Abstract
Background:
There is an acute need to uncover biomarkers that reflect the molecular pathologies, underpinning prostate cancer progression and poor patient outcome. We have previously demonstrated that in prostate cancer cell lines PDE4D7 is downregulated in advanced cases of the disease. To investigate further the prognostic power of PDE4D7 expression during prostate cancer progression and assess how downregulation of this PDE isoform may affect disease outcome, we have examined PDE4D7 expression in physiologically relevant primary human samples.
Methods:
About 1405 patient samples across 8 publically available qPCR, Affymetrix Exon 1.0 ST arrays and RNA sequencing data sets were screened for PDE4D7 expression. The TMPRSS2-ERG gene rearrangement status of patient samples was determined by transformation of the exon array and RNA seq expression data to robust z-scores followed by the application of a threshold >3 to define a positive TMPRSS2-ERG gene fusion event in a tumour sample.
Results:
We demonstrate that PDE4D7 expression positively correlates with primary tumour development. We also show a positive association with the highly prostate cancer-specific gene rearrangement between TMPRSS2 and the ETS transcription factor family member ERG. In addition, we find that in primary TMPRSS2-ERG-positive tumours PDE4D7 expression is significantly positively correlated with low-grade disease and a reduced likelihood of progression after primary treatment. Conversely, PDE4D7 transcript levels become significantly decreased in castration resistant prostate cancer (CRPC).
Conclusions:
We further characterise and add physiological relevance to PDE4D7 as a novel marker that is associated with the development and progression of prostate tumours. We propose that the assessment of PDE4D7 levels may provide a novel, independent predictor of post-surgical disease progression.
Similar content being viewed by others
Main
Prostate cancer is the most commonly occurring non-skin malignancy in men, with an estimated 900 000 new cases diagnosed world-wide in 2013 (Ferlay et al, 2015). However, reactive clinical intervention after routine diagnosis often leads to significant over-treatment of non-aggressive tumours. This has severe negative impacts on both patient quality of life and the medical resources of healthcare institutions (Andriole et al, 2009; Schröder et al, 2009). Therefore, the characterisation of new biomarkers and methods of clinical assessment is of significant importance when assessing the need for different forms of clinical intervention.
Previous studies have shown that signalling pathways mediated by the second messenger cAMP have various roles in the development and progression of prostate cancer (Merkle and Hoffmann, 2011). Cyclic nucleotide phosphodiesterases (PDEs) (Conti and Beavo, 2007; Maurice et al, 2014) provide the sole means of degrading cAMP and cGMP in cells, and are pivotally placed to regulate cAMP signalling by virtue of their intracellular location and post-translational modification (Lugnier, 2006; Houslay, 2010). Each of the 11 PDE genes encode for a series of isoform variants, thereby greatly increasing the diversity of unique regulatory mechanisms, intracellular targeting and kinetic properties, which define functionally independent and unique signalling roles within the cell (Houslay et al, 2007; Houslay, 2010; Francis et al, 2011). This diversity underpins a paradigm of compartmentalised, temporally gated cyclic nucleotide signalling. Due to the complexity of these orchestrated signalling events, any change in PDE isoform expression or regulation can functionally contribute to disease onset (Lee et al, 2012; Michot et al, 2012; Apuhan et al, 2013; Kaname et al, 2014; Yoon et al, 2014). The molecular characterisation of these changes can be expected to provide means for the development of novel therapeutics and diagnostics (Houslay et al, 2005; Houslay, 2010).
Members of the PDE4D subfamily have been implicated as underpinning the molecular pathology of various diseases including prostate cancer (Rahrmann et al, 2009; Henderson et al, 2014), stroke (Gretarsdottir et al, 2003), acrodysostosis (Kaname et al, 2014) and COPD (Yoon et al, 2014). The PDE4D gene encodes a cohort of isoforms that are classified as long, short and super-short. Long isoforms possess two conserved regulatory domains, called UCR1 and UCR2, which allow long isoforms to be phosphorylated and activated by PKA (3′,5′ cAMP-dependent protein kinase) after cAMP elevation in cells (Hoffmann et al, 1998), as well as being functionally regulated through phosphorylation by activated forms of ERK, MK2 and AMPK (MacKenzie et al, 2011; Sheppard et al, 2014).
