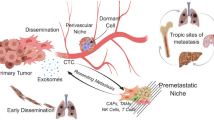
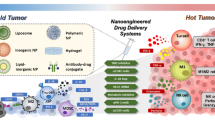
Abstract
Cancer immunotherapeutic strategies have shifted the focus of cancer treatment from eradicating the tumor cell by conventional cytotoxic chemotherapy, to educating the immune system to eliminate tumor, thereby preventing the recurrence of cancer. The understanding of tumor microenvironment and its components which generate an immunosuppressive environment is critical in further develo** efficient immunotherapies. In this review, we have classified the current immunotherapies based on their effect in modulating the tumor microenvironment. Additionally, we propose the inclusion of nanotechnology and tissue engineering approaches, which provide unique strategies to enhance the therapeutic efficacy and could lead to develo** nano/engineered immunotherapies for improved clinical outcomes. Specifically, we focus on criteria for designing nano/engineered immunotherapies and discuss targeted delivery strategies that can optimize the bioavailability of immunotherapies and, in turn, improve the therapeutic outcomes in the treatment of cancer.
Lay Summary
Several strategies aimed to exploit the therapeutic benefits of immunotherapy are based on alterations of the complex immunosuppressive tumor microenvironment. Such targeted approaches have also been significantly improved by various design criteria based on the concepts of nanotechnology and tissue engineering. The properties of specific targeting, controlled release, and ability to attain enhanced therapeutic effects with low doses conferred by these approaches have immensely helped to surmount the side effects and off-target issues of existing methods. Incorporation of these design criteria while develo** various carrier systems for targeted immunotherapy would certainly enhance their clinical translation potential, eventually augmenting anti-tumor responses.
Future Work
Modulation of tumor microenvironment with strategies discussed in this review will provide additional opportunities to improve cancer immunotherapy, especially in challenging diseases such as pancreatic and brain tumors. Various design attributes with targeted systems would provide numerous advantages, widening the scope of clinical translation and benefits to cancer patients.







Similar content being viewed by others
Abbreviations
- TME:
-
tumor microenvironment
- CAF:
-
cancer-associated fibroblasts
- Tregs :
-
regulatory T cells
- MDSC:
-
myeloid-derived suppressor cells
- TAM:
-
tumor-associated macrophages
- DCs:
-
dendritic cells
- FAPa:
-
fibroblast activation protein
- TCR:
-
T cell receptor
- CAR:
-
chimeric antigen receptor
- CAR-T:
-
CAR-expressing T cells
- DAMP:
-
damage-associated molecular patterns
- CpG-ODNs:
-
Cytosine-guanosine oligodeoxynucleotide
- GPI:
-
glycosyl-phosphatidylinositol
- pal-prot A:
-
palmitated protein A
- HER2:
-
human epidermal growth factor receptor 2
- ADCC:
-
antibody-dependent cell-mediated cytotoxicity
- TDLN:
-
tumor-draining lymph nodes
- APC:
-
antigen presenting cells
- PPS:
-
poly(propylene) sulfide
- TLR:
-
toll-like receptor
- TRP2:
-
tyrosine-related protein 2
- TADCs:
-
tumor-associated dendritic cells
- SLBs:
-
supported lipid bilayers
- MSRs:
-
mesoporous silica microrods
- APC-ms:
-
APC-mimetic scaffolds
- MSNP:
-
mesoporous silica nanoparticles
- CRS:
-
cytokine-release syndrome
- ROS:
-
reactive oxygen species
- GEM:
-
gemcitabine
- GM-CSF:
-
granulocyte-macrophage colony stimulating factor
- HA:
-
hyaluronic acid
- PEI:
-
poly(ethyleneimine)
- NSCLC:
-
non-small cell lung cancer
- GAC:
-
gastric adenocarcinoma
- IFP:
-
interstitial fluid pressure
- IDO1:
-
indoleamine 2,3-dioxygenase-1
- ALL:
-
acute lymphoblastic leukemia
- NK:
-
natural killer cells
- TILs:
-
tumor infiltrating lymphocytes
- PEGPH20:
-
PEGylated recombinant human hyaluronidase PH20
References
https://www.cancer.org/research/cancer-facts-statistics/global.html. Global cancer facts & figures: American Cancer Society.
Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat. 2017;3:250.
Weiden J, Tel J, Figdor CG. Synthetic immune niches for cancer immunotherapy. Nat Rev Immunol. 2018;18:212–9.
Cheung AS, Mooney DJ. Engineered materials for cancer immunotherapy. Nano Today. 2015;10:511–31.
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9.
Yu Y, Cui J. Present and future of cancer immunotherapy: a tumor microenvironmental perspective. Oncol Lett. 2018;16:4105–13.
Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74.
TL W. The tumor microenvironment and its role in promoting tumor growth. Oncogene Oncogene. 2008;27:5904–12.
Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2013;125:5591–6.
Malik R, Lelkes PI, Cukierman E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015;33:230–6.
Alexander B, Bloom M, Zaman H. Influence of the microenvironment on melanoma cell fate determination and phenotype. Physiol Genomics. 2014;46:309–14.
Junttila MR, De Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54.
Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23:8–11.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98.
Joyce J, Quail D. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37.
Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72:3125–30.
Gabriel A, Rabinovich D, Gabrilovich EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96.
Arina A, Corrales L, Bronte V. Enhancing T cell therapy by overcoming the immunosuppressive tumor microenvironment. Semin Immunol. 2016;28:54–63.
Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81.
Shimizu K, Iyoda T, Okada M, Yamasaki S, Fujii SI. Immune suppression and reversal of the suppressive tumor microenvironment. Int Immunol. 2018;30:445–55.
Nishikawa H, Shimon S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:109–18.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30.
Bruni D, Galon J. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218.
Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2:187–93.
Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science (80). 2010;330:827–30.
Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138:1058–66.
Calcinotto A, Filipazzi P, Grioni M, Iero M, de Milito A, Ricupito A, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–56.
Wu AA, Drake V, Huang HS, et al. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4:1–14.
Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51.
Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Ann Transl Med. 2016;4:261.
Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy--revisited. Nat Rev Drug Discov 2011/08/02. 2011;10:591–600.
Brinkman JA, Fausch SC, Weber JS, et al. Peptide-based vaccines for cancer immunotherapy. Expert Opin Biol Ther. 2004;4:181–98.
Hobernik D, Bros M. DNA vaccines-how far from clinical use? Int J Mol Sci. 2018;2018:19.
Melief CJ, van der Burg SH. Immunotherapy of established (pre) malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–60.
Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62–84.
McNamara MA, Nair SK, Holl EK. RNA-based vaccines in cancer immunotherapy. J Immunol Res. 2015;2015:794528.
Lundstrom K. Latest development on RNA-based drugs and vaccines. Futur Sci OA. 2018;4:FSO300.
Banday AH, Jeelani S, Hruby VJ. Cancer vaccine adjuvants--recent clinical progress and future perspectives. Immunopharmacol Immunotoxicol. 2014;37:1–11.
Hong E, Usiskin IM, Bergamaschi C, et al. Configuration-dependent presentation of multivalent IL-15:IL-15Ralpha enhances the antigen-specific T cell response and anti-tumor immunity. J Biol Chem. 2016;291:8931–50.
Luo Z, Wang C, Yi H, et al. Nanovaccine loaded with poly I:C and STAT3 siRNA robustly elicits anti-tumor immune responses through modulating tumor-associated dendritic cells in vivo. Biomaterials. 2014;38:50–60.
Molino NM, Neek M, Tucker JA, Nelson EL, Wang SW. Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials. 2016;86:83–91.
Galluzzi L, Buque A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2016/11/01. 2017;17:97–111.
Fucikova J, Kralikova P, Fialova A, et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–33.
Martins I, Kepp O, Schlemmer F, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2010/12/15. 2011;30:1147–58.
Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–9.
Mazumder A, Lee J, Talhi O, et al. Hydroxycoumarin OT-55 kills CML cells alone or in synergy with Imatinib or Synribo: involvement of ER stress and DAMP release. Cancer Lett. 2018;438:197–218.
Menger L, Vacchelli E, Adjemian S, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4:143ra99.
Mazumder A, Cerella C, Diederich M. Natural scaffolds in anticancer therapy and precision medicine. Biotechnol Adv. 2018;36:1563–85.
Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010/04/24. 2010;10:317–27.
