Abstract
Background:
Tumour biopsy for pharmacodynamic (PD) study is increasingly common in early-phase cancer trials. As they are non-diagnostic, the ethical justification for such procedures rests on their knowledge value. On the premise that knowledge value is related to reporting practices and outcome diversity, we assessed in a sample of recent invasive PD studies within cancer trials.
Methods:
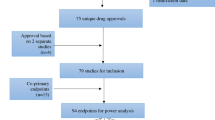
We assessed reporting practices and outcomes for PD studies in a convenience sample of cancer trials published from 2000 to 2010 that employed invasive, non-diagnostic tissue procurement. Extracted data were used to measure outcome reporting in individual trials. Using a reporting scale we developed for exploratory purposes, we tested whether reporting varied with study characteristics, such as funding source or drug novelty.
Results:
Reporting varied widely within and across studies. Some practices were sporadically reported, including results of all planned tests (78% trials reporting), use of blinded histopathological assessment (43% trials reporting), biopsy dimensions (38% trials reporting), and description of patient flow through PD analysis (62%). Pharmacodynamic analysis as a primary end point and mandatory biopsy had statistically significant positive relationships with overall quality of reporting. A preponderance of positive results (61% of the studies described positive PD results) suggests possible publication bias.
Conclusion:
Our results highlight the need for PD-reporting guidelines, and suggest several avenues for improving the risk/benefit for studies involving invasive, non-diagnostic tissue procurement.
Similar content being viewed by others
Main
Biopsy for pharmacodynamic (PD) and biomarker analysis is increasingly common in early-phase cancer trials (Twelves, 2006; Goulart et al, 2007). In principle, PD end points can provide evidence of target effects for a drug, and support decision making for subsequent trials (Workman, 2003; Sarker et al, 2007; Sarker and Workman, 2007; Tan et al, 2009). However, many PD studies require invasive procedures like tumour biopsy.
Studies find that many patients are willing to undergo research biopsy (Seah et al, 2013) and that ethics review committees and oncologists may overestimate patient anxiety associated with biopsies (Agulnik et al, 2006). In one study, overall and major complication rates for tumour biopsies were 5.2% and 0.8%, respectively (Overman et al, 2012). However, the majority of patients describe their biopsies as being painful (Agulnik et al, 2006) and other studies indicate that 10% of patients receiving one common procedure – breast tumour biopsy – report moderate-to-severe pain (a more extended discussion of tumour biopsy risk and burden is available at Brown et al (2008); Hemmer et al (2008); Kimmelman et al (2012)). As biopsies often have no value for subjects in terms of clinical management, their ethical justification rests on an expectation that their performance will be redeemed by the value of the knowledge accrued (Olson et al, 2011).
Given that the burdens of such procedures are well understood, debates concerning their application revolve around conflicting views about the scientific utility of PD evidence. Some commentators question whether research biopsies return sufficient knowledge to justify their risks (Dowlati et al, 2001; Parulekar and Eisenhauer, 2004; This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication Agulnik M, Oza A, Pond G, Siu L (2006) Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. J Clin Oncol 24: 4801–4807. Andre F, McShane LM, Michiels S, Ransohoff DF, Altman DG, Reis-Filho JS, Pusztai L (2011) Biomarker studies: a call for a comprehensive biomarker study registry. Nat Rev Clin Oncol 8 (3): 171–176. Brown A, Wendler D, Camphausen K, Miller F, Citrin D (2008) Performing nondiagnostic research biopsies in irradiated tissue: a review of scientific, clinical, and