochrome c causes cell death by binding to Apaf-1, an event that leads to recruitment and activation of pro-caspase-9 5. Unlike cytochrome c, mitochondrial-released Smac/DIABLO, Omi/HtrA2 and GSPT1/eRF3 induce caspase activation and apoptosis through their interactions with proteins of the IAP family 6, 7, 8.
IAP proteins are universally present in organisms from yeast to humans. All IAPs contain at least one baculovirus IAP repeat (BIR) domain and most also contain a C-terminal RING finger domain, which has E3 ubiquitin ligase activity. Although some IAP proteins have other activities, most IAPs inhibit apoptosis 9. Among the human IAPs, XIAP is the most extensively studied and the most potent inhibitor of apoptosis. XIAP binds to active caspase-9 with high affinity, and with lower affinity to caspase-3 and -7. Binding of XIAP to active caspases prevents their cleavage of cellular substrates, and thus inhibits apoptosis 10. XIAP may also target active caspase-3 for ubiquitination and degradation by the proteasome, thereby inhibiting apoptosis 11. Binding of mitochondrial-released Smac/Diablo, Omi/HtrA2 and GSPT1/eRF3 to IAPs causes release of active caspases from inhibitory IAPs and/or inhibition of ubiquitination and degradation of caspases, and therefore promotes apoptosis 9. Recently, it has been shown that XIAP and the baculovirus Op-IAP can ubiquitinate Smac/Diablo and target it for degradation by the proteasome, suggesting that IAPs can also inhibit apoptosis through degradation of their antagonists, rather than by directly inhibiting caspases 12. Whatever the mechanisms, the balance between mitochondrial-released death factors and cytosolic IAPs appears to be critically involved in the modulation of apoptosis.
Many extrinsic and intrinsic signals can trigger apoptosis. For example, JNK, a mitogen-activated protein kinase (MAPK) that is activated by stimuli that cause cell stress, such as DNA damaging reagents, UV light and proinflammatory cytokines, is pro-apoptotic under some circumstances in some cell types 13, 14. In Drosophila, the JNK ortholog, DJNK, is required for apoptosis during embryonic patterning of the wing, eye and gut 15. DJNK phosphorylates DJun, which promotes transcription of Hid and Rpr, two proteins that bind to the Drosophila IAP ortholog DIAP1 and prevent DIAP1 from inhibiting the Drosophila caspase, DRONC, thus triggering apoptosis 16.
In mammals, there is evidence for both pro-apoptotic and anti-apoptotic roles of JNK. JNK activity is required for apoptosis of PC12 neuronal cells induced by NGF withdrawal, of MEFs in response to many stimuli and of thymocytes in response to T cell receptor ligation Full size image