Abstract
Phytopathogens develop specialized infection-related structures to penetrate plant cells during infection. Different from phytopathogens that form appressoria or haustoria, the soil-borne root-infecting fungal pathogen Verticillium dahliae forms hyphopodia during infection, which further differentiate into penetration pegs to promote infection. The molecular mechanisms underlying the regulation of hyphopodium formation in V. dahliae remain poorly characterized. Mitogen-activated protein kinases (MAPKs) are highly conserved cytoplasmic kinases that regulate diverse biological processes in eukaryotes. Here we found that deletion of VdKss1, out of the five MAPKs encoded by V. dahliae, significantly impaired V. dahliae hyphopodium formation, in vitro penetration, and pathogenicity in cotton plants. Constitutive activation of MAPK kinase (MAPKK) VdSte7 and MAPK kinase kinase (MAPKKK) VdSte11 specifically activate VdKss1. Deletion of VdSte7 or VdSte11 resulted in a phenotype similar to that of the mutant with VdKss1 deletion. Thus, this study demonstrates that VdSte11-VdSte7-VdKss1 is a core MAPK cascade that regulates hyphopodium formation and pathogenicity in V. dahliae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In eukaryotes, mitogen-activated protein kinase (MAPK) cascades are highly conserved cytoplasmic kinases that integrate extracellular signaling and transduce to downstream substrates to regulate a wide range of biological processes (Bigeard and Hirt 2018; Dixon et al. 1999; Gustin et al. 1998). The MAPK cascades consist of MAPK kinase kinases (MAPKKKs, MAPK/extracellular signal-regulated kinase (ERK)-kinase kinases/MEKKs), MAPK kinases (MAPKKs, MAPK/ERK- kinases/MEKs), and MAPKs that are sequentially phosphorylated (Widmann et al. 1999). In general, MAPKKKs phosphorylate Ser and/or Thr residues located within the activation loop of MAPKKs, which in turn trigger dual phosphorylation of a highly conserved TXY motif located within the activation loop of MAPKs to activate MAPKs (Bigeard and Hirt 2018; Dixon et al. 1999; Gustin et al. 1998; Segmuller et al. 2007).
In fungi, MAPKs regulate growth and development, maintenance of cellular integrity, and responses to stress (Gustin et al. 1998; Jiang et al. 2018; Wang and Dohlman 2002; Zhao et al. 2005). In pathogenic fungi that infect plants, MAPK cascades regulate the pathogenesis and development of infection-related structures (Jiang et al. 2018; Li et al. 2019; Zhao et al. 2005). For instance, in the ascomycete Magnaporthe oryzae, which infects rice, barley, and other crops causing disease, pathogenicity MAP kinase 1 (Pmk1) is crucial for appressorium formation. MEKK MST11-MEK MST7-MAPK PMK1 constitutes a MAPK cascade regulating infection-related morphogenesis (Jiang et al. 2018; Zhao et al. 2005). In Colletotrichum gloeosporioides, Cgl-Slt2 MAPK plays an important role in the early stage of appressorium formation (Yong et al. 2013).
Verticillium dahliae infects a broad range of plants and causes severe wilt disease and agricultural losses. The development of infection-related structure is crucial for successful infection of host plants. Unlike M. oryzae and C. gloeosporioides, which form appressoria during infection, V. dahliae adheres tightly around the plant root surface and forms hyphopodia, which further develop into penetration pegs during infection (Zhao et al. 2014; Zhou et al. 2017). This process involves calcium accumulation, NADPH oxidase-dependent reactive oxygen species burst, and cAMP-mediated signaling (Sun et al. 2019; Zhao et al. 2014; Zhou et al. 2017); however, additional signaling components as well as the molecular mechanisms regulating these events remain elusive. The MAPKKK VdSte11, but not VdSsk2, plays a major role in regulating V. dahliae penetration (Yu et al. 2019). However, the core MAPK and complete MAPK cascade regulating hyphopodium formation in V. dahliae remain uncharacterized.
In this study, we investigate the functions of MAPKs, MAPKKs, and MAPKKKs in hyphopodium development in V. dahliae. Mutations in VdKss1, VdSte7, or VdSte11, respectively, significantly disrupted hyphopodium formation, in vitro penetration, and pathogenicity in cotton plants. The constitutive active forms of VdSte11 and VdSte7 specifically induced the phosphorylation of VdKss1. Thus, VdSte11-VdSte7-VdKss1 constitutes a complete MAPK cascade that regulates hyphopodium formation and pathogenicity of V. dahliae in host plants.