ethical considerations. J Clin Oncol 26: 3987–3994. Cannistra S (2007) Performance of biopsies in clinical research. J Clin Oncol 25: 1454–1455. Davis DW, Takamori R, Raut CP, **ong HQ, Herbst RS, Stadler WM, McConkey DJ (2005) Pharmacodynamic analysis of target inhibition and endothelial cell death in tumors treated with the vascular endothelial growth factor receptor antagonists SU5416 or SU6668. Clin Cancer Res 11 (2 Pt 1): 678–689. Dowlati A, Haaga J, Remick SC, Spiro TP, Gerson SL, Liu L, Willson JK (2001) Sequential tumor biopsies in early phase clinical trials of anticancer agents for pharmacodynamic evaluation. Clin Cancer Res 7 (10): 2971–2976. Eisenhauer E, Twelves C, Buyse M (2006) Phase 1 Cancer Clinical Trials: A Practical Guide. Oxford University Press: New York, NY, USA. Fleiss JL (1981) Statistical Methods For Rates And Proportions 2nd edn. Wiley: New York, NY, USA. Freeman GA, Kimmelman J (2012) Publication and reporting conduct for pharmacodynamic analyses of tumor tissue in early phase oncology trials. Clin Cancer Res 18: 6478–6484. Goulart BH, Clark JW, Pien HH, Roberts TG, Finkelstein SN, Chabner BA (2007) Trends in the use and role of biomarkers in phase I oncology trials. Clin Cancer Res 13 (22 Pt 1): 6719–6726. Harris AL (2005) REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93 (4): 385–386. Helft P, Daugherty C (2006) Are we taking without giving in return? The ethics of research-related biopsies and the benefits of clinical trial participation. J Clin Oncol 24: 4793–4795. Hemmer JM, Kelder JC, van Heesewijk HP (2008) Stereotactic large-core needle breast biopsy: analysis of pain and discomfort related to the biopsy procedure. Eur Radiol 18 (2): 351–354. How CONSORT began (2008) Available from http://www.consort-statement.org/about-consort/history/how-consort-began/. Huwiler-Muntener K, Juni P, Junker C, Egger M (2002) Quality of reporting of randomized trials as a measure of methodologic quality. JAMA 287 (21): 2801–2804. Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG (2001) Replication validity of genetic association studies. Nat Genet 29 (3): 306–309. Kelloff GJ, Sigman CC (2005) New science-based endpoints to accelerate oncology drug development. (Review). Eur J Cancer 41 (4): 491–501. Kimmelman J, Lemmens T, Kim SY (2012) Analysis of consent validity for invasive, nondiagnostic research procedures. (Research Support, Non-U.S. Gov′t). IRB 34 (5): 1–7. Kyzas PA, Denaxa-Kyza D, Ioannidis JPA (2007) Almost all articles on cancer prognostic markers report statistically significant results. Eur J Cancer 43 (17): 2559–2579. Kyzas PA, Loizou KT, Ioannidis JPA (2005) Selective reporting biases in cancer prognostic factor studies. J Natl Cancer Inst 97 (14): 1043–1055. Lai R, Chu R, Fraumeni M, Thabane L (2006) Quality of randomized controlled trials reporting in the primary treatment of brain tumors. J Clin Oncol 24 (7): 1136–1144. McDonald J. H (2009) Handbook of Biological Statistics 2nd edn. Sparky House Publishing: Baltimore, MD, USA. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 97 (16): 1180–1184. Olson EM, Lin NU, Krop IE, Winer EP (2011) The ethical use of mandatory research biopsies. Nat Rev Clin Oncol 8 (10): 620–625. Overman MJ, Modak J, Kopetz S, Murthy R, Yao JC, Hicks ME, Tam AL (2012) Use of research biopsies in clinical trials: are risks and benefits adequately discussed? J Clin Oncol 31 (1): 17–22. Parulekar WR, Eisenhauer EA (2004) Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst 96 (13): 990–997. Peppercorn J, Shapira I, Collyar D, Deshields T, Lin N, Krop I, Bertagnolli MM (2010) Ethics of mandatory research biopsy for correlative end points within clinical trials in oncology. J Clin Oncol 28 (15): 2635–2640. Ratain MJ, Glassman RH (2007) Biomarkers in phase I oncology trials: signal, noise, or expensive distraction? Clin Cancer Res 13 (22 Pt 1): 6545–6548. Rios LP, Odueyungbo A, Moitri MO, Rahman MO, Thabane L (2008) Quality of reporting