Abstract
Two cases of disseminated cryptococcosis are described. The first was an HIV-infected patient where cryptococcosis was diagnosed as “unmasking immune reconstitution syndrome”; the second was an immunosuppressed patient with multiple myeloma. In both cases, a definitive healing could not be reached despite long therapeutic approaches. This review summarizes both the most recent and relevant studies about disseminated and refractory form of cryptococcal infections and identifies research gaps. Given the limited data, we draw some conclusions with respect to management from literature: not clear and accepted indication are available regarding disseminated cryptococcosis, no specific schemes were identified, and the duration of therapy is usually decided case by case and supported only by case reports. In this perspective, usually standard therapeutic schemes and duration of induction depend on multiple factors (e.g., neurologic deficit, non-HIV/non transplant status, CSF culture positivity at 2 weeks, etc.). We found that there are no empiric and literature data that support a role of cryptococcal serum antigen (CRAG) in guiding the antifungal therapy; with the data collected, we think that although is possible, it is very rare to find disseminated cryptococcosis with negative CRAG. We looked also for the more important risk factor of recurrence. Some possible causes explored are risk of azole resistant strains, pre-existent conditions of patients that play a permissive role and the common situation where flucytosine is unavailable that led to suboptimal induction phase of therapy. Herein, we discuss disseminated cryptococcosis with a particular attention to antifungal therapy, role of cryptococcal antigen, and risk factors for recurrence of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryptococcus neoformans is an encapsulated yeast, which is present in environment, causing life-threatening infections such as meningitis, pulmonary cryptococcosis, or disseminated form of the disease [1]. Predominantly, it affects patients with impairment of immune status such as human immunodeficiency virus (HIV) infection. Before the introduction of highly active antiretroviral therapy (HAART), as many as 5% of all HIV-infected persons developed cryptococcosis [2]. Since then, the incidence has decreased by approximately one-half [3]. With the improvement of antiretroviral therapy (ART) and reduction of AIDS diagnoses, more cases are related to other cause of immunosuppression, like solid organ transplantation (SOT), systemic lupus erythematosus (LES), malignancy, and sarcoidosis [4, 5, 6, 7, 8]. Nationally representative estimates for the incidence of cryptococcosis are difficult to obtain because cryptococcosis is only reportable in a few countries. More data are available regarding cryptococcal meningitis: the regions with the highest number of estimated cases were sub-Saharan Africa (162,500 yearly cases), followed by South and Southeast Asia (43,200 yearly cases), while Europe accounts for nearly 4,400 yearly cases [9].
The diagnostic criteria of disseminated cryptococcosis have been defined as two or more non-adjacent organs being simultaneously affected with Cryptococcus spp. [10] and its management remains a challenge for doctors: persistence or recurrence of disease despite adequate therapy is indeed very common.
We present two cases of disseminated cryptococcosis focusing on clinical management and duration of therapy, infection control, and risk of relapse.
Case 1
HIV infection was diagnosed in a 49-year-old woman in September 2018 as a result of checks for weight loss and asthenia. Baseline CD4+ cells were 22/μL, HIV-RNA 1,360,000 copies/mL. Antiretroviral therapy (ART) with dolutegravir (DTG) + tenofovir disoproxil fumarate (TDF) + emtricitabine (FTC) has been started the same day when HIV was diagnosed according to the test and treat strategy [11]. After 1 month of therapy, the CD4 cell count was 182/μL and the viral load was of 478 copies/mL. In November 2018, she was hospitalized at our division for confusion, asthenia, and visual hallucination. Upon admission, she underwent lumbar puncture. Cerebrospinal fluid (CSF) was cloudy, white cells 240/μL (mainly monocytes), glucose 19 mg/dl, ratio blood glucose and CSF glucose 20 %, and protein 1.74 g/L; opening pressure was not reported during the procedure. Polymerase chain reaction (PCR) by FilmArray™ (Biomerieux, Ponte a Ema, Florence, Italy) for meningitis/encephalitis was positive for Cryptococcus neoformans/gattii; CrAg was positive (>1:512). Microscopic examination and CSF fungal culture were both negative.