Materials and methods
Fungal strains, plant materials and culture conditions
Colonies of V. dahliae V592 (Gao et al. 2010). Cotton plants then were photographed and subjected to disease index analyses 3–4 weeks post inoculation. The disease index was classified as follows: 0 (no symptoms), 1 (0% – 25% wilted leaves), 2 (25% – 50% wilted leaves), 3 (50% – 75% wilted leaves) and 4 (75% – 100% wilted leaves). The disease index was calculated as 100 × [sum (number of plants × disease grade)]/[(total number of plants) × (maximal disease grade)] (Xu et al. 2014).
Detection of MAPK phosphorylation
V. dahliae protoplasts were isolated from 36 h vegetative hyphae grown in CM medium and transfected with plasmid as described (Rehman et al. 2016). Fourteen hours after transfection, proteins of transfected protoplasts were extracted with extraction buffer containing 50 mM HEPES (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid sodium salt) (pH 7.5), 150 mM KCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM DTT (dithiothreitol), 0.2% Triton X-100, and 1 × proteinase inhibitor cocktail and subjected to IP and immunoblot assay with anti-pERK, anti-HA or anti-FLAG.
The presence of VdKss1-HA, VdMAPKKs-FLAG and VdMAPKKKs-FLAG was detected by anti-HA or anti-FLAG immunoblot. For anti-HA IP, total proteins were incubated with 2 µg of anti-HA antibody together with protein A agarose at 4 °C for 4 h. The agarose beads were collected and boiled for 5 min with 1 × protein loading buffer. Total proteins were separated in a 10% SDS-PAGE gel and transferred to the PVDF membrane. The phosphorylation of VdKss1 TEY motif was detected by anti-pERK immunoblot.
Results
Expression of a MAPK inhibitor compromises hyphopodium formation in V. dahliae
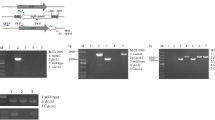
MAPK cascades are conserved signaling kinases that regulate diverse biological processes. HopAI1, a Pseudomonas syringae effector protein, has a unique phosphothreonine lyase activity towards plant, animal and fungal MAPKs (Li et al. 2007a; Zhang et al. 2007, 2017). Expression of HopAI1 in M. oryzae inhibits MAPK activation and appressorium formation (Zhang et al. 2017). To examine whether MAPK activation contributes to hyphopodium formation in V. dahliae, HopAI1 was expressed in V. dahliae. The expression of HopAI1 in the HopAI1-expressing strain was confirmed by anti-GFP immunoblot (Fig. S1). Similar to that in M. oryzae, expression of HopAI1 in V. dahliae can inhibit MAPK activity (Fig. S2). Compared with the wild-type (WT) V. dahliae strain, hyphopodium formation was significantly reduced in the HopAI1-expressing V. dahliae strain (Fig. 1A, B), suggesting that MAPK activation is crucial for V. dahliae hyphopodium development. WT and HopAI1-expressing V. dahliae strains were cultured on minimal medium (MM) covered with cellophane and examined for in vitro penetration (Zhao et al. 2016). Consistent with the deficiency of hyphopodium formation, the HopAI1-expressing strain displayed defects in in vitro penetration compared to the WT strain (Fig. 1C). WT and HopAI1-expressing V. dahliae strains were inoculated into cotton plants. Four weeks post inoculation, cotton plants infected with HopAI1-expressing strain exhibited much weaker disease symptoms than those infected with WT V592 (Fig. 1D). Disease index analyses indicated reduced pathogenicity of the HopAI1-expressing strain relative to the WT strain (Fig. 1E). These results strongly suggested that suppression of MAPK compromises hyphopodium formation and penetration, indicating an important role of MAPK activation in regulating hyphopodium formation of V. dahliae.
