Abstract
Aim:
The dorsal striatum has been proposed to contribute to the formation of drug-seeking behaviors, leading to excessive and compulsive drug usage, such as addiction. The current study aimed to investigate the involvement of extracellular signal-regulated kinase (ERK) pathway in the modification of striatal synaptic plasticity.
Methods:
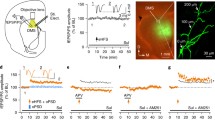
Ethanol was administered to rats in drinking water at concentration of 6% (v/v) for 30 days. Rats were sacrificed on day 10, 20, or 30 during ethanol intake or on withdrawal day 1, 3, or 7 following 30-d ethanol intake. The striata were removed either for electrophysiological recording or for protein immuno-blot analysis. Extracellular recording technique was used to record population spikes (PS) induced by high-frequency stimulation (HFS) in the dorsolateral striatum (DLS).
Results:
Corticostriatal long-term depression (LTD) was determined to be dependent upon ERK signaling. Chronic ethanol intake (CEI) attenuated ERK phosphorylation and LTD induction, whereas withdrawal for one day (W1D) potentiated ERK phosphorylation and LTD induction. These results showed that the impact of chronic ethanol intake and withdrawal on corticostriatal synaptic plasticity was associated with ethanol's effect on ERK phosphorylation. In particular, pharmacological inhibition of ERK hyper-phosphorylation by U0126 prevented LTD induction in the DLS and attenuated ethanol withdrawal syndrome as well.
Conclusion:
In rat DLS, chronic ethanol intake and withdrawal altered LTD induction via ERK signaling pathway. Ethanol withdrawal syndrome is mediated, at least partly, by ERK hyper-phosphorylation in the DLS.
Similar content being viewed by others
Introduction
Addiction is increasingly viewed as a pathological process of learning, involving cell signaling and synaptic mechanisms similar to those implicated in neural models of learning and memory1, 2, 3, 4. Synaptic plasticity is required for neuroadaptations that result from a variety of environmental stimuli. Therefore, it is attractive to hypothesize that drug abuse causes long-term changes in behavior by altering synaptic function and plasticity in relevant brain circuits.
The involvement of the ventral striatum, or nucleus accumbens (NAc), in mediating drug reward and reinforcement is well established2, 4, 5, 6. Recently, it has also been proposed that the dorsal striatum (caudate nucleus and putamen) is likely to be involved in advanced stages of addiction when drug use progresses toward a compulsive, habitual pathology2, 5, 6. Previous work from our lab25. Nevertheless, it is not surprising that LTD was invariably recorded in our experiment, given the use of older rats (2-3 months) and the recording subregion of striatum (DLS).
Although it has been previously shown that ERK phosphorylation is required for both LTP9, 10 and LTD26, 11 in hippocampal CA1 as well as LTD in the cerebellum8 and LTP in other cortical areas12, we provide the first evidence that ERK activation is required for striatal LTD induction.
The ethanol-induced modulation of ERK activity in vitro has been controversial, with potentiation reported by some27, 28 and depression by others29, 30, 31. However, in vivo exposure data are more consistent, revealing an ethanol-induced decrease in ERK activation13, 14, 15, except in the report from Bachtell et al32. Our data are in agreement with previous reports in which chronic ethanol exposure in vivo decreased ERK phosphorylation. It should be noted that in CEI30 groups, the suppression of phospho-ERK levels was, while still significant, attenuated, which may reflect the adaptation or tolerance to long-term alcohol consumption. We also demonstrated an enhancement of ERK phosphorylation briefly after withdrawal14. This may be related to increased neural activity following disinhibition or hyperexcitability caused by ethanol withdrawal.
The mechanisms underlying the effects of chronic ethanol exposure on ERK phosphorylation are still under investigation. However, studies showing the activation of MAP kinase by Ca2+ via NMDA receptors or voltage-dependent Ca2+ channels have been very well documented33, 34, 35. Since ethanol inhibits both voltage-gated Ca2+ channels and NMDA receptor-associated Ca2+ influx36, 37, 38, it is possible that a decrease in the Ca2+ concentration in striatal neurons might decrease the phosphorylation of MAP kinase. Future studies will be required to identify the site and mechanism underlying the effects of ethanol on this pathway.