Treatment with liposomal amphotericin B (L-Amb) 4 mg/kg/day plus fluconazole (200mg ×3/day) and dexamethasone 8mg/day was carried out for 2 weeks and ART stopped. 5-flucytosine was not used because it was not available. The symptoms disappeared and the woman was discharged after 2 weeks of therapy with home therapy based on fluconazole 400 mg/day prescribed for 8 weeks and with an indication to resume ART and follow fluconazole secondary prophylaxis (200mg/day orally).
In January 2019, under fluconazole prophylaxis, a magnetic resonance imaging (MRI) of the brain was performed and a 6-mm lesion in the corpus striatum was observed, compatible with cryptococcoma. A computerized tomography (CT) total body was therefore performed. It showed a subpleural nodular lesion in the left lung associated with enlarged node; both lesions were examined by positron-emission tomography (PET) revealing an intense metabolic activity. In the same month, a fine-needle biopsy was performed in the lung lesion with evidence of focal necrotic areas and a Gorcott-positive and PAS-positive microscopic formation, compatible with cryptococcosis.
In July 2019, a new CT scan showed new nodules in the left lung. Histological examination of the aspirate obtained by transbronchial needle aspiration (TBNA) showed a PAS-positive lesion suggesting a probable cryptococcal etiology.
Moreover, a new brain MRI showed enlargement of the aforementioned lesion and appearance of new lesions, so a new therapeutic attempt with L-Amb (at the dosage used previously) was started. Unfortunately, despite the maximal antifungal treatment with L-Amb 4 mg/kg and fluconazole 400mg administered periodically and ongoing at the time of the writing (September 2020), cerebral lesions are progressively worsening, and clinical conditions are deteriorated despite CD4+ cells increased at 270/μL, and HIV-RNA are <20 copies/mL.
Case 2
In July 2019, a 70-year-old man with multiple myeloma (MM) started having episodes of epilepsy treated with long-term levetiracetam.
Previously, in 2010, he was treated with an unsuccessful autologous hemopoietic stem cell transplantation (HSTC) followed by lenalidomide plus steroid treatment. In July 2019, a brain CT scan performed due to progressive headache was negative; conversely a concomitant chest X-ray showed opacity of the lower right lung lobe in the absence of pneumonia symptoms. In August 2019, the patient had new episodes of seizures, and a brain CT scan showed brain injuries (microangiopathic phenomena probably not related to the cryptococcal disease).
The patient, immediately hospitalized, underwent lumbar puncture: the cerebrospinal fluid was clear, proteins 0.30 g/l, leukocytes 9/μL and glucose 61 mg/dl, ratio blood glucose and CSF glucose 20 %, and India ink smear and cryptococcal antigen (CrAg) in CSF tested positive (>1:512); opening pressure was not reported during the procedure.
A prompt treatment with L-Amb (3.5 mg/kg/die) and fluconazole (200 mg × 3/die) for the first 2 weeks was started; as soon as flucytosine was available, we introduced it (dose 2.5 g × 2/die), while lenalidomide was stopped due to severe leukopenia. A new lumbar puncture was done 4 weeks apart and the culture for Cryptococcus spp. was negative; CrAg was still positive in CSF (titer >512).
After 60 days of treatment, an encephalic MRI did not show improvement. Moreover, a chest CT scan confirmed the previous right lung opacity, and through bronchoalveolar lavage and TBNA, C. neoformans infection was demonstrated (histologically). The hospital stays lasted 72 days, and during the antifungal treatment, several analyses of the cerebrospinal fluid were repeated with persistently positive results of CrAg> 1: 512, PCR for C. neoformans and ink test.
As the clinical conditions improved, despite the failure to eradicate the Cryptococcus from the CSF, the patient was discharged with fluconazole at the 400-mg daily dose, but at the moment of writing this manuscript, he was lost at clinical follow-up.