Expression of a MAPK inhibitor in Verticillium dahliae compromises hyphopodium formation. A Hyphopodium formation is suppressed by HopAI1. V592 and HopAI1-expressing strains were grown on MM covered with a cellophane for 5 days. Hyphopodium formation (red arrow) on the cellophane was observed by confocal laser scanning microscopy (CLSM). B Hyphopodium quantification of V592 and HopAI1-expressing strains. The number of hyphopodium in three fields was counted for each strain. Error bars indicate the standard deviation of three fields. Student’s t-test was carried out to determine the significance of difference. **Indicates significant difference at P-value of < 0.01. C The HopAI1-expressing strain displays a defect in penetration. The spores of V592 and HopAI1-expressing strains were grown on minimal medium (MM) covered with a cellophane for 5 days and photographed (above). The cellophane was removed and the culture was continued for 3 days and photographed (below). D Suppression of MAPK compromises V. dahliae virulence. Disease symptoms of upland cotton plants infected with the V592 and HopAI1-expressing strains were photographed and subjected to disease index analyses 3 weeks post inoculation. E Disease index analyses of upland cotton infected with the indicated strains. The disease indexes were evaluated with three replicates generated from 18 plants for each inoculum. Error bars indicate the standard deviation of three biological replicates. Student’s t-test was carried out to determine the significance of difference. **Indicates significant difference at P-value of < 0.01
Deletion of a specific MAPK compromises V. dahliae hyphopodium formation and penetration
Based on its homology with yeast homologs, V. dahliae encodes the following five putative MAPKs (Hamel et al. 2012): the Kss1 type VdKss1 (VDAG_09461), Hog1 types VdHog1-1 (VDAG_08982) and VdHog1-2 (VDAG_02354), Ime type VdIme2 (VDAG_06935), and Slt2 type VdSlt2 (VDAG_02584). To further confirm the requirement of MAPK activation for hyphopodium formation, V. dahliae mutants with gene deletion of individual MAPKs in the V592 WT strain were generated by homologous recombination (Fig. S3) using the ATMT-DS-vector (Wang et al. 2016a).
The Vd∆kss1, Vd∆hog1-1, Vd∆hog1-2, Vd∆ime2, Vd∆slt2, and WT V. dahliae strains were grown on PDA medium, transferred to MM covered with cellophane, and tested for their ability to penetrate artificial membrane. At 4 days after growth, hyphal growth of all mutants and the WT strain, except for the Vd∆kss1 mutant, was observed on the medium when the cellophane membrane was removed (Fig. 2A), suggesting that mutation of VdKss1 compromised the capacity for membrane penetration. Next, we examined hyphopodium formation in these mutants. Mutation of VdKss1 impaired hyphopodium formation (Fig. 2B, C). The complementation of VdKss1 in the Vd∆kss1 mutant restored membrane-penetration (Fig. 2A) and hyphopodium formation (Fig. 2B, C). Expression of VdKss1-GFP in the complementary strain was confirmed by anti-GFP immunoblot (Fig. S4A). Mutation of VdIme2 and VdSlt2 exhibited reduced hyphopodium formation (Fig. 2B, C), suggesting a contribution of VdIme2 and VdSlt2 to that process. These results indicated that VdKss1 plays a major role, whereas VdIme2 and VdSlt2 play minor roles, in regulating hyphopodium formation.
Deletion of a specific MAPK significantly compromises Verticillium dahliae hyphopodium formation and in vitro penetration. A Penetration phenotypes of VdMAPKs deletion mutants and Vd∆kss1/VdKss1-GFP complementary strain. The spores of indicated strains were grown on MM covered with a cellophane for 5 days and photographed (above). The cellophane was removed and the culture was continued for 3 days and photographed (below). B Hyphopodium formation of VdMAPKs deletion mutants and Vd∆kss1/VdKss1-GFP complementary strain. Indicated strains were grown on MM covered with a cellophane for 5 days. Hyphopodium formation (red arrow) on the cellophane was observed by confocal laser scanning microscopy (CLSM). C Hyphopodium quantification of VdMAPKs deletion mutants and Vd∆kss1/VdKss1-GFP complementary strain. The number of hyphopodium in three fields was counted for each strain. Error bars indicate the standard deviation of three fields. Student’s t-test was carried out to determine the significance of difference. *Indicates significant difference at P-value of < 0.05. **Indicates significant difference at P-value of < 0.01
The Vd∆kss1 mutant and WT strains were tested for pathogenicity in cotton plants. The Vd∆kss1 mutant displayed much less severe disease symptoms than the WT strain (Fig. 3A, B). The virulence of the Vd∆kss1 mutant was restored upon complementation with VdKss1 (Fig. 3A, B). These results indicate that VdKss1 plays an important role in regulating the development of hyphopodium and is required for the full pathogenicity of V. dahliae.