PDE4D7 is a long isoform member of this subfamily (Li et al, 2010).
Positive TMPRSS2-ERG fusion status was estimated in general by transformation to robust z-scores. Positive TMPRSS2-ERG fusion status was estimated by transformation to robust z-scores, utilising robust statistical measures, namely median and median absolute deviation, to replace mean and s.d., which are sensitive to outliers. Thus, log2-transformed expression values were converted by z-score=(expression−median(expression))/(MAD(expression)), and a threshold of >3 was applied to define samples with positive fusion events. Subsequently, a threshold of >3 was applied to define samples with positive fusion events. For the Erho et al (2013) data set, we applied a supervised clustering algorithm (Partitioning Around Medoids) to assign prostate cancer samples in one of the two clusters (high ERG or low ERG) based on the log2-transformed expression values of ERG. High ERG expression was subsequently assumed as representative for the presence of a positive TMPRSS2-ERG fusion event.
To assess whether any evidence of ERG binding in the genomic region of PDE4D could be observed, we utilised public ChIP-seq data (GSE14092) from the VCaP prostate cancer cell line after liftOver (https://genome.ucsc.edu/cgi-bin/hgLiftOver) to hg19 and found 43 peaks overlap** PDE4D when including 50-kb flanking regions. One of these peaks overlapped the PDE4D7 promoter region, while another was located in close proximity (<200 bases distance), which may hint towards an involvement of ERG binding in regulation of PDE4D7 expression.
Statistical data analysis
For ROC analysis, calculation of AUC under the ROC, ROC P-values and Box-and-Whisker plots the statistical software package MedCalc (MedCalc Software BVBA, Ostend, Belgium) was used. P-values for differences of mean expression were calculated by using Wilcoxon–Mann–Whitney testing unless mentioned otherwise.
Kaplan–Meier Survival curves have been generated by the medical statistical software package MedCalc based on the time to event for those patients who experienced the respective event (e.g., biochemical recurrence (BCR) or clinical recurrence (CR) of disease after surgery) and for those patients who did not suffer from the event at the time of follow-up (censored data). Further, to segregate the analysed patient cohort into two survival groups we determined a cut-off of PDE4D7 expression from a ROC curve analysis. The respective cut-off was objectively determined from the ROC curve at the unique point in the curve, where the sum of sensitivity and specificity reached a maximum.
Results
We have recently provided evidence, suggesting that PDE4D7 may play an important role in regulating cAMP signalling during prostate cancer progression (Henderson et al, 2014). To further explore this finding, we have evaluated the expression of PDE4D7 in a total of eight clinically relevant patient data sets. These data sets comprised a total of 1405 patient samples stratified into 8 sample categories listed in Figure 1A. Three different technology platforms were also leveraged to ensure reproducibility and significance of the gene expression data for PDE4D7, namely: (1) qPCR; (2) Affymetrix Human Exon Array 1.0 ST; (3) RNA seq (see Supplementary Table 1). More details of the data sets used within this study can be found in Supplementary Tables 1 and 2.
PDE4D7 expression correlates with primary localised prostate tumours and is significantly downregulated in CRPC
Our previous investigation in cell lines and xenograft material found that PDE4D7 was differentially expressed between androgen sensitive/responsive and CRPC cells (Henderson et al, 2014). To assess if this finding is physiologically relevant, we thought it prudent to examine PDE4D7 transcript expression in primary patient samples. We selected three prostate cancer exon array data sets (Figure 3. It is significant that in TMPRSS2-ERG-positive tumour samples the expression of PDE4D7 is negatively correlated with increasing pGleason, highlighting the transient nature of PDE4D7 upregulation. This finding bears a striking resemblance to our previous observations in cell lines and xenografts (Henderson et al, 2014).
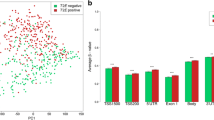
Correlation of PDE4D7 expression to pathology gleason score. (A and B) Box-and-Whisker plots of the normalised PDE4D7 transcript expression in various prostate cancer tissues. For all data sets, and all P-values see Supplementary Table 8 (data sets Figure 4C). We concluded from this that low PDE4D7 expression values in patient samples with low pGleason scores (6 and 7) are associated with an increased likelihood of biochemical failure after primary intervention.