Discussion
The diagnostic criteria of disseminated cryptococcosis have been defined as 2 or more non-adjacent organs being simultaneously affected with cryptococcosis [10]. Disseminated cryptococcosis is a real challenge for clinicians and its management continues to be unclear. Both cases show the paradigm that immune regulation is necessary to control the infection: in Case 1, the low CD4 +T cell count facilitated the spreading of infection. Moreover, it is a paradigmatic example of unmasking immune reconstitution syndrome (IRIS). In Case 2, the impairment of immune system made the patient prone to infection due to the underlying cellular and humoral immunodeficiency associated with multiple myeloma.
We managed both patients tailoring the antifungal therapy to the single patient, but such strategy has been ineffective and left us full of questions unsolved such as: How long the antifungal therapy should be prolonged? Is there a role for Cryptococcus antigen title in guiding the duration of therapy? Which are the main causes of relapsing infection? We reviewed what literature says focusing on these three aspects of management of disseminated cryptococcosis.
Antifungal Therapy
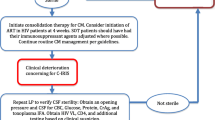
The optimal approach to antifungal therapy for C. neoformans meningoencephalitis and/or disseminated disease involves three phases: induction therapy with intravenous amphotericin B liposomal plus flucytosine (if available) or fluconazole for at least 2 weeks, followed by consolidative therapy for eight weeks, and then maintenance (i.e., suppressive) therapy for at least 1 year to decrease the risk of relapse with fluconazole. Table 1 and Table 2 summarize antifungal therapy both in HIV-infected individuals and transplant recipient ones.
Considering that no clinical trial has evaluated the optimal therapy of non-meningeal disease, the treatment regimen suggested is based on expert opinion, and induction phase should be prolonged for at least 6 weeks [13].
Treatment of disseminated cases of the disease should have an induction phase of four weeks at minimum (in HIV patients and non-HIV as well); this duration should be prolonged to 6 weeks if neurological forms are present.
In the studies found, these schemes appear to reduce mortality from 14–25% to 6% and relapses from 17–24% to 2–4% [14]. In all cases which flucytosine could not be used, it is suggested to add fluconazole amphotericin B. Although some articles of low quality revealed good outcomes for cryptococcal meningitis in HIV-negative patient treated with only fluconazole [2], this monotherapy remain suboptimal in HIV-positive patient [15].
In both presented cases, induction therapy with L-Amb was prolonged for more than 2 weeks: in Case 1, it was re-administered due to relapse of infection for further 2 months, while in Case 2, it was never stopped for 10 weeks due to the persistence of infection in the CSF until the discharge of the patient. Anyway, it is not always possible to prolong the antifungal therapy due to possible drug toxicity like nephrotoxicity, hypokalemia, and hypomagnesemia, so that infection remains uncontrolled.
It follows that regarding disseminated cryptococcosis, no specific schemes about induction, consolidation, and maintenance were identified by clinical trial, and duration of therapy is usually decided case by case and supported only by case report and case series [13]. A Chinese retrospective study made on 48 patients less than 18 years old with disseminated cryptococcosis showed good results (overall mortality rate of pediatric patients 11.5%) with amphotericin B deoxycholate (0.7–1.0 mg/kg/die), 5-flucytosine (100 mg/kg/die), and fluconazole (6 mg/kg/die) followed by fluconazole (6 mg/kg/die) for 6–12 months [16].
As seen in Table 3, different therapeutic schemes were used recently in disseminated cryptococcosis with heterogeneous results.
Never a doubt that amphotericin B is the milestone of anticryptococcal therapy, but half a century after the introduction of it, the management of cryptococcosis remains indeed unsatisfactory. Research about innovative drugs is warranted, with several antimicrobial agents showing in vitro activity against Cryptococcus [38] but such discussion is beyond the scope of this review.
Role of Cryptococcal Antigen
In both our cases, CrAg was useless as a follow-up marker of infection control despite the therapy: in Case 1, the titer (starting from >1:512) dropped in just one evaluation (1:128) before rising up again, while a titer of >1:512 was always found in Case 2.