VdKss1 contributes to Verticillium dahliae virulence in cotton plants. A Disease symptoms of upland cotton. Plants infected with indicated strains were photographed and subjected to disease index analyses 3–4 weeks post inoculation. B Disease index analyses of upland cotton infected with the indicated strains. The disease indexes were evaluated with three replicates generated from 24 plants for each inoculum. Error bars indicate the standard deviation of three biological replicates. Student’s t-test was carried out to determine the significance of difference. **Indicates significant difference at P-value of < 0.01
VdSte7 phosphorylates VdKss1 and regulates hyphopodium formation
Within the MAPK cascade, MAPKKK, MAPKK, and MAPK are sequentially phosphorylated. To determine the upstream MAPKKs responsible for phosphorylating VdKss1, V. dahliae MAPKK genes were cloned and analyzed for their ability to activate VdKss1. The genome of V. dahliae contains three putative MAPKK genes, VdSte7 (VDAG_08626), VdMKK1 (VDAG_09823), and VdPbs2 (VDAG_02783) (Hamel et al. 2012). Double mutation of the conserved serine and/or threonine residues located in the kinase-activation loop converts MAPKKs into their constitutive active (CA) forms (Asai et al. 2002; Liu and Zhang 2004; Ren et al. 2002). CA mutants of each of the above MAPKKs were generated and co-expressed with VdKss1 in V. dahliae protoplasts. The phosphorylation of VdKss1 TEY motif was examined using an anti-pERK immuno-blot. VdSte7CA, but not VdMKK1CA or VdPbs2CA, specifically induces the phosphorylation of VdKss1 (Fig. 4A), indicating that VdSte7 can phosphorylate VdKss1.
VdSte7 phosphorylates VdKss1 and regulates hyphopodium formation. A The constitutive active (CA) mutant VdSte7CA specifically phosphorylates VdKss1. Verticillium dahliae protoplasts were transfected with VdKss1-HA alone or together with VdSte7CA-FLAG, VdMKK1CA-FLAG, or VdPbs2CA-FLAG. Proteins were extracted 16 h post transfection and subjected to Co-immunoprecipitation, then followed by anti-pERK or anti-HA immunoblot. The experiments were repeated three times with similar results. B Penetration phenotypes of VdMAPKKs deletion mutants and Vd∆ste7/VdSte7-GFP complementary strain. The spores of indicated strains were grown on MM covered with a cellophane for 5 days and photographed (above). The cellophane was removed and the culture was continued for 3 days and photographed (below). C Hyphopodium formation of VdMAPKKs deletion mutants and Vd∆ste7/VdSte7-GFP complementary strain. Indicated strains were grown on MM covered with a cellophane for 5 days. Hyphopodium formation (red arrow) on the cellophane was observed by confocal laser scanning microscopy (CLSM). D Hyphopodium quantification of VdMAPKKs deletion mutants and Vd∆ste7/VdSte7-GFP complementarystrain. The number of hyphopodium in three fields was counted for each strain. Error bars indicate the standard deviation of three fields. Student’s t-test was carried out to determine the significance of difference. **Indicates significant difference at P-value of < 0.01
We next generated mutants with gene deletion of individual MAPKK genes (Fig. S5) and examined the penetration and development of hyphopodium in these mutants. Consistent with the specific phosphorylation of VdKss1 induced by VdSte7CA, the Vd∆ste7 mutant, but neither the Vd∆mkk1 nor the Vd∆pbs2 mutants, exhibited compromised membrane penetration (Fig. 4B) and significantly reduced hyphopodium formation (Fig. 4C, D). The complementation of VdSte7 in the Vd∆ste7 mutant restored membrane penetration in the medium (Fig. 4B) and hyphopodium formation (Fig. 4C, D). Expression of VdSte7-GFP in the complementary strain was confirmed by anti-GFP immunoblot (Fig. S4B). The results indicating that VdSte7 acts as the upstream MAPKK to phosphorylate VdKss1 and contributes to the development of hyphopodium in V. dahliae.
VdSte11 activates VdKss1 and regulates hyphopodium formation
VdSte11 (VDAG_05822), VdBck1 (VDAG_00874), and VdSsk2 (VDAG_08787) are three putative MAPKKKs encoded by V. dahliae (Hamel et al. 2012). MAPKKK usually contains an N-terminal auto-inhibitory domain that negatively regulates kinase activity. Removal of the N-terminal inhibitory domain results in activation of MAPKKKs in the absence of upstream kinases (Asai et al. 2002; Bergmann et al. 2004). Deletion of VdSte11, a V. dahliae MAPKKK, has been reported to impair hyphopodium formation in V. dahliae (Yu et al. 2019), suggesting an important role of VdSte11 in regulating V. dahliae hyphopodium development. Whether VdSte11 activates VdKss1 and whether additional MAPKKKs are involved in hyphopodium development remain undetermined. The truncated C-terminal forms of the individual MAPKKKs, VdSte11CA, VdBck1CA, and VdSsk2CA, were then constructed and co-expressed with VdKss1 in V. dahliae protoplasts. Co-expression of VdSte11CA, but not VdBck1CA or VdSsk2CA, induced phosphorylation of VdKss1 (Fig. 5A), indicating that VdSte11 acts as the upstream MAPKKK that induces VdKss1 phosphorylation.