A graphical representation of PDE4D7 expression in various cell and tissue types including AR negative/AR positive cell lines and xenografts, primary prostate cancer with and without progression to biochemical or CR, metastases and CRPC is shown in Figure 5A (cell lines and xenograft samples) and Figure 5B (patient samples; Supplementary Table 4). The samples are ordered based on their normalised PDE4D7 expression. For the cell lines, xenografts, primary tumours without progression and primary tumours with progression to BCR or CR, as well as CRPC tumours, the status of the TMPRSS2-ERG rearrangement is indicated. In general, the more aggressive type of samples are represented by low expression levels of PDE4D7, while less aggressive samples show elevated PDE4D7 expression. It is evident from the depicted cell lines and xenografts that the expression level of PDE4D7 is largely influenced by its TMPRSS2-ERG rearrangement status rather than its AR expression status, where AR positive cell lines without gene fusion show low PDE4D7 expression, while cell lines of the same category but positive gene translocation demonstrate high PDE4D7 expression levels (Figure 5A). It is also of importance to note that this effect seems to be very specific to the ERG translocation as cell lines or xenograft samples with ETV1 or ETV4 translocations do not show elevated PDE4D7 transcription (Figure 5A). Also, looking at the samples collected from patients without disease progression during follow-up reveals that those samples that were positively tested for TMPRSS2-ERG in general show increased expression of PDE4D7 (Figure 5B). This was also the case for primary tumour samples where patients progressed to either biochemical or CR as well as for CRPC. We further annotated for patients who experienced a biochemical relapse the time to PSA recurrence as two categories—relapse <24 months vs relapse >24 months after primary treatment. We observed a clear association between an increased PDE4D7 expression level and an elevated time to recurrence (P=1.72E−02; eight out of nine patients with normalised PDE4D7 expression >0 had a BCR recurrence event >24 months after primary therapy; Figure 5B). Furthermore, we noticed that from eight patients with clinical disease recurrence during follow-up seven patients showed normalised PDE4D7 expression values <0 (Figure 5B) while only in one patient tissue we could measure PDE4D7 expression values >0 (Figure 5B).
Correlation of PDE4D7 expression in cancerous human prostate tissues to patient outcome. A range of prostate Cancer Cell lines, xenografts, as well as patient prostate cancer tissues (data Rahrmann et al, 2009; Henderson et al, 2014). Specifically, we reported for the first time the downregulation of PDE4D7 in hormone-refractory prostate disease represented by a wide range of both cellular and xenograft models (Henderson et al, 2014). Here, we set out to discern whether the differential regulation of PDE4D7 could be verified in human tissue samples collected from primary, as well as metastatic and castration resistant tumours. Encouragingly, across multiple data sets we were able to detect a clear and significant downregulation of PDE4D7 transcript abundance correlating with increasing prostate disease aggressiveness (as assessed by increasing pGleason score and disease stage).
We previously demonstrated that selective knockdown of PDE4D7 expression in androgen-sensitive cell line models led to a more aggressive phenotype, while its overexpression in CRPC cells had the opposite effect (Henderson et al, 2014). The precise details of the cAMP signalling pathways regulated by PDE4D7 during the development of aggressive prostate cancer remain to be uncovered and are subject to future research. However, we would like to propose that PDE4D7 has a contributing role in initial prostate cancer cell states rather than having a ‘passenger effect’ occurring as a consequence of the molecular changes induced by other factors. To understand the baseline for PDE4D7 expression, and thereby contextualise the differential regulation of this particular PDE isoform during prostate cancer development and progression, we examined its expression status in normal prostate tissue compared with primary and advanced prostate cancers. Notably, the expression of the PDE4D7 transcript was significantly lower in normal, as well as tissue of benign origin compared with low-grade prostate tumours. This leads us to propose a model, where PDE4D7 expression becomes upregulated in primary disease. This, perhaps, reflects an attempt by cells to counteract the proliferative phenotype, before the failure/overcoming of this response leads to PDE4D7 downregulation, which characterises the more aggressive prostate tumours. Thus PDE4D7 appears to be functionally involved in the primary development of prostatic tumours. However, our data suggests that future cellular and molecular studies could usefully be directed to ascertain whether the initial upregulation of PDE4D7 is intimately involved in the initial stage of prostate tumorigenesis.