CrAg level was demonstrated to be related to the presence of symptoms of cryptococcosis more often than asymptomatic form of the disease, which appears to anticipate death. The literature showed that several cohorts underline a connection among plasma titers and mortality: when the first increase, the latter is higher as well [39]. Another relevant point is that symptoms appear to be linked to survival only for certain titers of CrAg; in fact, titers <1:80 plus symptoms like headache did not correlate to survival, and on the other hand, titers between 1:160 and 1:320 in presence of symptoms correlate with lower survival more often than same titers but in asymptomatic patients. In case of very high titers (e.g., 1:640), survival appears to be poor independently of presence of symptoms [39].
The importance of asymptomatic CrAg as a precursor to symptomatic meningitis and death has also already well been defined [40]. In fact, CrAg is detectable in blood weeks to months before onset of meningitis symptoms [41], and long-term maintenance therapy with fluconazole is indicated to prevent relapse after an episode of cryptococcal meningitis in patients with advanced HIV infection and asymptomatic presence of cryptococcal antigen [42].
Moreover, is not well established if there is a value of CrAg titer that can predict the disseminated form of the disease. As is possible to see in Table 4, among the most recent case report, the titer had different values (mean 858, median 384). There was also a report of a very high titer (>1:100.000) [43]. These data suggest that although is possible, it is very rare to find disseminated form with negative CrAg.
Among asymptomatic CrAg-positive persons, CrAg titers of ≥1:160 are associated with increased mortality despite receiving fluconazole pre-emptive therapy [48].
The WHO, indeed, recommends routine serum or plasma CrAg screening in ART (antiretroviral therapy)-naïve adults a CD4 counts < 100 cells/μL, followed by pre-emptive anti-fungal therapy if CrAg positive, to reduce the development of cryptococcal disease. It is proved that this strategy can prevent clinical disease, avoid hospitalization, and improve long-term survival to be equivalent to CRAG-negative persons, and it is also regarded as cost effective for specific groups [49, 50]. In a cluster randomized study that enrolled ART-naive participants with CD4 ≤100x106/L, asymptomatic CrAg + participants received pre-emptive fluconazole therapy; the results showed that 6-month mortality of participants with CrAg titers ≤1:160 and CRAG-negative patients did not differ, but patients with CrAg titers >1:160 had 2.6-fold higher 6-month mortality than patients with titers ≤1:160 suggesting that pre-emptive antifungal therapy for asymptomatic cryptococcosis seemed to be effective in patients with CrAg titer ≤1:160 and a more aggressive approach is required for persons with CrAg titer >1:160 [51].
A Cochrane systematic review showed that antifungal prophylaxis reduced the risk of develo** and dying from cryptococcal disease. Therefore, where CrAg screening is not available, antifungal prophylaxis may be used in patients with low CD4 counts at diagnosis and who are at risk of develo** cryptococcal disease [52].
There are no empiric and literature data that support a role of cryptococcal serum antigen (CRAG) in guiding the antifungal therapy. Future areas of research should include evaluation of customized therapy according to titer in persons with cryptococcal infection.
Recurrence of Disease
Increasing concerns are spreading about high level of persistence and frequency of relapse after a case of cryptococcal meningitis or disseminated cryptococcosis [12].
We summarized three aspects that may explain recurrence of disease.
On the one hand, the widespread use of fluconazole for long-term suppressive therapy of cryptococcal infection may cause the development of fluconazole resistance, especially among the relapsing patients [53].
The importance of susceptibility testing of C. neoformans isolates in all cases of meningeal cryptococcosis, even without fluconazole exposure, has been stressed out in several works and case reports, but in real-life scenarios, the methods are not always feasible. In both our cases, unfortunately, it has not been carried out because of the absence of growth of the microorganism, and we cannot exclude a fluconazole resistance as trigger of relapsing infection (especially in Case 1).
However, the unavailability of MIC interpretive breakpoints for any antifungal against Cryptococcus spp. together with discrepancies between the available methods makes it difficult to correlate in vitro MICs and clinical outcome when a single episode is tested [53].
In the literature, this problem was already took in account: a systematic review with a collection of 4995 isolates of Cryptococcus from 3210 patients, with 248 (5%) of the isolates from relapsing episode, showed resistance level of 12.1% (95% CI: 5.5–15.6) in whom had no relapse and 24.1% (95% CI: 3.1–51–2) in relapsing case [54]. A gradual increase in fluconazole resistance appeared over the years in USA: data reported in 1993 and 2001 demonstrated zero (0%) and 1.1% resistance, respectively [55, 56]. However, data are uncertain since some studies suggest that C. neoformans appeared not to increase the risk of failure or relapse during treatment [21]. Notably, some serotype of the fungus shows less susceptibility to azoles (serotype A is less susceptible compared to serotype D) [57].