VdSte11 activates VdKss1 and regulates hyphopodium formation. A The CA mutant VdSte11CA activates VdKss1. Verticillium dahliae protoplasts were transfected with VdKss1-HA alone or together with VdSte11CA-FLAG, VdBck1CA-FLAG, or VdSsk2CA-FLAG. Proteins were extracted 16 h post transfection and subjected to Co-immunoprecipitation, then followed by anti-pERK or anti-HA immunoblot. The experiments were repeated three times with similar results. B Penetration phenotypes of VdMAPKKKs deletion mutants and Vd∆ste11/VdSte11-GFP complementary strain. The spores of indicated strains were grown on MM covered with a cellophane for 5 days and photographed (above). The cellophane was removed and the culture was continued for 3 days and photographed (below). C Hyphopodium formation of VdMAPKKKs deletion mutants and Vd∆ste11/VdSte11-GFP complementary strain. Indicated strains were grown on MM covered with a cellophane for 5 days. Hyphopodium formation (red arrow) on the cellophane was observed by confocal laser scanning microscopy (CLSM). D Hyphopodium quantification of VdMAPKKKs deletion mutants and Vd∆ste11/Ste11-GFP complementary strain. The number of hyphopodium in three fields was counted for each strain. Error bars indicate the standard deviation of three fields. Student’s t-test was carried out to determine the significance of difference. **Indicates significant difference at P-value of < 0.01
To further examine the role of MAPKKKs in the regulation of hyphopodium development, mutants with deletion of individual MAPKKKs were generated (Fig. S5). While the Vd∆bck1 and Vd∆ssk2 mutants exhibited normal penetration, the Vd∆ste11 mutant exhibited deficient cellophane penetration (Fig. 5B). Expression of VdSte11-GFP in the complementary strain was confirmed by anti-GFP immunoblot (Fig. S4C). All three mutants generated fewer hyphopodia than the WT strains, among which the Vd∆ste11 mutant was almost impaired in hyphopodium formation (Fig. 5C, D). The results indicated that VdSte11, VdBck1, and VdSsk2 are all involved in regulating hyphopodium formation, and that VdSte11 plays a greater role than VdBck1 and VdSsk2.
VdSte7 and VdSte11 are required for full pathogenicity of V. dahliae
To determine the role of VdSte7 and VdSte11 in pathogenicity, Vd∆ste7, Vd∆ste11 mutants and WT strains were subjected to pathogenicity assays in cotton plants. Reduced pathogenicity was observed in both the Vd∆ste7 (Fig. 6A, B) and Vd∆ste11 mutants (Fig. 6C, D). Complementation of VdSte7 in Vd∆ste7 mutant (Fig. 6A, B) and VdSte11 in Vd∆ste11 (Fig. 6C, D) restored their pathogenicity to the level of the WT strain. These results suggest that both VdSte7 and VdSte11 are required for full pathogenicity of V. dahliae. Thus, the results indicate that VdSte11-VdSte7-VdKss1 constitutes a complete MAPK cascade that regulates the hyphopodium formation and pathogenicity of V. dahliae.
Deletion of VdSte7 or VdSte11 compromises virulence of Verticillium dahliae in cotton plants. A, C. Disease symptoms of upland cotton. Plants infected with indicated strains were photographed and subjected to disease index analyses 3–4 weeks post inoculation. B, D Disease index analyses of upland cotton infected with the indicated strains. The disease indexes were evaluated with three replicates generated from 24 plants for each inoculum. Error bars indicate the standard deviation of three biological replicates. Student’s t-test was carried out to determine the significance of difference. **Indicates significant difference at P-value of < 0.01
Discussion
V. dahliae infects more than 200 kinds of plants, causing Verticillium wilt, leading to severe yield losses worldwide. Formation of hyphopodium is crucial for the establishment of V. dahliae infection, and the underlying regulatory mechanisms remain poorly characterized. We have previously reported the cAMP-mediated regulation of hyphopodium formation (Sun et al. 2019). VdSho1, a tetraspan transmembrane protein, regulates V. dahliae cellophane penetration and virulence in plants via the downstream MAPK signaling adaptor Vst50 (Li et al. 2019). Saccharomyces cerevisiae transmembrane mucin Msb2, which is widely conserved in fungi, functions upstream of the Kss1 MAPK cascade to regulate filamentous growth (Cullen et al. 2004; Perez-Nadales and Di Pietro 2011). In V. dahliae, VdMsb, has been reported to be required for plant infection and microsclerotia formation (Jiang et al. 2018; Tian et al. 2014). Moreover, mutation of VdSte11 has also been shown to impair hyphopodium formation (Yu et al. 2019), indicating the involvement of MAPK cascades in regulating hyphopodium formation. However, a complete MAPK cascade has not been characterized yet.