Anatomical studies have shown projections from the cortex to the medial striatum, including visual, auditory and limbic (ie, hippocampus, entorhinal and piriform cortices) afferents. In contrast, most cortical projections to the lateral striatum are of sensorimotor origin and, to a lesser degree, auditory and visual afferents39. Accordingly, recent studies suggest that the dorsomedial and dorsolateral striata have differential roles in different learning and memory paradigms. The dorsomedial striatum, in particular, has been shown to be critical for the learning of goal-directed actions. In contrast, the dorsolateral striatum appears to be involved in the formation of habits40, 41. By showing that chronic ethanol intake and withdrawal differentially altered synaptic plasticity in dorsolateral striatum through ERK signaling pathway, our findings suggest that chronic alcohol abuse could disrupt the habit-formation process, and this neural maladaptation may consequently lead to habitual drug-seeking behavior.
To summarize, our current studies have shown that induction of corticostriatal LTD in rat is differentially altered by chronic ethanol intake and withdrawal, which occurs through ERK signaling pathway. Understanding the biochemical mechanism underlying chronic ethanol treatment-induced changes in striatal synaptic plasticity may aid in the development of more effective therapeutic agents for alcohol abuse.
Author contribution
**ao-ru YUAN and **g LI designed the research; Sheng-zhong CUI, Shen-jun WANG, Rong ZHOU, and Gui-qin XIE performed the research; Sheng-zhong CUI, **ao-ru YUAN, and Ling CHEN analyzed the data and wrote the paper.
References
Nestler EJ . Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2001; 2: 119–28.
Berke JD, Hyman SE . Addiction, dopamine, and the molecular mechanisms of memory. Neuron 2000; 25: 515–32.
Kelley AE . Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 2004; 44: 161–79.
Hyman SE, Malenka RC, Nestler EJ . Neural mechanisms of addiction: the role of reward–related learning and memory. Annu Rev Neurosci 2006; 29: 565–98.
Everitt BJ, Wolf ME . Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 2002; 22: 3312–20.
Koob GF, Le Moal M . Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001; 24: 97–129.
**a JX, Li J, Zhou R, Zhang XH, Ge YB, Yuan XR . Alterations of rat corticostriatal synaptic plasticity after chronic ethanol exposure and withdrawal. Alcohol Clin Exp Res 2006; 30: 819–24.
English JD, Sweatt JD . A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem 1997; 272: 19103–6.
Kanterewicz BI, Urban NN, McMahon DB, Norman ED, Giffen LJ, Favata MF, et al. The extracellular signal-regulated kinase cascade is required for NMDA receptor-independent LTP in area CA1 but not area CA3 of the hippocampus. J Neurosci 2000; 20: 3057–66.
Kawasaki H, Fujii H, Gotoh Y, Morooka T, Shimohama S, Nishida E, et al. Requirement for mitogen-activated protein kinase in cerebellar long-term depression. J Biol Chem 1999; 274: 13498–502.
Gallagher SM, Daly CA, Bear MF, Huber KM . Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci 2004; 24: 4859–64.
Di Cristo G, Berardi N, Cancedda L, Pizzorusso T, Putignano E, Ratto GM, et al. Requirement of ERK activation for visual cortical plasticity. Science 2001; 292: 2337–40.
Kalluri HS, Ticku MK . Ethanol-mediated inhibition of mitogen-activated protein kinase phosphorylation in mouse brain. Eur J Pharmacol 2002; 439: 53–8.
Sanna PP, Simpson C, Lutjens R, Koob G . ERK regulation in chronic ethanol exposure and withdrawal. Brain Res 2002; 948: 186–91.
Roberto M, Nelson TE, Ur CL, Brunelli M, Sanna PP, Gruol DL . The transient depression of hippocampal CA1 LTP induced by chronic intermittent ethanol exposure is associated with an inhibition of the MAP kinase pathway. Eur J Neurosci 2003; 17: 1646–54.
Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 1998; 273: 18623–32.
Charpier S, Deniau JM . In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci USA 1997; 94: 7036–40.
Charpier S, Mahon S, Deniau JM . In vivo induction of striatal long-term potentiation by low-frequency stimulation of the cerebral cortex. Neurosci 1999; 91: 1209–22.
Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G . Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci 1992; 12: 4224–33.
Lovinger DM, Tyler EC, Merritt A . Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol 1993; 70: 1937–49.