of randomized controlled trials in general endocrinology literature. J Clin Endocrinol Metab 93 (10): 3810–3816. Sarker D, Pacey S, Workman P (2007) Use of pharmacokinetic/pharmacodynamic biomarkers to support rational cancer drug development. Biomark Med 1 (3): 399–417. Sarker D, Workman P (2007) Pharmacodynamic biomarkers for molecular cancer therapeutics. Adv Cancer Res 96: 213–268. Seah DS, Scott SM, Najita J, Openshaw T, Krag K, Frank E, Sohl J, Stadler ZK, Garrett M, Silverman SG, Peppercorn J, Winer EP, Come SE, Lin NU (2013) Attitudes of patients with metastatic breast cancer toward research biopsies. Ann Oncol 24 (7): 1853–1859. Tan DS, Thomas GV, Garrett MD, Banerji U, De Bono JS, Kaye SB, Workman P (2009) Biomarker-driven early clinical trials in oncology: a paradigm shift in drug development. Cancer J 15 (5): 406–420. Toulmonde M, Bellera C, Mathoulin-Pelissier S, Debled M, Bui B, Italiano A (2011) Quality of randomized controlled trials reporting in the treatment of sarcomas. J Clin Oncol 29 (9): 1204–1209. Twelves C (2006) Practical aspects of pharmacokinetics and pharmacodynamics. In Eisenhauer EA, Twelves C, Buyse M (eds) Phase 1 Cancer Clinical Trials: A Practical Guide pp 209–243. Oxford University Press: New York, NY, USA. Weijer C, Miller PB (2004) When are research risks reasonable in relation to anticipated benefits? Nat Med 10 (6): 570–573. Workman P (2003) How much gets there and what does it do?: the need for better pharmacokinetic and pharmacodynamic endpoints in contemporary drug discovery and development. Curr Pharm Des 9 (11): 891–902. This work was funded by the Canadian Institutes of Health Research (EOG 102824). The authors declare no conflict of interest. This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. Adjei AA, RB Cohen, WFranklin, CMorris, DWilson, JRMolina, LJHanson, L Gore, L Chow, S Leong, L Maloney, G Gordon, H Simmons, A Marlow, K Litwiler, S Brown, G Poch, K Kane, J Haney, SG Eckhardt (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor Azd6244 (Arry-142886) in patients with advanced cancers. J Clin Oncol 26(13): 2139–2146. Agulnik M, EW Cohen, RB Cohen, EX Chen, EE Vokes, SJ Hotte, EWinquist, S Laurie, DN Hayes, JE Dancey, S Brown, GR Pond, I Lorimer, M Daneshmand, J Ho, MS Tsao, LL Siu (2007) Phase Ii study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or Erbb2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol 25(25): 3978–3984. Agulnik M, G da Cunha Santos, D Hedley, T Nicklee, PP Dos Reis, J Ho, GR Pond, H Chen, S Chen, Y Shyr, E Winquist, D Soulieres, EX Chen, JA Squire, P Marrano, S Kamel-Reid, J Dancey, LL Siu, MS Tsao (2007) Predictive and pharmacodynamic biomarker studies in tumor and skin tissue samples of patients with recurrent or metastatic squamous cell carcinoma of the head and neck treated with erlotinib. J Clin Oncol 25(16): 2184–2190. Albanell J, F Rojo, S Averbuch, A Feyereislova, JM Mascaro, R Herbst, P LoRusso, D Rischin, S Sauleda, J Gee, RI Nicholson, J Baselga (2002) Pharmacodynamic studies of the epidermal growth factor receptor inhibitor zd1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 20(1): 110–124. Annunziata CM, AJ Walker, L Minasian, M Yu, H Kotz, BJ Wood, K Calvo, P Choyke, D Kimm, SM Steinberg, EC Kohn (2010) Vandetanib, designed to inhibit Vegfr2 and Egfr signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of Vegfr2. Clin Cancer Res 16(2): 664–672. Badros A, AM Burger, S Philip, R Niesvizky, SS Kolla, O Goloubeva, C Harris, J Zwiebel, JJ Wright, I Espinoza-Delgado, MR Baer, JL Holleran, MJ Egorin, S Grant (2009) Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res 15(16): 5250–5257. Baselga J, J Albanell, A Ruiz, A Lluch, P Gascon, V Guillem, S Gonzalez, S Sauleda, I Marimon, JM Tabernero, MT Koehler, F Rojo (2005) Phase Ii and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol 23(23): 5323–5333. Baselga J, D Rischin, M Ranson, H Calvert, E Raymond, DG Kieback, SB Kaye, L Gianni, A Harris, T