In this study, we explored the function of the MAPK pathway and identified VdSte11-VdSte7-VdKss1 as a complete MAPK cascade that regulates V. dahliae hyphopodium formation and virulence. Among the five MAPKs encoded by V. dahliae, VdKss1 and VdHog1-1 have been shown to regulate microsclerotia formation and pathogenicity (Rauyaree et al. 2005; Wang et al. 2016b). Our study indicated that VdKss1, but not VdHog1-1, is essential for hyphopodium formation. In addition, VdBck1 and VdSsk2 are involved in hyphopodium formation. However, the CA forms of VdBck1 and VdSsk2 did not induce VdKss1 phosphorylation, suggesting the existence of additional regulatory mechanisms of hyphopodium formation that are mediated by VdBck1 and VdSsk2.
Although MAPK cascades are highly conserved in fungi, differential MAPK cascade specificities also occurs in different fungi. In S. cerevisiae, the high osmolarity glycerol (HOG) pathway activates the Ste11/Ssk2/Ssk22-Pbs2-Hog1 MAPK cascades (Hamel et al. 2012). In V. dahliae, however, VdSsk2, but not VdSte11, induces the phosphorylation of VdHog1 in response to stress. Also, a differential contribution between VdSsk2 and VdSte11 in V. dahliae pathogenesis has been reported (Yu et al. 2019).
The MAPK cascade regulating V. dahliae hyphopodium formation is highly homologous to that utilized by M. oryzae in the regulation of appressorium formation. Hog homologs are widely found in fungi including M. oryzae (Dixon et al. 1999), Bipolaris oryzae (Moriwaki et al. 2006), Botrytis cinerea (Segmuller et al. 2007), and the oomycete Phytophthora sojae (Li et al. 2010). Hog pathway is vital for the accumulation of osmo-protectant molecules and thus required for responses to environmental stresses in fungi (Hamel et al. 2012). Hog pathway also regulates virulence in some phytopathogens but not in M. oryzae (Dixon et al. 1999; Wang et al. 2016b). Our study showed that VdHog1-1 and VdHog1-2 are dispensable for hyphopodium formation in V. dahliae. The evidence indicates the convergence of MAPK cascades in M. oryzae and V. dahliae, although different infection-related structures are developed by the two pathogens during infection.
In phytopathogenic fungi M. oryzae, the appressorium formation is regulated by the conserved MAPK pathway Mst11-Mst7-Pmk1. As the major intracellular MAPK that is targeted by HopAI in M. oryzae, Mps1 (the ortholog of Slt2 in S. cerevisiae) is important for appressorium penetration and plant infection but is not necessary for appressorium formation (Xu et al. 1998; Zhang et al. 2017). In M. oryzae, G protein-coupled receptors (GPCRs) (Li et al. 2012, 2007b) and cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) signaling pathways are involved in the regulation of appressoria formation (** et al. 2013; Zhao et al. 2007).
We have showed that VdKss1 plays an important role in regulating the development of hyphopodium and is required for the full pathogenicity of V. dahliae. In addition, the VdSlt2 also plays an important role in hyphopodium formation and pathogenicity of V. dahliae. Our results suggested that both VdKss1 and VdSlt2 contribute to V. dahliae hyphopodium formation and pathogenicity, with VdKss1 plays a greater role. Whether and how GPCRs and cAMP pathways interplay with the VdSte11-VdSte7-VdKss1 cascade to regulate hyphopodium formation in V. dahliae remains to be further investigated.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415:977–983. https://doi.org/10.1038/415977a
Bergmann DC, Lukowitz W, Somerville CR (2004) Stomatal development and pattern controlled by a MAPKK kinase. Science 304:1494–1497. https://doi.org/10.1126/science.1096014
Bigeard J, Hirt H (2018) Nuclear signaling of plant MAPKs. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2018.00469