The second point regards the importance of combining flucytosine to amphotericin B in the treatment strategy. Some authors stressed that without flucytosine as backbone, patients did not negativize cultures easily with the possibility that the pathogen may be still isolated in different samples after 2 or more months of adequate therapy (amphotericin B and fluconazole). Probably, the absence of flucytosine may cause the development of resistance in course of therapy: a study tried to demonstrate this possibility, and it was found that in a group with relapses or those who did not negativize cultures, one isolate (out of 256 strains) became resistant after therapy (MIC ≤64 mg/ml) and other four showed dose-dependent susceptibility (MIC 16–32 mg/ml) [58].
A third element that could determine the risk of relapse or treatment failure is the underlying condition that plays a role in the onset of the disease. Beyond the typical subset of people living with HIV and transplant recipients, it has been pointed out that even non-HIV non-transplant (NHNT) patients suffer from a high mortality form of this disease suggesting that cryptococcosis in NHNT patients appears to be a distinct entity that needs further study and requires a higher level of clinical suspicion than it currently receives [59].
Obviously, in transplant recipients with cryptococcosis, the outcome appears to be influenced by the type of immunosuppressive agent employed [60].
A possible and much rare cause could be the presence of sanctuary district for Cryptococcus where level of drugs remains too low to eradicate the fungus.21 36
Conclusion
Definitive treatment recommendations for disseminated cryptococcosis are hampered by the absence of prospective, randomized controlled trials or prospective cohort studies of patient outcomes. Treatment guidelines are based on retrospective case series, expert opinion, and are inferred from studies of CNS cryptococcosis, especially those of cryptococcal meningitis in HIV-infected patients.
There is also an urgent need to establish antifungal breakpoints for Cryptococcus spp. For the moment, it could be reasonable to obtain new specimens at each relapse and retest the MIC of the isolate to identify treatment failure early and reduce the risks of further relapses; in this setting, measurement of antifungal drug levels could also help.
The use of non-azole therapy early in the course of the disease for such patients could potentially improve clinical outcome and prognosis.
Given the importance of CRAG titer in predicting meningitis and/or death, CRAG titer will likely be used in the future to customize therapy both for prevention and treatment of cryptococcal meningitis.
References
Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS — 100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8(4):515–48.
Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence : findings from 4961 suspected cases. 2010.
Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:1098–107.
Beji S, Hajji M, El Kateb H, et al. Disseminated cryptococcosis as a complication of lupus nephritis. Saudi J Kidney Dis Transpl. 2017;28(6):1435–9.
Baddley JW, Forrest GN. Cryptococcosis in solid organ transplantation infectious diseases community of practice. 2013. https://doi.org/10.1111/ajt.12116.
Wang LR, Barber CE, Johnson AS, Barnabe C. Invasive fungal disease in systemic lupus erythematosus: a systematic review of disease characteristics, risk factors, and prognosis. Semin Arthritis Rheum. 2014;44(3):325–30. https://doi.org/10.1016/j.semarthrit.2014.06.001.
Cancelli I, Merlino G, Serafini A, Valente M, Luigi G. Sarcoidosis as risk factor for cryptococcal meningitis in an apparently immunocompetent patient. 2008;29:33–5. https://doi.org/10.1007/s10072-008-0856-y.
Lin Y, Shiau S, Fang C. Risk factors for invasive Cryptococcus neoformans diseases: a case-control study. 2015;10:1–13. https://doi.org/10.1371/journal.pone.0119090.
Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 17(8):873–81. https://doi.org/10.1016/S1473-3099(17)30243-8.
Ben PD, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer / Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group EORTC / M. Clin Infect Dis. 2008;46:1813–21. https://doi.org/10.1086/588660.
Nachega JB, Uthman OA, del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis. 2014;59:S21–7.
Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of. America. 2010;50:291–322. https://doi.org/10.1086/649858.
The diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva: World Health Organization; 2018 Mar.
Dromer F, Mathoulin S, Dupont B, Brugiere O, Letenneur L. Comparison of the efficacy of amphotericin B and fluconazole in the treatment of cryptococcosis in human immunodeficiency virus-negative patients: retrospective analysis of 83 cases. Clin Infect Dis. January 1985;1993:154–60.
Beyene T, Zewde AG, Balcha A, Hirpo B. Inadequacy of high-dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg )– positive human immunodeficiency virus-infected persons in an Ethiopian CrAg screening program. Clin Infect Dis. 2017;65(August 2014):2014–7. https://doi.org/10.1093/cid/cix613.
Gao L, Jiao A, Wu X, et al. Clinical characteristics of disseminated cryptococcosis in previously healthy children in China. BMC Infect Dis. 2017;17:1–10. https://doi.org/10.1186/s12879-017-2450-5.
Chavapradit N, Angkasekwinai N. Disseminated cryptococcosis in Crohn’s disease: a case report. BMC Infect Dis. 2018;3:1–5.
Han G, Kwon SH, Song HJ, Jeon J. Disseminated cryptococcosis presenting as cellulitis. Indian J Dermatol Venereol Leprol. 2017;83(1):89–91.
Vechi HT, Theodoro RC, Oliveira AL. De, et al. Invasive fungal infection by Cryptococcus neoformans var. grubii with bone marrow and meningeal involvement in a HIV-infected patient: a case report. BMC Infect Dis. 2019;19(1):1–8.
Sato S, Kambe E, Tamai Y. Disseminated cryptococcosis in a patient with multiple myeloma treated with daratumumab, lenalidomide , and dexamethasone. Intern Med. 2019;58(6):843–7. https://doi.org/10.2169/internalmedicine.1726-18.
Ito M, Hinata T, Tamura K, Koga A, Ito T, Fujii H. Disseminated cryptococcosis with adrenal insufficiency and meningitis in an immunocompetent individual. Intern Med. 2016;56(10):1259–64. https://doi.org/10.2169/internalmedicine.56.7356.
Pal P, Ray S, Patra SK, Mukherjee D. Disseminated cryptococcosis in an apparently immunocompetent patient presenting with primary intraventricular haemorrhage. BMJ Case Rep. 2015:10–3. https://doi.org/10.1136/bcr-2015-210250.
Sacht GL, de Lima AM, Perdomo YC, Boigues RS, Takita LC, Filho GH. Disseminated cryptococcosis with cutaneous involvement in an immunocompetent patient. An Bras Dermatol. 2016;91(6):832–4.
Huang D. Disseminated cryptococcosis in a patient with multiple sclerosis treated with fingolimod. Neurology. 2015;85(11):1001–3.
Slawinska M, Hlebowicz M, Iżycka-Świeszewska E, et al. Dermoscopic observations in disseminated cryptococcosis with cutaneous involvement. J Eur Acad Dermatol Venereol. 2018;32(6):e223–4. https://doi.org/10.1111/jdv.14744.
Qu F, Qu Z, Lv Y, Song B, Wu B. Disseminated Cryptococcosis revealed by transverse myelitis in Immunocompetent patient: a case report and review of the literature. BMC Neurol. 2020;20(1):13.
Inaba A, Okada A, Yoshida T, Itoyama S. Disseminated cryptococcosis with rapidly growing lung nodules in an end-stage renal disease patient. Intern Med. 2016;56(3):377–80. https://doi.org/10.2169/internalmedicine.56.7438.
Ni W, Huang Q, Cui J. Disseminated cryptococcosis initially presenting as cellulitis in a patient suffering from nephrotic syndrome. BMC Nephrol. 2013;14(20):3–6.
Adzic-vukicevic T, Cevik M, Poluga J, et al. An exceptional case report of disseminated cryptococcosis in a hitherto immunocompetent patient. Rev Inst Med Trop Sao Paulo. 2020;62(e3):2–5.
Suner L, Mathis S. Disseminated Cryptococcosis in Bone Marrow. Blood. 2014;123(20):3070.