Bjork, SD Averbuch, A Feyereislova, H Swaisland, F Rojo, J Albanell (2002) Phase I safety, pharmacokinetic, and pharmacodynamic trial of zd1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol 20(21): 4292–4302. Baselga J, V Semiglazov, P van Dam, A Manikhas, M Bellet, J Mayordomo, M Campone, E Kubista, R Greil, G Bianchi, J Steinseifer, B Molloy, E Tokaji, H Gardner, P Phillips, M Stumm, HA Lane, JM Dixon, W Jonat, HS Rugo (2009) Phase Ii randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27(16): 2630–2637. Borghaei H, K Alpaugh, G Hedlund, G Forsberg, C Langer, A Rogatko, R Hawkins, S Dueland, U Lassen, RB Cohen (2009) Phase I dose escalation, pharmacokinetic and pharmacodynamic study of naptumomab estafenatox alone in patients with advanced cancer and with docetaxel in patients with advanced non-small-cell lung cancer. J Clin Oncol 27(25): 4116–4123. Boss DS, GK Schwartz, MR Middleton, DD Amakye, H Swaisland, RS Midgley, M Ranson, S Danson, H Calvert, R Plummer, C Morris, RD Carvajal, LR Chirieac, JH Schellens, GI Shapiro (2010) Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral cyclin-dependent kinase inhibitor azd5438 when administered at intermittent and continuous dosing schedules in patients with advanced solid tumours. Ann Oncol 21(4): 884–894. Busam KJ, P Capodieci, R Motzer, T Kiehn, D Phelan, AC Halpern (2001) Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol 144(6): 1169–1176. Byrd JC, G Marcucci, MR Parthun, JJ **ao, RB Klisovic, M Moran, TS Lin, S Liu, AR Sklenar, ME Davis, DM Lucas, B Fischer, R Shank, SL Tejaswi, P Binkley, J Wright, KK Chan, MR Grever (2005) A phase 1 and pharmacodynamic study of depsipeptide (Fk228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood 105(3): 959–967. Camidge DR, MN Pemberton, JW Growcott, D Johnstone, PJ Laud, JR Foster, KJ Randall, AM Hughes (2005) Assessing proliferation, cell-cycle arrest and apoptotic end points in human buccal punch biopsies for use as pharmacodynamic biomarkers in drug development. Br J Cancer 93(2): 208–215. Decensi A, R Torrisi, S Bruno, M Costantini, A Curotto, G Nicolo, B Malcangi, L Baglietto, GP Bruttini, B Gatteschi, G Rondanina, M Varaldo, M Perloff, WF Malone, P Bruzzi (2000) Randomized trial of fenretinide in superficial bladder cancer using DNA flow cytometry as an intermediate end point. Cancer Epidemiol Biomarkers Prev 9(10): 1071–1078. Dowlati A, K Robertson, T Radivoyevitch, J Waas, NP Ziats, P Hartman, FW Abdul-Karim, JK Wasman, J Jesberger, J Lewin, K McCrae, P Ivy, SC Remick (2005) Novel phase i dose de-escalation design trial to determine the biological modulatory dose of the antiangiogenic agent Su5416. Clin Cancer Res 11(21): 7938–7944. Duran I, J Kortmansky, D Singh, H Hirte, W Kocha, G Goss, L Le, A Oza, T Nicklee, J Ho, D Birle, GR Pond, D Arboine, J Dancey, S Aviel-Ronen, MS Tsao, D Hedley, LL Siu (2006) A phase Ii clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer 95(9): 1148–1154. Dy GK, JP Thomas, G Wilding, L Bruzek, S Mandrekar, C Erlichman, D Alberti, K Binger, HC Pitot, SR Alberts, LJ Hanson, R Marnocha, K Tutsch, SH Kaufmann, AA Adjei (2005) A phase I and pharmacologic trial of two schedules of the proteasome inhibitor, Ps-341 (Bortezomib, Velcade), in patients with advanced cancer. Clin Cancer Res 11(9): 3410–3416. Eder JP, GI Shapiro, LJ Appleman, AX Zhu, D Miles, H Keer, B Cancilla, F Chu, S Hitchcock-Bryan, L Sherman, S McCallum, EI Heath, SA Boerner, PM LoRusso (2010) A phase I study of foretinib, a multi-targeted inhibitor of C-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res 16(13): 3507–3516. Fakih MG, L Pendyala, G Fetterly, K Toth, JA Zwiebel, I Espinoza-Delgado, A Litwin, YM Rustum, ME Ross, JL Holleran, MJ Egorin (2009) A phase I, pharmacokinetic and pharmacodynamic study on vorinostat in combination with 5-fluorouracil, leucovorin, and oxaliplatin in patients with refractory colorectal cancer. Clin Cancer Res 15(9): 3189–3195. Fakih MG, A Rajput, GY Yang, L