Abstract
Metabolic-associated fatty liver disease (MAFLD) is the most prevalent chronic liver disease worldwide, affecting 25% of people globally and up to 80% of people with obesity. MAFLD is characterised by fat accumulation in the liver (hepatic steatosis) with varying degrees of inflammation and fibrosis. MAFLD is strongly linked with cardiometabolic disease and lifestyle-related cancers, in addition to heightened liver-related morbidity and mortality. This position statement examines evidence for exercise in the management of MAFLD and describes the role of the exercise professional in the context of the multi-disciplinary care team. The purpose of these guidelines is to equip the exercise professional with a broad understanding of the pathophysiological underpinnings of MAFLD, how it is diagnosed and managed in clinical practice, and to provide evidence- and consensus-based recommendations for exercise therapy in MAFLD management. The majority of research evidence indicates that 150–240 min per week of at least moderate-intensity aerobic exercise can reduce hepatic steatosis by ~ 2–4% (absolute reduction), but as little as 135 min/week has been shown to be effective. While emerging evidence shows that high-intensity interval training (HIIT) approaches may provide comparable benefit on hepatic steatosis, there does not appear to be an intensity-dependent benefit, as long as the recommended exercise volume is achieved. This dose of exercise is likely to also reduce central adiposity, increase cardiorespiratory fitness and improve cardiometabolic health, irrespective of weight loss. Resistance training should be considered in addition to, and not instead of, aerobic exercise targets. The information in this statement is relevant and appropriate for people living with the condition historically termed non-alcoholic fatty liver disease (NAFLD), regardless of terminology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Metabolic-associated fatty liver disease (MAFLD) affects one in four adults globally and is linked with type 2 diabetes, cardiovascular disease and lifestyle-related cancers, as well as progressive liver disease and liver cancer. |
This position statement collates evidence on the role of exercise in the management of MAFLD including referral pathways, assessment and management priorities, exercise prescription recommendations, action planning and considerations for clinicians working with people with MAFLD. The information in this statement is relevant and appropriate for people living with the condition historically termed non-alcoholic fatty liver disease (NAFLD), regardless of terminology. |
The majority of research evidence indicates that 150–240 min per week of at least moderate-intensity aerobic exercise can reduce liver fat by ~ 2–4%, but as little as 135 min/week has been shown to be effective. This dose of exercise is likely to improve central adiposity, cardiorespiratory fitness and cardiometabolic health, irrespective of weight loss. |
1 Background
Metabolic-associated fatty liver disease (MAFLD), historically known as non-alcoholic fatty liver disease (NAFLD), represents the hepatic manifestation of a multi-system metabolic dysfunction-driven disorder [1]. MAFLD affects at least 25% of people globally and within Australia [2,3,4]. In addition to increasing the risk of end-stage liver disease and primary liver cancer, MAFLD plays a central role in the development of type 2 diabetes (T2D), cardiovascular disease (CVD) and extrahepatic lifestyle-related cancers [5], reducing life expectancy by 4 years and increasing time spent living with high metabolic burden [6]. In Australia, it is expected that MAFLD cases will increase by 25% between 2019 and 2030 to over 7 million cases [4], considerably elevating the related disease burden. Globally, from 1991 to 2019, MAFLD increased from 22 to 37%, with an annual increase of 0.7% [7].
Given its strong association with obesity, and in the absence of pharmacological agents approved for the long-term management of MAFLD, lifestyle modifications with dietary changes and increased physical activity/exercise remain the cornerstone of MAFLD management. Allied health professionals (e.g. dietitians, exercise physiologists, physiotherapists, health psychologists) play an integral role in managing the burden of MAFLD. The aim of this position statement was to synthesise the literature on the role of exercise for the management of MAFLD and to produce evidence-based statements to be used to develop recommendations for exercise prescription. Evidence to facilitate the translation, implementation and uptake of these recommendations was also reviewed.
1.1 Definitions of Metabolic-Associated Fatty Liver Disease (MAFLD)
Until recently, MAFLD was known as non-alcoholic fatty liver disease (NAFLD) and was diagnosed if there was evidence of steatosis in ≥ 5% of hepatocytes and the exclusion of other chronic liver diseases and ‘excess’ alcohol intake. NAFLD was further dichotomised into NAFL (referring to simple steatosis) and non-alcoholic steatohepatitis (NASH, referring to the more progressive condition characterised by hepatocyte ballooning, inflammation and varying degrees of fibrosis). Concerns with this terminology included stigma relating to the term ‘alcohol’ and the reprioritisation of disease significance with the term ‘non’. Given the metabolic dysfunction underlying the pathophysiology the term ‘MAFLD’ has been proposed and increasingly used, although a global consensus has not been reached at the time of this publication. MAFLD is defined by the presence of hepatic steatosis in the setting of metabolic dysfunction characterised by having overweight/obesity or T2D, or specific features of metabolic dysregulation [1] (Fig. 1).
Schematic definition of MAFLD. Adapted from Eslam 2020 [1], with permission. BMI body mass index, HDL-cholesterol high-density lipoprotein-cholesterol, HbA1c glycated haemoglobin, HOMA homeostatic model assessment, hs-CRP high-sensitivity C-reactive protein. *Relates to liver histology from liver biopsy, with ≥ 5% referring to the proportion of hepatocytes containing visible intracellular lipid droplets. Other modalities (imaging, blood biomarkers/scores) have assigned thresholds to detect steatosis at ≥ 5%
MAFLD can coexist with any liver disease (including alcohol related liver disease) and disease severity is further categorised by grade of activity and stage of fibrosis. Importantly for this position statement, many of the citations and reported studies have used the term NAFLD. There is marked overlap between NAFLD as previously defined and MAFLD [1, 8] with high overall concordance of the two definitions (Cohen’s kappa of up to 0.92) [9]. For consistency throughout the statement, we have used the term MAFLD with appropriate clarifications where necessary.