Sciaudone G, Pellino G, Guadagni I, Somma A, Armiento FPD, Selvaggi F. Disseminated Cryptococcus neoformans infection and Crohn ’ s disease in an immunocompetent patient. J Crohn’s Colitis. 2011;5(1):60–3. https://doi.org/10.1016/j.crohns.2010.08.003.
Chan IS, Bs RC, Burrows AB. Delayed diagnosis of disseminated cryptococcosis with associated meningoencephalitis in an immunocompetent Septuagenarian host. Am J Med. 130(1):e31–2. https://doi.org/10.1016/j.amjmed.2016.05.044.
Hirai Y, Ainoda Y, Shoji T, Fujita T, Yoshinaga K. Disseminated cryptococcosis in a non-Hodgkin’s lymphoma patient with late-onset neutropenia following rituximab-CHOP chemotherapy: a case report and literature review. Mycopathologia. 2011;172(3):227–32. https://doi.org/10.1007/s11046-011-9423-9.
Matsuda Y, Kawate H, Okishige Y, Abe I, Adachi M, Ohnaka K, et al. Successful management of cryptococcosis of the bilateral adrenal glands and liver by unilateral adrenalectomy with antifungal agents: a case report. BMC Infect Dis. 2011;14(11):6–10. https://doi.org/10.1186/1471-2334-11-340.
Friedman DK, K Oberdorfer P. Disseminated cryptococcosis in an HIV-positive boy. BMJ Case Rep. 2012;2012:bcr2012007036.
Chaya R, Padmanabhan S, Anandaswamy V, Moin A. Disseminated Cryptococcosis presenting as cellulitis in a renal transplant recipient. J Infect Dev Ctries. 2013:1–4.
Nankeu S, Djaha J, aint Marcoux B, Kaci R, Debandt M. Disseminated cryptococcosis revealed by vertebral osteomyelitis in an immunocompetent patient. Jt Bone Spine. 2012;79:29–31. https://doi.org/10.1016/j.jbspin.2012.01.014.
Santos-gandelman J, Rodrigues ML, Machado A, Santos-gandelman J, Lourenço M, Silva AM. Future perspectives for cryptococcosis treatment. Expert Opin Ther Pat. 2018;00(00):1–10. https://doi.org/10.1080/13543776.2018.1503252.
Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR. Cryptococcal meningitis diagnostics and screening in the era of point-of-care laboratory testing. J Clin Microbiol. September 2018;2019:1–8.
Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4 + cell count р 100 cells / m L who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51(4):448–55. https://doi.org/10.1086/655143.
Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. 2007;12(8):929–35. https://doi.org/10.1111/j.1365-3156.2007.01874.x.
Diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. WHO. 2011;(December).
Ferraioli G, Cavanna C, Ricciardi M, et al. Retroperitoneal Cryptococcoma in a Case of Disseminated Cryptococcosis on Antifungal Maintenance Therapy. Curr HIV Res. October 2008;2011:28–30.
Saini AG, Patil S, Agrawal T, Basha A, Garg R, Rudramurthy SM, et al. Cryptococcosis in an immune-competent child. J Infect Public Health. 2018;11(3):436–8. https://doi.org/10.1016/j.jiph.2017.09.015.
Chen M, Wang X, Yu X, Dai C, Chen D, Yu C, et al. Pleural effusion as the initial clinical presentation in disseminated cryptococcosis and fungaemia: an unusual manifestation and a literature review. BMC Infect Dis. 2015;15:4–11. https://doi.org/10.1186/s12879-015-1132-4.
Haraga J, Leblanc M, Chiovaro J. Not all it’s CrAged up to be: disseminated cryptococcosis. Am J Med. 2018;131(12):1452–5. https://doi.org/10.1016/j.amjmed.2018.07.015.
Hung Z, Lai Y, Hsu Y, Wang C, Fang T, Hsu B. Disseminated cryptococcosis causes adrenal insufficiency in an immunocompetent individual. Intern Med. 2010;49(11):1023–6. https://doi.org/10.2169/internalmedicine.49.3051.