Vacchelli E, Pol J, Bloy N, et al. Trial watch: tumor-targeting monoclonal antibodies for oncological indications. Oncoimmunology. 2015;4:e985940.
Galluzzi L, Vacchelli E, Bravo-San Pedro JM, et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5:12472–508.
Holubec L, Polivka J Jr, Safanda M, et al. The role of cetuximab in the induction of anticancer immune response in colorectal cancer treatment. Anticancer Res. 2016;36:4421–6.
Vermorken JB, Herbst RS, Leon X, et al. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112:2710–9.
Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res. 2017;120:116–32.
Forero-Torres A, Shah J, Wood T, Posey J, Carlisle R, Copigneaux C, et al. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5). Cancer Biother Radiopharm 2010/03/02. 2010;25:13–9.
Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand J Immunol. 2000;51:634–41.
Naito K, Takeshita A, Shigeno K, et al. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab zogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia. 2000;14:1436–43.
Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130:2373–6.
Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2014;93:290–6.
Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–33.
Hansson V, Djoseland O, Torgersen O, et al. Hormones and hormonal target cells in the testis. Andrologia. 1976;8:195–202.
Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–40.
Abbot Bitao Liang, and Tianjian Li (2015).“.” Application no. 14/653,650. S. Chimeric antigen receptors. application US patent, editor. U.S
Fournier C, Martin F, Zitvogel L, et al. Trial Watch: adoptively transferred cells for anticancer immunotherapy. Oncoimmunology. 2017;6:e1363139.
Boyiadzis MM, Dhodapkar MV, Brentjens RJ, Kochenderfer JN, Neelapu SS, Maus MV, et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother cancer. 2018;6:137.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
Verneris MR, June CH, Myers GD, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Bird L. Calming the cytokine storm. Nat Rev Immunol. 2018;18:417.
Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–48.
Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–8.
Bol KF, Schreibelt G, Gerritsen WR, et al. Dendritic cell-based immunotherapy: state of the art and beyond. Clin Cancer Res. 2016;22:1897–906.
Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in. J Clin Oncol 2010/12/15. 2011;29:330–6.
Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–302.
Boczkowski D, Nair SK, Nam JH, et al. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–34.
Garg NK, Dwivedi P, Prabha P, et al. RNA pulsed dendritic cells: an approach for cancer immunotherapy. Vaccine. 2013;31:1141–56.
Irvine AS, Trinder PK, Laughton DL, et al. Efficient nonviral transfection of dendritic cells and their use for in vivo immunization. Nat Biotechnol. 2000;18:1273–8.
Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:9482–7.
Handy CE, Antonarakis ES. Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol. 2017/12/21. 2018;14:907–17.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010/09/08. 2010;363:411–22.
Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009/09/29. 2009;10:1185–92.
Walker LSK. PD-1 and CTLA4: Ttwo checkpoints, one pathway? Sci Immunol. 2017;2017:2.
Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–63.
Aspeslagh S, Postel-Vinay S, Rusakiewicz S, et al. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2015/12/10. 2016;52:50–66.
Barbee MS, Ogunniyi A, Horvat TZ, et al. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015/05/21. 2015;49:907–37.
Cabo M, Offringa R, Zitvogel L, et al. Trial Watch: immunostimulatory monoclonal antibodies for oncological indications. Oncoimmunology. 2017;6:e1371896.
Segal NH, He AR, Doi T, Levy R, Bhatia S, Pishvaian MJ, et al. Phase i study of single-agent utomilumab (PF-05082566), a 4-1bb/cd137 agonist, in patients with advanced cancer. Clin Cancer Res. 2018;24:1816–23.
Caux C, Ramos RN, Prendergast GC, et al. A milestone review on how macrophages affect tumor growth. Cancer Res. 2016;76:6439–42.
Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73.
Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216.
Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer 2013/06/21. 2014;134:32–42.
Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–62.
Wiehagen KR, Girgis NM, Yamada DH, et al. Combination of CD40 agonism and CSF-1R blockade reconditions tumor-associated macrophages and drives potent antitumor immunity. Cancer Immunol Res. 2017;5:1109–21.
Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86.
Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72.
Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–94.
Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–92.
Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65.
Allard B, Longhi MS, Robson SC, et al. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–44.
Bastid J, Regairaz A, Bonnefoy N, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res. 2014/11/19. 2015;3:254–65.