Interestingly, we uncover here a novel link between AR signalling and PDE4D7 expression by correlating the incidence of TMPRSS2-ERG gene fusion and PDE4D7 transcript levels. The TMPRSS2-ERG gene fusion between the prostate specific serine protease TMPRSS2 and the ETS transcription factor family member ERG was first detected in 2005 by a statistical outlier approach (Tomlins et al, 2005). Subsequently, this gene fusion has been shown to be present in ∼50% of prostate cancer patients and is, consequently, one of the most prominent genomic fusion events reported in prostate cancer (Kumar-Sinha et al, 2008). This translocation results in androgen-regulated ERG expression such that the androgen-responsive promoter of TMPRSS2 now drives TMPRSS2-ERG expression, resulting in an upregulation in both the expression and activity of the transcription factor, ERG (Tomlins et al, 2005). However, despite numerous studies the clinical implications and functional consequences of the genomic fusion remain to be fully understood (Petrovics et al, 2005; Mosquera et al, 2007; Saramäki et al, 2008; Hermans et al, 2009). Here, we uncover a remarkably significant difference in PDE4D7 expression between TMPRSS2-ERG-negative and TMPRSS2-ERG-positive tumour samples. Indeed, when stratified by TMPRSS2-ERG incidence it is clear that PDE4D7 is most significantly upregulated in low-grade TMPRSS2-ERG-positive tumours. This raises the possibility that PDE4D7 expression may be directly or indirectly regulated by the aberrant transcriptional activity of the TMPRSS2-ERG fusion protein. Inspection of the PDE4D gene reveals several putative binding sites for ERG, one within the promoter region of PDE4D7 (Materials and Methods). It would therefore seem logical that if PDE4D7 is regulated by ERG transcription, an increase in the expression of the androgen-regulated TMPRSS2-ERG factor would lead to a concurrent androgen-driven increase in PDE4D7 expression.
To date, most newly detected prostate cancer cases are clinically classified low-risk diseases (Bangma and Roobol, 2012). It is crucial to understand the natural history of these tumours as it is under considerable debate whether and to what extent low-risk Gleason 6 tumours are able to progress to higher grade tumours leading to metastatic spread or even cancer-specific death (Whittemore et al, 1991; Sowalsky et al, 2013). Interestingly, our data may indicate that reduced expression of PDE4D7 in low to intermediate Gleason tumours is correlated to progression after primary treatment. Although initially positively correlated with tumour development, the expression of PDE4D7 actually appears to be protective against further disease progression, which is in line with the data previously obtained regarding the cellular functioning of PDE4D7 (Henderson et al, 2014).
As new strategies for targeted pharmacological manipulation of specific PDE4D transcripts become available then PDE4D7 likely provides a promising future target in the treatment of primary and/or advanced prostate cancer. Our data indicate that during tumour progression the risk of fast recurrence to clinical endpoints like biochemical or clinical disease is correlated to the level of PDE4D7 expression in the primary tumour. Consequently, patients with a low expression level of PDE4D7 in their primary cancers after surgical resection may very well be candidates for immediate adjuvant treatment like radiotherapy and/or androgen ablation. Furthermore, the manipulation of PDE4D7 suggests a strategy to selectively treat TMPRSS2-ERG fusion-positive prostate cancers. However, the success of such strategy may depend on the stratification into molecular sub-types according to the status of the TMPRSS2-ERG gene translocation.
The data presented here demonstrates the relevance of PDE4D7 as a potential biomarker for more accurate prostate cancer diagnostics. In particular, we have demonstrated the potential role of this specific splice variant of the PDE4D gene for prognosis of aggressive prostate cancer in the molecular sub-type of TMPRSS2-ERG-positive prostate tumours as well as its role as a putative target gene for therapy of primary vs late-stage, hormone-refractory disease.
References
Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD (2009) Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 360 (13): 1310–1319.
Apuhan T, Gepdiremen S, Arslan AO, Aktas G (2013) Evaluation of patients with nasal polyps about the possible association of desmosomal junctions, RORA and PDE4D gene. Eur Rev Med Pharmacol Sci 17 (19): 2680–2683.
Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, Park K, Kitabayashi N, MacDonald TY, Ghandi M, Van Allen E, Kryukov GV, Sboner A, Theurillat J-P, Soong TD, Nickerson E, Auclair D, Tewari A, Beltran H, Onofrio RC, Boysen G, Guiducci C, Barbieri CE, Cibulskis K, Sivachenko A, Carter SL, Saksena G, Voet D, Ramos AH, Winckler W, Cipicchio M, Ardlie K, Kantoff PW, Berger MF, Gabriel SB, Golub TR, Meyerson M, Lander ES, Elemento O, Getz G, Demichelis F, Rubin MA, Garraway LA (2013) Punctuated evolution of prostate cancer genomes. Cell 153 (3): 666–677.
Baillie GS, MacKenzie SJ, McPhee I, Houslay MD (2000) Sub-family selective actions in the ability of Erk2 MAP kinase to phosphorylate and regulate the activity of PDE4 cyclic AMP-specific phosphodiesterases. Br J Pharmacol 131 (4): 811–819.
Bangma CH, Roobol MJ (2012) Defining and predicting indolent and low risk prostate cancer. Crit Rev Oncol 83 (2): 235–241.
Böttcher R, Hoogland AM, Dits N, Verhoef EI, Kweldam C, Waranecki P, Bangma CH, van Leenders GJ, Jenster G (2015) Novel long non-coding RNAs are specific diagnostic and prognostic markers for prostate cancer. Oncotarget 6 (6): 4036–4050.
Boormans JL, Korsten H, Ziel-van der Made AJC, van Leenders GJLH, de Vos CV, Jenster G, Trapman J (2013) Identification of TDRD1 as a direct target gene of ERG in primary prostate cancer. Int J Cancer 133 (2): 335–345.
Brase J, Johannes M, Mannsperger H, Falth M, Metzger J, Kacprzyk L, Andrasiuk T, Gade S, Meister M, Sirma H, Sauter G, Simon R, Schlomm T, BeiSZbarth T, Korf U, Kuner R, Sultmann H (2011) TMPRSS2-ERG -specific transcriptional modulation is associated with prostate cancer biomarkers and TGF-beta signaling. BMC Cancer 11 (1): 507.
Byrne AM, Elliott C, Hoffmann R, Baillie GS (2015) The activity of cAMP-phosphodiesterase 4D7 (PDE4D7) is regulated by protein kinase A-dependent phosphorylation within its unique N-terminus. FEBS Lett 589 (6): 750–755.
Conti M, Beavo J (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76 (1): 481–511.
Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C, Bergstralh EJ, Kollmeyer T, Fink S, Haddad Z, Zimmermann B, Sierocinski T, Ballman KV, Triche TJ, Black PC, Karnes RJ, Klee G, Davicioni E, Jenkins RB (2013) Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS One 8 (6): e66855.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136 (5): E359–E386.
Francis SH, Blount MA, Corbin JD (2011) Mammalian cyclic nucleotide phosphodiesterases. Physiol Rev 91 (5): 651–690.
Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, Jonsdottir T, Gudmundsdottir T, Bjarnadottir SM, Einarsson OB, Gudjonsdottir HM, Hawkins M, Gudmundsson G, Gudmundsdottir H, Andrason H, Gudmundsdottir AS, Sigurdardottir M, Chou TT, Nahmias J, Goss S, Sveinbjornsdottir S, Valdimarsson EM, Jakobsson F, Agnarsson U, Gudnason V, Thorgeirsson G, Fingerle J, Gurney M, Gudbjartsson D, Frigge ML, Kong A, Stefansson K, Gulcher JR (2003) The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet 35 (2): 131–138.
Henderson DJP, Byrne A, Dulla K, Jenster G, Hoffmann R, Baillie GS, Houslay MD (2014) The cAMP phosphodiesterase-4D7 (PDE4D7) is downregulated in androgen-independent prostate cancer cells and mediates proliferation by compartmentalising cAMP at the plasma membrane of VCaP prostate cancer cells. Br J Cancer 110 (5): 1278–1287.