Letang E, Müller MC, Ntamatungiro AJ, et al. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis. 2015:1–8. https://doi.org/10.1093/ofid/ofv046.
Patel S, Shin GYWI, Vitharana SR, et al. The prevalence of cryptococcal antigenemia in newly diagnosed HIV patients in a Southwest London. J Infect. 2013;66(1):75–9. https://doi.org/10.1016/j.**f.2012.09.014.
Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label , randomised controlled trial. Lancet. 2015;385:2173–82. https://doi.org/10.1016/S0140-6736(15)60164-7.
Meya DB, Kiragga AN, Nalintya E, Morawski BM, Rajasingham R, Park BJ, et al. Reflexive laboratory-based cryptococcal antigen screening and preemptive fluconazole therapy for cryptococcal antigenemia in HIV-infected individuals with CD4 <100 cells/μL: a stepped-wedge, cluster-randomized trial. J Acquir Immune Defic Syndr. 2019;80(2):182–9.
Awotiwon AA, Johnson S, Rutherford GW, Meintjes G, Eshun-Wilson I. Primary antifungal prophylaxis for cryptococcal disease in HIV- positive people. Cochrane Database Syst Rev. 2018. https://doi.org/10.1002/14651858.CD004773.pub3.www.cochranelibrary.com.
Wan J, Cheong SAI, JOEMC C. Fluconazole resistance in cryptococcal disease: emerging or intrinsic? 2013;(April):261–9. https://doi.org/10.3109/13693786.2012.715763.
Bongomin F, Oladele RO, Gago S, Moore CB, Richardson MD. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. October 2017;2018:290–7. https://doi.org/10.1111/myc.12747.
Casadevall A, Spitzer ED, Webb D, Rinaldi MG. Susceptibilities of serial Cryptococcus neoformans isolates from patients with recurrent cryptococcal meningitis to amphotericin b and fluconazole. 1993;37(6):1383–6.
Brandt ME, Pfaller MA, Hajjeh RA, et al. Trends in antifungal drug susceptibility of Cryptococcus neoformans Isolates in the United States: 1992 to 1994 and 1996 to 1998. 2001;45(11):3065–9. https://doi.org/10.1128/AAC.45.11.3065.
Ramírez A, María A, Andrés C, et al. Response to therapy in patients with cryptococcosis and AIDS: association with in vitro susceptibility to fluconazole. Rev Iberoam Micol. 2015;32(4):214–20. https://doi.org/10.1016/j.riam.2014.07.006.
Ochiuzzi E, Borgnia D, Marı G, AIA Ã. Fluconazole and amphotericin B susceptibility testing of Cryptococcus neoformans: Results of minimal inhibitory concentrations against 265 isolates from HIV-positive patients before and after two or more months of antifungal therapy. Rev Iberoam ´ a Micol. 2009;26(3):194–7. https://doi.org/10.1016/j.riam.2009.02.001.
Hevey MA, Ms IAG, Raval K, Powderly WG, Msci AS. Presentation and mortality of cryptococcal infection varies by predisposing illness: a retrospective cohort study. Am J Med. 2019;132(8):977–983.e1. https://doi.org/10.1016/j.amjmed.2019.04.026.
Singh N, Alexander BD, Lortholary O, Dromer F, Gupta KL, John GT, et al. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis. 2007;195:756–64. https://doi.org/10.1086/511438.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
A.B. and M.P. had the idea for the article, performed the literature search, and drafted the work. F.L., L.Z., B.B., and G.S. critically revised the work. A.B. provided academic leadership.
Corresponding author
Ethics declarations
Ethical Approval and Informed Consent
The study does not need ethics committee approval because all data were collected for clinical purpose in adherence with ethical standards. We obtained the patient informed consent for publication in anonymous form.
Consent to Participate and Publication
Both patients gave consent to participate and make a publication regarding their clinical data.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Botta, A., Piccica, M., Lagi, F. et al. Disseminated and Relapsing Cryptococcosis: What We Still Have to Learn—a Case Series and Review of Literature. SN Compr. Clin. Med. 3, 1914–1922 (2021). https://doi.org/10.1007/s42399-021-00981-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-021-00981-6