Antonioli L, Yegutkin GG, Pacher P, et al. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer. 2016;2:95–109.
Sek K, Molck C, Stewart GD, et al. Targeting adenosine receptor signaling in cancer immunotherapy. Int J Mol Sci. 2018;2018:19.
Hatfield SM, Sitkovsky M. A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1alpha driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol. 2016;29:90–6.
Belladonna ML, Puccetti P, Orabona C, et al. Immunosuppression via tryptophan catabolism: the role of kynurenine pathway enzymes. Transplantation. 2007/08/19. 2007;84:S17–20.
Platten M, von Knebel Doeberitz N, Oezen I, et al. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2015/01/30. 2014;5:673.
Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010/03/04. 2010;115:3520–30.
Yue EW, Douty B, Wayland B, et al. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. J Med Chem. 2009;52:7364–7.
Banerjee T, Duhadaway JB, Gaspari P, et al. A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. Oncogene. 2008;27:2851–7.
Prendergast GC, Malachowski WP, DuHadaway JB, et al. Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res. 2017;77:6795–811.
Long GV, Dummer R, Hamid O, Gajewski T, Caglevic C, Dalle S, et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: results of the phase 3 ECHO-301/KEYNOTE-252 study. J Clin Oncol. 2018;36:108.
Labadie BW, Bao R, Luke JJ. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan–kynurenine–aryl hydrocarbon axis. Clin Cancer Res. 2019;25:1462–71.
Bonifaz L, Bonnyay D, Mahnke K, et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38.
Schreibelt G, Klinkenberg LJ, Cruz LJ, et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. 2012;119:2284–92.
Pitt JM, Andre F, Amigorena S, et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016;126:1224–32.
Escudier B, Dorval T, Chaput N, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10.
Tacken PJ, de Vries IJ, Torensma R, et al. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802.
Stylianopoulos T, Poh MZ, Insin N, Bawendi MG, Fukumura D, Munn LL, et al. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys J. 2010;99:1342–9.
Martino MM, Hubbell JA. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24:4711–21.
Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99:853–60.
Martino MM, Tortelli F, Mochizuki M, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3:100ra89.
Martino MM, Briquez PS, Güç E, et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science (80-). 2014;343:885–8.
Stylianopoulos T, Munn LL, Jain RK. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends in Cancer. 2018;4:292–319.
Li H-J, Zhang Y-R, Wang J, et al. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;11:e1519.
Singha NC, Nekoroski T, Zhao C, Symons R, Jiang P, Frost GI, et al. Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol Cancer Ther. 2015;14:523–32.
Connor RJ, Bookbinder LH, Shepard HM, et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010;9:3052–64.
Thompson CB, Clift R, Rosengren S, et al. Increasing tumor-infiltrating CD8+ T cell response and checkpoint inhibitor efficacy by enzymatic reduction of tumor hyaluronan in a murine syngeneic pancreatic cancer model, In: Proceedings of the AACR Special Conference on Tumor Immunology and Immunothe. Cancer Immunol. Res. 2018;6:Abstract nr B38.
Doherty GJ, Tempero M, Corrie PG. HALO-109-301: a phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018;14:13–22.
Berdov BA, Korn R, Holcombe RF, et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res. 2016;22:2848–54.
Rosen LS, Ramanathan RK, LoRusso P, et al. Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br J Cancer. 2017;118:153–61.
Phase 1b open-label study of PEGylated recombinant human hyaluronidase (PEGPH20) with pembrolizumab. Available from: https://clinicaltrials.gov/ct2/show/NCT02563548; U.S. National Library of Medicine, ClinicalTrials.gov.
Alwan LM, Grossmann K, Sageser D, van Atta J, Agarwal N, Gilreath JA. Comparison of acute toxicity and mortality after two different dosing regimens of high-dose interleukin-2 for patients with metastatic melanoma. Target Oncol. 2014;9:63–71.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34.
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32.
Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–86.
Milling L, Zhang Y, Irvine JD. Delivering safer immunotherapies for cancer. Adv Drug Deliv Rev. 2017;22:194–213.
Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol. 2007;178:4194–213.
Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20:1747–56.
Weide B, Eigentler TK, Pflugfelder A, Zelba H, Martens A, Pawelec G, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014;2:668–78.
Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–82.
Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJM. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17:2270–80.