Hermans KG, Boormans JL, Gasi D, van Leenders GJHL, Jenster G, Verhagen PCMS, Trapman J (2009) Overexpression of prostate-specific TMPRSS2(exon 0)-ERG fusion transcripts corresponds with favorable prognosis of prostate cancer. Clin Cancer Res 15 (20): 6398–6403.
Hoffmann R, Baillie GS, MacKenzie SJ, Yarwood SJ, Houslay MD (1999) The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. EMBO J 18 (20): 893–903.
Hoffmann R, Wilkinson IR, McCallum JF, Engels P, Houslay MD (1998) cAMP-specific phosphodiesterase HSPDE4D3 mutants which mimic activation and changes in rolipram inhibition triggered by protein kinase A phosphorylation of Ser-54: generation of a molecular model. Biochem J 333 (Pt 1): 139–149.
Houslay MD (2010) Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci 35 (2): 91–100.
Houslay MD, Baillie GS, Maurice DH (2007) cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res 100 (7): 950–966.
Houslay MD, Schafer P, Zhang KYJ (2005) Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today 10 (22): 1503–1519.
Kaname T, Ki C-S, Niikawa N, Baillie GS, Day JP, Yamamura K-i, Ohta T, Nishimura G, Mastuura N, Kim O-H, Sohn YB, Kim HW, Cho SY, Ko A-R, Lee JY, Kim HW, Ryu SH, Rhee H, Yang K-S, Joo K, Lee J, Kim CH, Cho K-H, Kim D, Yanagi K, Naritomi K, Yoshiura K-i, Kondoh T, Nii E, Tonoki H, Houslay MD, ** D-K (2014) Heterozygous mutations in cyclic AMP phosphodiesterase-4D (PDE4D) and protein kinase A (PKA) provide new insights into the molecular pathology of acrodysostosis. Cell Signal 26 (11): 2446–2459.
Kumar-Sinha C, Tomlins SA, Chinnaiyan AM (2008) Recurrent gene fusions in prostate cancer. Nat Rev Cancer 8 (7): 497–511.
Lee H, Graham John M, Rimoin David L, Lachman Ralph S, Krejci P, Tompson Stuart W, Nelson Stanley F, Krakow D, Cohn Daniel H (2012) Exome sequencing identifies PDE4D mutations in acrodysostosis. Am J Hum Genet 90 (4): 746–751.
Li B, Ruotti V, Stewart RM, Thomson JA, Dewey CN (2010) RNA-Seq gene expression estimation with read map** uncertainty. Bioinformatics 26 (4): 493–500.
Lugnier C (2006) Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Therap 109 (3): 366–398.
MacKenzie KF, Wallace DA, Hill EV, Anthony DF, Henderson DJP, Houslay DM, Arthur JSC, Baillie GS, Houslay MD (2011) Phosphorylation of cAMP-specific PDE4A5 (phosphodiesterase-4A5) by MK2 (MAPKAPK2) attenuates its activation through protein kinase A phosphorylation. Biochem J 435 (Article): 755–769.
Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC (2014) Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov 13 (4): 290–314.
Merkle D, Hoffmann R (2011) Roles of cAMP and cAMP-dependent protein kinase in the progression of prostate cancer: Cross-talk with the androgen receptor. Cell Signal 23 (3): 507–515.
Michot C, Le Goff C, Goldenberg A, Abhyankar A, Klein C, Kinning E, Guerrot A-M, Flahaut P, Duncombe A, Baujat G, Lyonnet S, Thalassinos C, Nitschke P, Casanova J-L, Le Merrer M, Munnich A, Cormier-Daire V (2012) Exome sequencing identifies PDE4D mutations as another cause of acrodysostosis. Am J Hum Genet 90 (4): 740–745.
Mosquera JM, Perner S, Demichelis F, Kim R, Hofer MD, Mertz KD, Paris PL, Simko J, Collins C, Bismar TA, Chinnaiyan AM, Rubin MA (2007) Morphological features of TMPRSS2-ERG gene fusion prostate cancer. J Pathol 212 (1): 91–101.
Petrovics G, Liu A, Shaheduzzaman S, Furusato B, Sun C, Chen Y, Nau M, Ravindranath L, Chen Y, Dobi A, Srikantan V, Sesterhenn IA, McLeod DG, Vahey M, Moul JW, Srivastava S (2005) Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene 24 (23): 3847–3852.