Pendyala, K Toth, JL Smith, DD Lawrence, YM Rustum (2006) A phase I study of weekly intravenous oxaliplatin in combination with oral daily capecitabine and radiation therapy in the neoadjuvant treatment of rectal adenocarcinoma. Int J Radiat Oncol Biol Phys 65(5): 1462–1470. Felip E, F Rojo, M Reck, A Heller, B Klughammer, G Sala, S Cedres, S Peralta, H Maacke, D Foernzler, M Parera, J Mocks, C Saura, U Gatzemeier, J Baselga (2008) A phase Ii pharmacodynamic study of erlotinib in patients with advanced non-small cell lung cancer previously treated with platinum-based chemotherapy. Clin Cancer Res 14(12): 3867–3874. Fracasso PM, H Burris 3rd, MA Arquette, R Govindan, F Gao, LP Wright, SA Goodner, FA Greco, SF Jones, N Willcut, C Chodkiewicz, A Pathak, GM Springett, GR Simon, DM Sullivan, R Marcelpoil, SD Mayfield, D Mauro, CR Garrett (2007) A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res 13(3): 986–993. Goss G, H Hirte, WH Miller Jr, IA Lorimer, D Stewart, G Batist, DA Parolin, P Hanna, S Stafford, J Friedmann, W Walsh, S Mathews, L Douglas, LK Seymour (2005) A phase I study of oral Zd 1839 given daily in patients with solid tumors: Ind.122, a study of the investigational new drug program of the national cancer institute of canada clinical trials group.’ Invest New Drugs 23(2): 147–155. Haddad RI, LJ Weinstein, TJ Wieczorek, N Bhattacharya, H Raftopoulos, MW Oster, X Zhang, VM Latham Jr, R Costello, J Faucher, C DeRosa, M Yule, LP Miller, M Loda, MR Posner, GI Shapiro (2004) A phase Ii clinical and pharmacodynamic study of E7070 in patients with metastatic, recurrent, or refractory squamous cell carcinoma of the head and neck: modulation of retinoblastoma protein phosphorylation by a novel chloroindolyl sulfonamide cell cycle inhibitor. Clin Cancer Res 10(14): 4680–4687. Herbst RS, AM Davies, RB Natale, TP Dang, JH Schiller, LL Garland, VA Miller, D Mendelson, AD Van den Abbeele, Y Melenevsky, DJ De Vries, DA Eberhard, B Lyons, SG Lutzker, BE Johnson (2007) Efficacy and safety of single-agent pertuzumab, a human epidermal receptor dimerization inhibitor, in patients with non small cell lung cancer. Clin Cancer Res 13(20): 6175–6181. Jansen B, V Wacheck, E Heere-Ress, H Schlagbauer-Wadl, C Hoeller, T Lucas, M Hoermann, U Hollenstein, K Wolff, H Pehamberger (2000) Chemosensitisation of malignant melanoma by Bcl2 antisense therapy. Lancet 356(9243): 1728–1733. Jatoi A, VJ Suman, P Schaefer, M Block, C Loprinzi, P Roche, S Garneau, R Morton, PJ Stella, SR Alberts, M Pittelkow, J Sloan, R Pagano (2003) A phase Ii study of topical ceramides for cutaneous breast cancer. Breast Cancer Res Treat 80(1): 99–104. Joensuu G, T Joensuu, P Nokisalmi, C Reddy, J Isola, M Ruutu, M Kouri, PA Kupelian, J Collan, S Pesonen, A Hemminki (2010) A phase I/Ii trial of gefitinib given concurrently with radiotherapy in patients with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys 78(1): 42–49. Koon HB, GJ Bubley, L Pantanowitz, D Masiello, B Smith, K Crosby, J Proper, W Weeden, TE Miller, P Chatis, MJ Egorin, SR Tahan, BJ Dezube (2005) Imatinib-induced regression of AIDS-related kaposi’s sarcoma. J Clin Oncol 23(5): 982–989. Kummar S, R Kinders, ME Gutierrez, L Rubinstein, RE Parchment, LR Phillips, J Ji, A Monks, JA Low, A Chen, AJ Murgo, J Collins, SM Steinberg, H Eliopoulos, VL Giranda, G Gordon, L Helman, R Wiltrout, JE Tomaszewski, JH Doroshow (2009) Phase 0 clinical trial of the poly (Adp-ribose) polymerase inhibitor Abt-888 in patients with advanced malignancies. J Clin Oncol 27(16): 2705–2711. Lin J, J Gilbert, MA Rudek, JA Zwiebel, S Gore, A Jiemjit, M Zhao, SD Baker, RF Ambinder, JG Herman, RC Donehower, MA Carducci (2009) A phase I dose-finding study of 5-azacytidine in combination with sodium phenylbutyrate in patients with refractory solid tumors. Clin Cancer Res 15(19): 6241–6249. LoRusso PM, SS Krishnamurthi, JJ Rinehart, LM Nabell, L Malburg, PB Chapman, SE DePrimo, S Bentivegna, KD Wilner, W Tan, AD Ricart (2010) Phase I pharmacokinetic and pharmacodynamic study of the oral Mapk/Erk kinase inhibitor Pd-0325901 in patients with advanced cancers. Clin Cancer Res 16(6): 1924–1937. Mackay H, D Hedley, P Major, C Townsley, M Mackenzie, M Vincent, P Degendorfer, MS Tsao, T Nicklee, D Birle, J Wright, L Siu, M Moore, A Oza (2005) A phase Ii trial with pharmacodynamic endpoints of the proteasome inhibitor bortezomib in patients with metastatic colorectal cancer. Clin Cancer Res 11(15): 5526–5533. Makower D, A Rozenblit, H Kaufman, M Edelman, ME Lane, J Zwiebel, H Haynes, S Wadler (2003) ‘Phase Ii clinical trial of intralesional administration of the oncolytic adenovirus onyx-015 in patients with hepatobiliary tumors with correlative P53 studies. Clin Cancer Res 9(2): 693–702. Mathew P, PF Thall, D Jones, C Perez, C Bucana, P Troncoso, SJ Kim, IJ Fidler, C Logothetis (2004) Platelet-derived growth factor receptor inhibitor imatinib mesylate and docetaxel: a modular phase I trial in androgen-independent prostate cancer. J Clin Oncol 22(16): 3323–3329. McDaid HM, S Mani, HJ Shen, F Muggia, D Sonnichsen, SB Horwitz (2002) Validation of the pharmacodynamics of Bms-247550, an analogue of epothilone B, during a phase I clinical study. Clin Cancer Res 8(7): 2035–2043. Millward MJ, A Joshua, R Kefford, S Aamdal, D Thomson, P Hersey, G Toner, K Lynch (2005) Multi-centre phase Ii trial of the polyamine synthesis inhibitor Sam486a (Cgp48664) in patients with metastatic melanoma. Invest New Drugs 23(3): 253–256. Moschos SJ, CA Sander, W Wang, SL Reppert, LM Drogowski, DM Jukic, UN Rao, C Athanassiou, M Buzoianu, M Mandic, L Richman, L McKinney, J Leininger, DA Tice, L Hammershaimb, JM Kirkwood (2010) Pharmacodynamic (phase 0) study using etaracizumab in advanced melanoma.’ J Immunother 33(3): 316–325. Moulder SL, WF Symmans, DJ Booser, TL Madden, C Lipsanen, L Yuan, AM Brewster, M Cristofanilli, KK Hunt, TA Buchholz, J Zwiebel, V Valero, GN Hortobagyi, FJ Esteva (2008) Phase I/Ii study of G3139 (Bcl-2 antisense oligonucleotide) in combination with doxorubicin and docetaxel in breast cancer. Clin Cancer Res 14(23): 7909–7916. Mullamitha SA, NC Ton, GJ Parker, A Jackson, PJ Julyan, C Roberts, GA Buonaccorsi, Y Watson, K Davies, S Cheung, L Hope, JW Valle, JA Radford, J Lawrance, MP Saunders, MC Munteanu, MT Nakada, JA Nemeth, HM Davis, Q Jiao, U Prabhakar, Z Lang, RE Corringham, RA Beckman, GC Jayson (2007) ‘Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (Cnto 95) in patients with advanced solid tumors. Clin Cancer Res 13(7): 2128–2135. O′Neil BH, L Raftery, BF Calvo, AB Chakravarthy, A Ivanova, MO Myers, HJ Kim, E Chan, PE Wise, LS Caskey, SA Bernard, HK Sanoff, RM Goldberg, JE Tepper (2010) A phase I study of bortezomib in combination with standard 5-fluorouracil and external-beam radiation therapy for the treatment of locally advanced or metastatic rectal cancer. Clin Colorectal Cancer 9(2): 119–125. Ord JJ, E Streeter, A Jones, K Le Monnier, D Cranston, J Crew, SP Joel, MA Rogers, RE Banks, IS Roberts, AL Harris (2005) Phase I trial of intravesical suramin in recurrent superficial transitional cell bladder carcinoma. Br J Cancer 92(12): 2140–2147. Overmoyer B, P Fu, C Hoppel, T Radivoyevitch, R Shenk, M Persons, P Silverman, K Robertson, NP Ziats, JK Wasman, FW Abdul-Karim, JA Jesberger, J Duerk, P Hartman, S Hanks, J Lewin, A Dowlati, K McCrae, P Ivy, SC Remick (2007) Inflammatory breast cancer as a model disease to study tumor angiogenesis: results of a phase Ib trial of combination Su5416 and doxorubicin. Clin Cancer Res 13(19): 5862–5868. Perez RP, LD Lewis, AP Beelen, AJ Olszanski, N Johnston, CH Rhodes, B Beaulieu, MS Ernstoff, A Eastman (2006) Modulation of cell cycle progression in human tumors: a pharmacokinetic and tumor molecular pharmacodynamic study of cisplatin plus the Chk1 inhibitor Ucn-01 (Nsc 638850). Clin Cancer Res 12(23): 7079–7085. Perotti A, A Locatelli, C Sessa, D Hess, L Vigano, G Capri, M Maur, T Cerny, S