Wickens JR, Begg AJ, Arbuthnott GW . Dopamine reverses the depression of rat corticostriatal synapses which normally follows high-frequency stimulation of cortex in vitro. Neuroscience 1996; 70: 1–5.
Calabresi P, Pisani A, Mercuri NB, Bernardi G . Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci 1992; 4: 929–35.
Smith R, Musleh W, Akopian G, Buckwalter G, Walsh JP . Regional differences in the expression of corticostriatal synaptic plasticity. Neuroscience 2001; 106: 95–101.
Partridge JG, Tang KC, Lovinger DM . Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol 2000; 84: 1422–9.
Butler AK, Uryu K, Chesselet M . A role for N-methyl-D-aspartate receptors in the regulation of synaptogenesis and expression of the polysialylated form of the neural cell adhesion molecule in the develo** striatum. Dev Neurosci 1998; 20: 253–62.
Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E . Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J Neurosci 2002; 22: 2054–62.
Roivainen R, Hundle B, Messing RO . Ethanol enhances growth factor activation of mitogen-activated protein kinases by a protein kinase C-dependent mechanism. Proc Natl Acad Sci USA 1995; 92: 1891–5.
Smith TL, Navratilova E . The effect of ethanol exposure on mitogen-activated protein kinase activity and expression in cultured rat astrocytes. Neurosci Lett 2003; 341: 91–4.
Hallak H, Seiler AE, Green JS, Henderson A, Ross BN, Rubin R . Inhibition of insulin-like growth factor-I signaling by ethanol in neuronal cells. Alcohol Clin Exp Res 2001; 25: 1058–64.
Ma W, Li BS, Maric D, Zhao WQ, Lin HJ, Zhang L, et al. Ethanol blocks both basic fibroblast growth factor- and carbachol-mediated neuroepithelial cell expansion with differential effects on carbachol-activated signaling pathways. Neuroscience 2003; 118: 37–47.
Seiler AE, Ross BN, Green JS, Rubin R . Differential effects of ethanol on insulin-like growth factor-I receptor signaling. Alcohol Clin Exp Res 2000; 24: 140–8.
Bachtell RK, Tsivkovskaia NO, Ryabinin AE . Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther 2002; 302: 516–24.
Orban CP, Chapman FP, Brambilla R . Is Ras-MAPK signaling pathway necessary for long term memory formation? Trends Neurosci 1999; 22: 38–44.
Rosen BL, Ginty DD, Weber JM, Greenberg EM . Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 1994; 12: 1207–21.
**a Z, Dudek H, Miranti KC, Greenberg ME . Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci 1996; 16: 5425–36.
Lovinger DM, White G, Weight FF . Ethanol inhibits NMDA activated ion current in hippocampal neurons. Science 1989; 243: 1721–4.
Lovinger DM, White G, Weight FF . NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci 1990; 10: 1372–9.
Walter HJ, Messing RO . Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem Int 1999; 35: 95–101.
McGeorge AJ, Faull RLM . The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 1989; 29: 503–37.
Yin HH, Knowlton BJ . The role of the basal ganglia in habit formation. Nature Rev Neurosci 2006; 7: 464–76.
Yin HH, Knowlton BJ, Balleine BW . Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 2004; 19: 181–9.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No 30572173) and the Natural Science Foundation of the Education Committee of Jiangsu Province, China (No 06KJB180071).
We thank Dr Shang SHEN at University of Nevada, Las Vegas, for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Sz., Wang, Sj., Li, J. et al. Alteration of synaptic plasticity in rat dorsal striatum induced by chronic ethanol intake and withdrawal via ERK pathway. Acta Pharmacol Sin 32, 175–181 (2011). https://doi.org/10.1038/aps.2010.199
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.199
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Ethanol Stimulation of Microglia Release Increases ERK1/2-Dependent Neuronal cPLA2 Activity in Immature Cultured Cortical Preparations
Neurochemical Research (2020)
-
Ethanol exposure in prenatal and early postnatal induced cardiac injury in rats: involvement of oxidative stress, Hsp70, ERK 1/2, JNK, and apoptosis in a 3-month follow-up study
Cell Stress and Chaperones (2019)
-
Dorsolateral striatal miR-134 modulates excessive methamphetamine intake in self-administering rats
Metabolic Brain Disease (2019)
-
Drug addiction: a curable mental disorder?
Acta Pharmacologica Sinica (2018)
-
Translational Research on Habit and Alcohol
Current Addiction Reports (2016)