Purdom E, Simpson KM, Robinson MD, Conboy JG, Lapuk AV, Speed TP (2008) FIRMA: a method for detection of alternative splicing from exon array data. Bioinformatics 24 (15): 1707–1714.
Rahrmann EP, Collier LS, Knutson TP, Doyal ME, Kuslak SL, Green LE, Malinowski RL, Roethe L, Akagi K, Waknitz M, Huang W, Largaespada DA, Marker PC (2009) Identification of PDE4D as a proliferation promoting factor in prostate cancer using a Slee** Beauty transposon based somatic mutagenesis screen. Cancer Res 69 (10): 4388–4397.
Saramäki OR, Harjula AE, Martikainen PM, Vessella RL, Tammela TLJ, Visakorpi T (2008) TMPRSS2:ERG fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin Cancer Res 14 (11): 3395–3400.
Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A (2009) Screening and prostate-cancer mortality in a randomized european study. N Engl J Med 360 (13): 1320–1328.
Sheppard CL, Lee LCY, Hill EV, Henderson DJP, Anthony DF, Houslay DM, Yalla KC, Cairns LS, Dunlop AJ, Baillie GS, Huston E, Houslay MD (2014) Mitotic activation of the DISC1-inducible cyclic AMP phosphodiesterase-4D9 (PDE4D9), through multi-site phosphorylation, influences cell cycle progression. Cell Signal 26 (9): 1958–1974.
Sowalsky AG, Ye H, Bubley GJ, Balk SP (2013) Clonal progression of prostate cancers from gleason grade 3 to grade 4. Cancer Res 73 (3): 1050–1055.
Taylor BS, Schultz N, Hieronymus H, Gopalan A, **ao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL (2010) Integrative genomic profiling of human prostate cancer. Cancer Cell 18 (1): 11–22.
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun X-W, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310 (5748): 644–648.
Wang D, Deng C, Bugaj-Gaweda B, Kwan M, Gunwaldsen C, Leonard C, **n X, Hu Y, Unterbeck A, De Vivo M (2003) Cloning and characterization of novel PDE4D isoforms PDE4D6 and PDE4D7. Cell Signal 15 (9): 883–891.
Whittemore AS, Keller JB, Betensky R (1991) Low-grade, latent prostate cancer volume: predictor of clinical cancer incidence? J Natl Cancer Inst 83 (17): 1231–1235.
Yoon H-K, Hu H-J, Rhee C-K, Shin S-H, Oh Y-M, Lee S-D, Jung S-H, Yim S-H, Kim T-M, Chung Y-J (2014) Polymorphisms in PDE4D are associated with a risk of COPD in non-emphysematous koreans. COPD 11 (6): 652–658.
Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, Lawrence MS, Zhang C-Z, Wala J, Mermel CH, Sougnez C, Gabriel SB, Hernandez B, Shen H, Laird PW, Getz G, Meyerson M, Beroukhim R (2013) Pan-cancer patterns of somatic copy number alteration. Nat Genet 45 (10): 1134–1140.
Acknowledgements
This study was supported by the framework of CTMM (The Netherlands), the Center for Translational Molecular Medicine, PCMM project (grant 03O-203). We would like to acknowledge support from the Biotechnology and Biological Sciences Research Council (UK) for award of a CASE studentship to DJPH. BBSRC funding to GSB (BB/G01647X/1). We also like to thank Drs Chris Bangma and Mark Wildhagen for their support regarding patient sample collection and annotation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Böttcher, R., Henderson, D., Dulla, K. et al. Human phosphodiesterase 4D7 (PDE4D7) expression is increased in TMPRSS2-ERG-positive primary prostate cancer and independently adds to a reduced risk of post-surgical disease progression. Br J Cancer 113, 1502–1511 (2015). https://doi.org/10.1038/bjc.2015.335
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.335
- Springer Nature Limited
Keywords
This article is cited by
-
Loss of PDE4D7 expression promotes androgen independence, neuroendocrine differentiation and alterations in DNA repair: implications for therapeutic strategies
British Journal of Cancer (2023)
-
Inhibition of TPL2 by interferon-α suppresses bladder cancer through activation of PDE4D
Journal of Experimental & Clinical Cancer Research (2018)