Cresta, F Rojo, J Albanell, S Marsoni, I Corradino, L Berk, VM Rivera, F Haluska, L Gianni (2010) Phase Ib study of the Mtor inhibitor ridaforolimus with capecitabine. J Clin Oncol 28(30): 4554–4561. Posadas EM, MS Liel, V Kwitkowski, L Minasian, AK Godwin, MM Hussain, V Espina, BJ Wood, SM Steinberg, EC Kohn (2007) A phase Ii and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer 109(7): 1323–1330. Puglisi F, GG Cardellino, D Crivellari, C Di Loreto, MD Magri, AM Minisini, M Mansutti, C Andreetta, S Russo, D Lombardi, T Perin, G Damante, A Veronesi (2008) Thymidine phosphorylase expression is associated with time to progression in patients receiving low-dose, docetaxel-modulated capecitabine for metastatic breast cancer. Ann Oncol 19(9): 1541–1546. Ramanathan RK, MJ Egorin, C Erlichman, SC Remick, SS Ramalingam, C Naret, JL Holleran, CJ TenEyck, SP Ivy, CP Belani (2010) Phase I pharmacokinetic and pharmacodynamic study of 17-dimethylaminoethylamino-17-demethoxygeldanamycin, an inhibitor of heat-shock protein 90, in patients with advanced solid tumors. J Clin Oncol 28(9): 1520–1526. Ranson M, P Hersey, D Thompson, J Beith, GA McArthur, A Haydon, ID Davis, RF Kefford, P Mortimer, PA Harris, S Baka, A Seebaran, A Sabharwal, AJ Watson, GP Margison, MR Middleton (2007) Randomized trial of the combination of lomeguatrib and temozolomide compared with temozolomide alone in chemotherapy naive patients with metastatic cutaneous melanoma. J Clin Oncol 25(18): 2540–2545. Rao S, N Starling, D Cunningham, M Benson, A Wotherspoon, C Lupfert, R Kurek, J Oates, J Baselga, A Hill (2008) Phase I study of epirubicin, cisplatin and capecitabine plus matuzumab in previously untreated patients with advanced oesophagogastric cancer. Br J Cancer 99(6): 868–874. Rojo F, E Gracias, N Villena, T Cruz, JM Corominas, I Corradino, M Cedeno, C Campas, M Osorio, N Iznaga, B Bellosillo, A Rovira, S Marsoni, P Gascon, S Serrano, C Sessa, T Crombet, J Albanell (2010) Pharmacodynamic trial of nimotuzumab in unresectable squamous cell carcinoma of the head and neck: a sendo foundation study. Clin Cancer Res 16(8): 2474–2482. Rojo F, J Tabernero, J Albanell, E Van Cutsem, A Ohtsu, T Doi, W Koizumi, K Shirao, H Takiuchi, S Ramon y Cajal, J Baselga (2006) Pharmacodynamic studies of gefitinib in tumor biopsy specimens from patients with advanced gastric carcinoma. J Clin Oncol 24(26): 4309–4316. Rothenberg ML, B LaFleur, DE Levy, MK Washington, SL Morgan-Meadows, RK Ramanathan, JD Berlin, AB Benson 3rd, RJ Coffey (2005) Randomized phase Ii trial of the clinical and biological effects of two dose levels of gefitinib in patients with recurrent colorectal adenocarcinoma. J Clin Oncol 23(36): 9265–9274. Siu LL, EK Rowinsky, LA Hammond, GR Weiss, M Hidalgo, GM Clark, J Moczygemba, L Choi, R Linnartz, NC Barbet, IT Sklenar, R Capdeville, G Gan, CW Porter, DD Von Hoff, SG Eckhardt (2002) A phase I and pharmacokinetic study of Sam486a, a novel polyamine biosynthesis inhibitor, administered on a daily-times-five every-three-week schedule in patients with advanced solid malignancies. Clin Cancer Res 8(7): 2157–2166. Stearns V, A Gallagher, CG Kleer, B Singh, M Freedman, BR Haddad, C Isaacs, R Warren, M Brown, J Cullen, B Trock, DF Hayes (2004) A pilot study to establish a clinical model to perform phase Ii studies of breast cancer chemopreventive agents in women at high risk with biomarkers as surrogate endpoints for activity. Clin Cancer Res 10(24): 8332–8340. Tabernero J, F Rojo, E Calvo, H Burris, I Judson, K Hazell, E Martinelli, S Ramon y Cajal, S Jones, L Vidal, N Shand, T Macarulla, FJ Ramos, S Dimitrijevic, U Zoellner, P Tang, M Stumm, HA Lane, D Lebwohl, J Baselga (2008) Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26(10): 1603–1610. Thomas F, P Rochaix, A Benlyazid, J Sarini, M Rives, JL Lefebvre, BC Allal, F Courbon, E Chatelut, JP Delord (2007) Pilot study of neoadjuvant treatment with erlotinib in nonmetastatic head and neck squamous cell