Summary
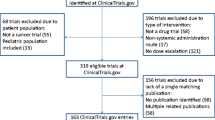
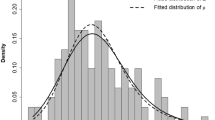
Introduction The role of phase I cancer trials is constantly evolving and they are increasingly being used in ‘go/no’ decisions in drug development. As a result, there is a growing need to ensure trials are published when completed. There are limited data on the publication rate and the factors associated with publication in phase I trials. Methods The ClinicalTrials.gov database was searched for completed adult phase I cancer trials with reported results. PubMed was searched for matching publications published prior to April 1, 2017. Logistic regression was used to identify factors associated with unpublished trials. Linear regression was used to explore factors associated with time lag from study database lock to publication for published trials. Results The study cohort included 319 trials. 95 (30%) trials had no matching publication. Thirty (9%) trials were not published in abstract form as well. On multivariable analysis, the most significant factor associated with unpublished trials was industry funding (odds ratio 3.3, 95% confidence interval 1.7-6.6, p=0.019). For published trials, time lag between database lock and publication was longer by 10.9 months (standard error 3.6, p<0.001) for industry funded trials compared with medical center funded trials. Conclusions Timely publishing of early cancer clinical trials results remains unsatisfactory. Industry funded phase I cancer trials were more likely to remain unpublished, and were associated with a longer time lag from database lock to publication. Policies that promote transparency and data sharing in clinical trial research might improve accountability among industry and investigators and improve timely results publication.

Similar content being viewed by others
References
Shepshelovich D, Goldvaser H, Wang L, Abdul Razak AR, Bedard PL (2017) Comparison of reporting phase I trial results in ClinicalTrials.gov and matched publications. Invest New Drugs 35:827–833
Camacho LH, Bacik J, Cheung A, Spriggs DR (2005) Presentation and subsequent publication rates of phase I oncology clinical trials. Cancer 104:1497–1504
Massey PR, Wang R, Prasad V, Bates SE, Fojo T (2016) Assessing the Eventual Publication of Clinical Trial Abstracts Submitted to a Large Annual Oncology Meeting. Oncologist 21:261–268
Kho ME, Brouwers MC (2009) Conference abstracts of a new oncology drug do not always lead to full publication: proceed with caution. J Clin Epidemiol 62:752–758
Riveros C, Dechartres A, Perrodeau E, Haneef R, Boutron I, Ravaud P (2013) Timing and completeness of trial results posted at ClinicalTrials.gov and published in journals. PLoS Med 10:e1001566 discussion e1001566
ClinicalTrials.gov (2017) About the Results Database. https://clinicaltrials.gov/ct2/about-site/results. Last Accessed Oct 30th, 2017
Decullier E, Chan AW, Chapuis F (2009) Inadequate dissemination of phase I trials: a retrospective cohort study. PLoS Med 17:e1000034
Chen R, Desai NR, Ross JS, Zhang W, Chau KH, Wayda B, Murugiah K, Lu DY, Mittal A, Krumholz HM (2016) Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ 17:352–i637
Ross JS, Gross CP, Krumholz HM (2012) Promoting transparency in pharmaceutical industry-sponsored research. Am J Public Health 102:72–80
Bourgeois FT, Murthy S, Mandl KD (2010) Outcome reporting among drug trials registered in ClinicalTrials.gov. Ann Intern Med 153:158–166
Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM (2012) Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ 3:344–d7292
Ross JS, Mocanu M, Lampropulos JF, Tse T, Krumholz HM (2013) Time to publication among completed clinical trials. JAMA Intern Med 173:825–828
Ioannidis JP (1998) Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA 279:281–286
Song SY, Koo DH, Jung SY, Kang W, Kim EY (2017) The significance of the trial outcome was associated with publication rate and time to publication. J Clin Epidemiol 84:78–84
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shepshelovich, D., Goldvaser, H., Wang, L. et al. Comparison of published and unpublished phase I clinical cancer trials: an analysis of the CliniclTrials.gov database. Invest New Drugs 36, 933–938 (2018). https://doi.org/10.1007/s10637-017-0549-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0549-6