Cullen PJ, Sabbagh W, Graham E, Irick MM, Van Olden EK, Neal C, Delrow J, Bardwell L, Sprague GF (2004) A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Genes Dev 18:1695–1708. https://doi.org/10.1101/gad.1178604
Dixon KP, Xu JR, Smirnoff N, Talbot NJ (1999) Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 11:2045–2058. https://doi.org/10.1105/tpc.11.10.2045
Gao F, Zhou BJ, Li GY, Jia PS, Li H, Zhao YL, Zhao P, **a GX, Guo HS (2010) A glutamic acid-rich protein identified in Verticillium dahliae from an insertional mutagenesis affects microsclerotial formation and pathogenicity. Plos One. https://doi.org/10.1371/journal.pone.0015319
Gustin MC, Albertyn J, Alexander M, Davenport K (1998) MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev 62:1264–1300. https://doi.org/10.1128/mmbr.62.4.1264-1300.1998
Hamel LP, Nicole MC, Duplessis S, Ellis BE (2012) Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell 24:1327–1351. https://doi.org/10.1105/tpc.112.096156
Jiang C, Zhang X, Liu HQ, Xu JR (2018) Mitogen-activated protein kinase signaling in plant pathogenic fungi. PLoS Pathog 14:8. https://doi.org/10.1371/journal.ppat.1006875
** QC, Li CY, Li YZ, Shang JJ, Li DB, Chen BS, Dong HT (2013) Complexity of roles and regulation of the PMK1-MAPK pathway in mycelium development, conidiation and appressorium formation in Magnaporthe oryzae. Gene Expr Patterns 13:133–141. https://doi.org/10.1016/j.gep.2013.02.003
Li CM, Hienonen E, Haapalainen M, Kontinen VP, Romantschuk M, Taira S (2007a) Type III secretion system-associated pilus of Pseudomonas syringae as an epitope display tool. FEMS Microbiol Lett 269:104–109. https://doi.org/10.1111/j.1574-6968.2006.00612.x
Li L, Wright SJ, Krystofova S, Park G, Borkovich KA (2007b) Heterotrimeric G protein signaling in filamentous fungi. Annu Rev Microbiol 61:423–452. https://doi.org/10.1146/annurev.micro.61.080706.093432
Li AN, Wang YL, Tao K, Dong SM, Huang QA, Dai TT, Zheng XB, Wang YC (2010) PsSAK1, a stress-activated MAP kinase of Phytophthora sojae, is required for zoospore viability and infection of soybean. Mol Plant Microbe Interact 23:1022–1031. https://doi.org/10.1094/mpmi-23-8-1022
Li GT, Zhou XY, Xu JR (2012) Genetic control of infection-related development in Magnaporthe oryzae. Curr Opin Microbiol 15:678–684. https://doi.org/10.1016/j.mib.2012.09.004
Li JJ, Zhou L, Yin CM, Zhang DD, Klosterman SJ, Wang BL, Song J, Wang D, Hu XP, Subbarao KV, Chen JY, Dai XF (2019) The Verticillium dahliae Sho1-MAPK pathway regulates melanin biosynthesis and is required for cotton infection. Environ Microbiol 21:4852–4874. https://doi.org/10.1111/1462-2920.14846
Liu YD, Zhang SQ (2004) Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16:3386–3399. https://doi.org/10.1105/tpc.104.026609
Moriwaki A, Kubo E, Arase S, Kihara J (2006) Disruption of SRM1, a mitogen-activated protein kinase gene, affects sensitivity to osmotic and ultraviolet stressors in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol Lett 257:253–261. https://doi.org/10.1111/j.1574-6968.2006.00178.x
Perez-Nadales E, Di Pietro A (2011) The membrane mucin Msb2 regulates invasive growth and plant infection in Fusarium oxysporum. Plant Cell 23:1171–1185. https://doi.org/10.1105/tpc.110.075093
Rauyaree P, Ospina-Giraldo MD, Kang S, Bhat RG, Subbarao KV, Grant SJ, Dobinson KF (2005) Mutations in VMK1, a mitogen-activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Curr Genet 48:109–116. https://doi.org/10.1007/s00294-005-0586-0
Rehman L, Su XF, Guo HM, Qi XL, Cheng HM (2016) Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae. BMC Biotechnol 16. https://doi.org/10.1186/s12896-016-0287-4.