carcinoma. Clin Cancer Res 13(23): 7086–7092. Ton NC, GJ Parker, A Jackson, S Mullamitha, GA Buonaccorsi, C Roberts, Y Watson, K Davies, S Cheung, L Hope, F Power, J Lawrance, J Valle, M Saunders, R Felix, JA Soranson, L Rolfe, K Zinkewich-Peotti, GC Jayson (2007) Phase I evaluation of Cdp791, a pegylated di-fab' conjugate that binds vascular endothelial growth factor receptor 2. Clin Cancer Res 13(23): 7113–7118. Townsley CA, P Major, LL Siu, J Dancey, E Chen, GR Pond, T Nicklee, J Ho, D Hedley, M Tsao, MJ Moore, AM Oza (2006) Phase Ii study of erlotinib (Osi-774) in patients with metastatic colorectal cancer. Br J Cancer 94(8): 1136–1143. Tse AN, DS Klimstra, M Gonen, M Shah, T Sheikh, R Sikorski, R Carvajal, J Mui, C Tipian, E O'Reilly, K Chung, R Maki, R Lefkowitz, K Brown, K Manova-Todorova, N Wu, MJ Egorin, D Kelsen, GK Schwartz (2008) A phase 1 dose-escalation study of irinotecan in combination with 17-allylamino-17-demethoxygeldanamycin in patients with solid tumors. Clin Cancer Res 14(20): 6704–6711. Van Waes C, CT Allen, D Citrin, D Gius, AD Colevas, NA Harold, S Rudy, L Nottingham, C Muir, Z Chen, AK Singh, J Dancey, JC Morris (2010) Molecular and clinical responses in a pilot study of gefitinib with paclitaxel and radiation in locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 77(2): 447–454. Watson AJ, MR Middleton, G McGown, M Thorncroft, M Ranson, P Hersey, G McArthur, ID Davis, D Thomson, J Beith, A Haydon, R Kefford, P Lorigan, P Mortimer, A Sabharwal, O Hayward, GP Margison (2009) O(6)-Methylguanine-DNA methyltransferase depletion and DNA damage in patients with melanoma treated with temozolomide alone or with lomeguatrib. Br J Cancer 100(8): 1250–1256. Welch S, HW Hirte, MS Carey, SJ Hotte, MS Tsao, S Brown, GR Pond, JE Dancey, AM Oza (2007) Ucn-01 in combination with topotecan in patients with advanced recurrent ovarian cancer: a study of the princess margaret hospital phase Ii consortium. Gynecol Oncol 106(2): 305–310. Weng DE, PA Masci, SF Radka, TE Jackson, PA Weiss, R Ganapathi, PJ Elson, WB Capra, VP Parker, JA Lockridge, JW Cowens, N Usman, EC Borden (2005) A phase I clinical trial of a ribozyme-based angiogenesis inhibitor targeting vascular endothelial growth factor receptor-1 for patients with refractory solid tumors. Mol Cancer Ther 4(6): 948–955. **ong HQ, R Herbst, SC Faria, C Scholz, D Davis, EF Jackson, T Madden, D McConkey, M Hicks, K Hess, CA Charnsangavej, JL Abbruzzese (2004) A phase I surrogate endpoint study of Su6668 in patients with solid tumors. Invest New Drugs 22(4): 459–466. Zhang D, T Pier, DG McNeel, G Wilding, A Friedl (2007) Effects of a monoclonal anti-alphavbeta3 integrin antibody on blood vessels: a pharmacodynamic study. Invest New Drugs 25(1): 49–55. Zhou EH, RJ Ellis, E Cherullo, V Colussi, F Xu, WD Chen, S Gupta, CC Whalen, D Bodner, MI Resnick, AA Rimm, SM Koroukian (2009) Radiotherapy and survival in prostate cancer patients: a population-based study. Int J Radiat Oncol Biol Phys 73(1): 15–23. From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Freeman, G., Kimmelman, J., Dancey, J. et al. Reporting practices of pharmacodynamic studies involving invasive research procedures in cancer trials.
Br J Cancer 109, 897–908 (2013). https://doi.org/10.1038/bjc.2013.417 Received: Revised: Accepted: Published: Issue Date: DOI: https://doi.org/10.1038/bjc.2013.417 Scientific Reports (2021) British Journal of Cancer (2017)Change history
20 August 2013
References
Acknowledgements
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Additional information
Appendices
Appendix 1




Appendix 2
Inventory of studies included in sample
Rights and permissions
About this article
Cite this article
Keywords
This article is cited by
Variability in biopsy quality informs translational research applications in hepatocellular carcinoma
Safety and utility of image-guided research biopsies in relapsed high-grade serous ovarian carcinoma—experience of the BriTROC consortium