Van Herpen CM, Huijbens R, Looman M, et al. Pharmacokinetics and immunological aspects of a phase Ib study with intratumoral administration of recombinant human interleukin-12 in patients with head and neck squamous cell carcinoma: a decrease of T-bet in peripheral blood mononuclear cells. Clin Cancer Res. 2003;9:2950–6.
Moritz T, Niederle N, Baumann J, May D, Kurschel E, Osieka R, et al. Phase I study of recombinant human tumor necrosis factor α in advanced malignant disease. Cancer Immunol Immunother. 1989;29:144–50.
Bartsch HH, Pfizenmaier K, Schroeder M, Nagel GA. Intralesional application of recombinant human tumor necrosis factor alpha induces local tumor regression in patients with advanced malignancies. Eur J Cancer Clin Oncol. 1989;25:287–91.
Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204.
**a Y, Gupta GK, Castano AP, Mroz P. Pinar Avci and MRH. CpG oligodeoxynucleotide as immune adjuvant enhances photodynamic therapy response in murine metastatic breast cancer. J Biophotonics. 2014;7:897–905.
Nierkens S, den Brok MH, Roelofsen T, Wagenaars JAL, Figdor CG, Ruers TJ, et al. Route of administration of the TLR9 agonist CpG critically determines the efficacy of cancer immunotherapy in mice. PLoS One. 2009;4:e8368.
Liu H, Kwong B, Irvine DJ. Membrane anchored immunostimulatory oligonucleotides for in vivo cell modification and localized immunotherapy. Angew Chem Int Ed. 2011;50:7052–5.
Kwong B, Liu H, Irvine DJ. Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials. 2011;32:5134–47.
McHugh RS, Nagarajan S, Wang YC, Sell KW, Selvaraj P. Protein transfer of glycosyl-phosphatidylinositol-B7-1 into tumor cell membranes: a novel approach to tumor immunotherapy. Cancer Res. 1999;59:2433–7.
Liu S, Breiter DR, Zheng G, Chen A. Enhanced antitumor responses elicited by combinatorial protein transfer of chemotactic and costimulatory molecules. J Immunol. 2007;178:3301–6.
Zheng G, Chen A, Sterner RE, Zhang PJ, Pan T, Kiyatkin N, et al. Induction of antitumor immunity via intratumoral tetra-costimulator protein transfer. Cancer Res. 2001;61:8127–34.
Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat Chem Biol. 2014;10:69–75.
**ao H, Woods EC, Vukojicic P, Bertozzi CR. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci. 2016;113:10304–9.
Kwong B, Gai SA, Elkhader J, Wittrup KD, Irvine DJ. Localized immunotherapy via liposome-anchored anti-CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Res. 2013;73:1547–58.
Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–96.
Dubrot J, Milheiro F, Alfaro C, Palazón A, Martinez-Forero I, Perez-Gracia JL, et al. Treatment with anti-CD137 mAbs causes intense accumulations of liver T cells without selective antitumor immunotherapeutic effects in this organ. Cancer Immunol Immunother. 2010;59:1223–33.
Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Frechet JMJ. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc Natl Acad Sci. 2009;106:5497–502.
Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–58.
van Mierlo GJD, Boonman ZFHM, Dumortier HMH, den Boer AT, Fransen MF, Nouta J, et al. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol. 2004;173:6753–9.
Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS One. 2013;8:e61646.
Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly (propylene sulfide) nanoparticles. J Control Release. 2006;112:26–34.
Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35:814–24.
Zwiorek K, Bourquin C, Battiany J, Winter G, Endres S, Hartmann G, et al. Delivery by cationic gelatin nanoparticles strongly increases the immunostimulatory effects of CpG oligonucleotides. Pharm Res. 2008;25:551–62.
Bourquin C, Anz D, Zwiorek K, Lanz AL, Fuchs S, Weigel S, et al. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J Immunol. 2008;181:2990–8.
Andorko JI, Jewell CM. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng Transl Med. 2017;2:139–55.
Padmakumar S, Paul-Prasanth B, Pavithran K, Vijaykumar DK, Rajanbabu A, Sivanarayanan TB, et al. Long-term drug delivery using implantable electrospun woven polymeric nanotextiles. Nanomed Nanotechnol, Biol. Med. 2019;15:274–84.