Ren DT, Yang HP, Zhang SQ (2002) Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem 277:559–565. https://doi.org/10.1074/jbc.M109495200
Segmuller N, Ellendorf U, Tudzynski B, Tudzynski P (2007) BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot Cell 6:211–221. https://doi.org/10.1128/ec.00153-06
Sun LF, Qin J, Rong W, Ni H, Guo HS, Zhang J (2019) Cellophane surface-induced gene, VdCSIN1, regulates hyphopodium formation and pathogenesis via cAMP-mediated signalling in Verticillium dahliae. Mol Plant Pathol 20:323–333. https://doi.org/10.1111/mpp.12756
Tian LL, Xu J, Zhou L, Guo WZ (2014) VdMsb regulates virulence and microsclerotia production in the fungal plant pathogen Verticillium dahliae. Gene 550:238–244. https://doi.org/10.1016/j.gene.2014.08.035
Wang YQ, Dohlman H (2002) Regulation of MAPK signaling by ubiquitination. FASEB J 16:A557–A557
Wang S, **ng HY, Hua CL, Guo HS, Zhang J (2016a) An improved single-step cloning strategy simplifies the Agrobacterium tumefaciens-mediated transformation (ATMT)-based gene-disruption method for Verticillium dahliae. Phytopathology 106:645–652. https://doi.org/10.1094/phyto-10-15-0280-r
Wang YL, Tian LY, **ong DG, Klosterman SJ, **ao SX, Tian CM (2016b) The mitogen-activated protein kinase gene, VdHog1, regulates osmotic stress response, microsclerotia formation and virulence in Verticillium dahliae. Fungal Genet Biol 88:13–23. https://doi.org/10.1016/j.fgb.2016.01.011
Widmann C, Gibson S, Jarpe MB, Johnson GL (1999) Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol Rev 79:143–180. https://doi.org/10.1152/physrev.1999.79.1.143
Xu JR, Staiger CJ, Hamer JE (1998) Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc Natl Acad Sci USA 95:12713–12718. https://doi.org/10.1073/pnas.95.21.12713
Xu L, Zhang WW, He X, Liu M, Zhang K, Shaban M, Sun LQ, Zhu JC, Luo YJ, Yuan DJ, Zhang XL, Zhu LF (2014) Functional characterization of cotton genes responsive to Verticillium dahliae through bioinformatics and reverse genetics strategies. J Exp Bot 65:6679–6692. https://doi.org/10.1093/jxb/eru393
Yong HY, Bakar FDA, Illias RM, Mahadi NM, Murad AMA (2013) Cgl-SLT2 is required for appressorium formation, sporulation and pathogenicity in Colletotrichum gloeosporioides. Braz J Microbiol 44:1241–1250. https://doi.org/10.1590/s1517-83822013000400031
Yu J, Li TY, Tian LY, Tang C, Klosterman SJ, Tian CM, Wang YL (2019) Two Verticillium dahliae MAPKKKs, VdSsk2 and VdSte11, have distinct roles in pathogenicity, microsclerotial formation, and stress adaptation. Msphere 4:18. https://doi.org/10.1128/mSphere.00426-19
Zhang J, Shao F, Cui H, Chen LJ, Li HT, Zou Y, Long CZ, Lan LF, Chai JJ, Chen S, Tang XY, Zhou JM (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1:175–185. https://doi.org/10.1016/j.chom.2007.03.006
Zhang X, Liu WD, Li Y, Li GT, Xu JR (2017) Expression of HopAI interferes with MAP kinase signalling in Magnaporthe oryzae. Environ Microbiol 19:4190–4204. https://doi.org/10.1111/1462-2920.13884
Zhao XH, Kim Y, Park G, Xu JR (2005) A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea. Plant Cell 17:1317–1329. https://doi.org/10.1105/tpc.104.029116
Zhao XH, Mehrabi R, Xu JR (2007) Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot Cell 6:1701–1714. https://doi.org/10.1128/Ec.00216-07
Zhao P, Zhao YL, ** Y, Zhang T, Guo HS (2014) Colonization process of Arabidopsis thaliana roots by a green fluorescent protein-tagged isolate of Verticillium dahliae. Protein Cell 5:94–98. https://doi.org/10.1007/s13238-013-0009-9
Zhao YL, Zhou TT, Guo HS (2016) Hyphopodium-specific VdNoxB/VdPls1-dependent ROS-Ca2+ signaling is required for plant infection by Verticillium dahliae. PLoS Pathog 12:23. https://doi.org/10.1371/journal.ppat.1005793
Zhou TT, Zhao YL, Guo HS (2017) Secretory proteins are delivered to the septin-organized penetration interface during root infection by Verticillium dahliae. PLoS Pathog 13:26. https://doi.org/10.1371/journal.ppat.1006275
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2022YFD1400800), the Chinese Natural Science Foundation (32172504), the CAS Projects for Young Scientists in Basic Research (YSBR-080), the Chinese Natural Science Foundation (32200241), the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDPB16) and the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ye, Z., Qin, J., Wang, Y. et al. A complete MAP kinase cascade controls hyphopodium formation and virulence of Verticillium dahliae. aBIOTECH 4, 97–107 (2023). https://doi.org/10.1007/s42994-023-00102-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42994-023-00102-y