Monette A, Ceccaldi C, Assaad E, Lerouge S, Lapointe R. Chitosan thermogels for local expansion and delivery of tumor-specific T lymphocytes towards enhanced cancer immunotherapies. Biomaterials. 2016;75:237–49.
Madhanagopal BR, Zhang S, Demirel E, Wady H, Chandrasekaran AR. DNA nanocarriers: programmed to deliver. Trends Biochem Sci. 2018;43:997–1013.
Wang J, Hu X, **ang D. Nanoparticle drug delivery systems: an excellent carrier for tumor peptide vaccines. Drug Deliv. 2018;25:1319–27.
Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, et al. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+T cell-mediated anti-tumor immunity. Vaccine. 2008;26:5046–57.
Cheung AS, Koshy ST, Stafford AG, et al. Adjuvant-loaded subcellular vesicles derived from disrupted cancer cells for cancer vaccination. Small. 2016;12:2321–33.
Lu J, Liu X, Liao YP, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8:1811.
Hansel TT, Kropshofer H, Singer T, et al. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–38.
Shahbazi M-A, Shrestha N, Mäkilä E, et al. A prospective cancer chemo-immunotherapy approach mediated by synergistic CD326 targeted porous silicon nanovectors. Nano Res. 2014;8:1505–21.
Wang C, Ye Y, Hochu GM, et al. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334–40.
Shahbazi MA, Fernandez TD, Makila EM, et al. Surface chemistry dependent immunostimulative potential of porous silicon nanoplatforms. Biomaterials. 2014;35:9224–35.
Wang C, Wang J, Zhang X, et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci Transl Med. 2018;10:1–13.
Li SY, Liu Y, Xu CF, et al. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J Control Release. 2016;231:17–28.
Hobo W, Novobrantseva TI, Fredrix H, et al. Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol Immunother. 2012/08/21. 2013;62:285–97.
Verma V, Kim Y, Lee M-C, Lee JT, Cho S, Park IK, et al. Activated dendritic cells delivered in tissue compatible biomatrices induce in-situ anti-tumor CTL responses leading to tumor regression. Oncotarget. 2016;7:39894–906.
Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–8.
Ali OA, Emerich D, Dranoff G, et al. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1:22–8.
Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66.
Zemon H. An artificial solution for adoptive immunotherapy. Trends Biotechnol. 2003;21:418–20.
Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic field-induced t cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano. 2014;8:2252–60.
Cheung AS, Zhang DKY, Koshy ST, Mooney DJ. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T-cells. Nat Biotechnol. 2018;36:160–9.
Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering using surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–41.
Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat Biotechnol. 2015;33:97–101.
Su MJ, Aldawsari H, Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of MicroRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci Rep. 2016;6:1–15.
Zhu S, Niu M, O’Mary H, Cui Z. Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Mol Pharm. 2013;10:3525–30.
Ganesh S, Iyer AK, Morrissey DV, et al. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34:3489–502.
Parayath NN, Parikh A, Amiji MM. Repolarization of tumor-associated macrophages in a genetically engineered nonsmall cell lung cancer model by intraperitoneal administration of hyaluronic acid-based nanoparticles encapsulating microRNA-125b. Nano Lett. 2018;18:3571–9.
Chen Y, **a R, Huang Y, et al. An immunostimulatory dual-functional nanocarrier that improves cancer immunochemotherapy. Nat Commun. 2016;7:13443.
Acknowledgments
SP acknowledges Department of Science & Technology DST-INSPIRE, Government of India for her Senior Research Fellowship and the additional funding from Department of Biotechnology, Government of India through Pilot Project Grants, Program for Young Investigators in Cancer Biology. She also acknowledges Amrita Vishwa Vidyapeetham for PhD Scholar’s Fellowship (2017) and the infrastructural support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Submission for the Special Issue of the journal “Regenerative Engineering and Translational Medicine” as a tribute to Professor Robert Langer on his 70th Birthday
Rights and permissions
About this article
Cite this article
Parayath, N., Padmakumar, S., Nair, S.V. et al. Strategies for Targeting Cancer Immunotherapy Through Modulation of the Tumor Microenvironment. Regen. Eng. Transl. Med. 6, 29–49 (2020). https://doi.org/10.1007/s40883-019-00113-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00113-6