1.2 Development and Clinical Impacts of MAFLD
1.2.1 Development
MAFLD develops when there is dysfunction in hepatic fuel utilisation resulting in excess storage of fat (as intrahepatic triglyceride) in the liver (hepatic steatosis) and reduced clearance [via oxidation or repackaging as very low-density lipoprotein (VLDL)-cholesterol]. Hepatic steatosis is a cause and a consequence of insulin resistance [10,11,12]. Increased free fatty acid (FFA) delivery to the liver primarily arises (59%) from insulin-resistant adipose tissue [13] [especially visceral adipose tissue (VAT) that delivers FFAs directly to the liver via the portal vein [10]]. Additionally, de novo lipogenesis, accounting for a further 26% of FFA flux, uses excess substrate from glucose metabolism (glycerol 3 phosphate), exacerbated by insulin resistance in skeletal muscles, to form intrahepatic triglyceride. Dietary fats, which deliver FFA from the gut via chylomicrons, account for the remaining 15% [13]. Within the liver, these FFAs are excreted by VLDL, oxidised through hepatic β-oxidation, or are synthesised to triglyceride for storage.
1.2.2 Clinical Impacts
MAFLD leads to a range of liver-related and extra-hepatic clinical consequences. Obesity and insulin resistance are the key pathophysiological drivers for both liver-related and extra-hepatic disease severity, underpinned by genetic predisposition and/or disruption to the microbiome [14]. People with MAFLD have increased overall mortality, with a 34% higher death rate observed over 7.5 years compared with age- and sex-matched individuals in the general population [15]. The primary causes of death in MAFLD are non-liver related, with CVD and lifestyle-related extra-hepatic cancers accounting for 37% and 21% of deaths, respectively [16]. Approximately 30% of people with MAFLD will develop metabolic-associated steatohepatitis (characterised by hepatocyte injury and death with associated inflammation and varying degrees of fibrosis, historically called non-alcoholic steatohepatitis/NASH); a burgeoning indication for liver transplantation [17, 18]. However, unlike the high incidence of progression from MAFLD to metabolic-associated steatohepatitis, the onset of cirrhosis is relatively low with slow progression (~ 3% in 15 years) [19]. People with histological evidence of steatohepatitis have an increased liver-related mortality rate that is dose-dependent based on the severity of liver fibrosis [20].
1.3 Definitions of Physical Activity, Exercise and Sedentary Behaviour
Physical activity is defined as bodily movement that increases the metabolic rate and can be categorised in relation to the metabolic demands of the activity termed ‘metabolic equivalents’ or METs. Exercise is considered as planned and structured physical activity, generally with a goal to improve or maintain health, wellbeing and/or performance. Exercise prescription centres on the manipulation of programming variables which include the mode of exercise, the frequency (number of sessions per week) of the exercise bouts, the duration (time of the individual exercise bout) and the intensity (the physiological effort/ energy demands of activity). The volume of exercise encompasses the total energy expended in kilojoules per exercise bout or per week, and is a function of the intensity, frequency and duration of weekly exercise. While there are different intensity domain cut points across different professional bodies, intensity is generally framed as ‘light’, ‘moderate’ and ‘vigorous’, and described by approach (e.g. continuous, interval) and modality (e.g. aerobic, anaerobic, resistance). Additionally, sedentary behaviour is classified as activities involving sitting or lying/reclined that have a low energy requirement and little additional movement [21]. Further guidance on exercise prescription variables for both aerobic and resistance training and definitions of intensity domains can be sought elsewhere [21, 22]. The focus of this position paper is on the health effects of exercise on the pathophysiological features of MAFLD, with a specific lens on those that are pertinent to the exercise professional.
1.4 Objectives of the Management of People with MAFLD
1.4.1 Clinical Presentation
Most people with MAFLD do not present with specific symptoms; MAFLD is often identified incidentally through routine assessment of blood biochemistry [e.g. abnormal liver enzymes: alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] or on imaging performed for another health reason. MAFLD may be suspected based on an individual’s medical history or the presence of central obesity, or on investigations including abdominal imaging (ultrasound or computed tomography) showing features of hepatic steatosis. There are established criteria to diagnose MAFLD (see Fig. 1) [8, 23], but in addition physicians may consider the following:
-
(i)
An assessment of the severity of liver injury including the degree of liver fibrosis. This is most often undertaken with multi-modal non-invasive approaches using bloodwork that relies on routine tests (such as a full blood count and standard biochemistry panels for the NAFLD fibrosis score and Fibrosis-4 (FIB-4) algorithms) and/or serum biomarkers [as used for the enhanced liver fibrosis (ELF) test], or assessments of liver stiffness based on ultrasound [such as vibration-controlled transient elastography (Fibroscan™)], shear wave elastography or acoustic radiation force imaging, or other imaging modalities (e.g. magnetic resonance elastography). Where these non-invasive tests suggest a patient has advanced disease, they may be referred to hepatology services for further evaluation including possible liver biopsy.
-
(ii)
Other co-morbid contributors to liver disease including hepatitis C and hepatitis B viruses, and hazardous alcohol consumption as these may require management in conjunction with MAFLD.
-
(iii)
Medication and over the counter preparations (including herbal remedies and traditional medicines), as well as surgical and general health history.
-
(iv)
Screening for cardiometabolic health risk and common comorbidities including personal and family history of CVD and T2D, metabolic syndrome, hyperglycaemia [e.g. elevated glycosylated haemoglobin (HbA1c), impaired fasting glucose], atherogenic dyslipidaemia, hypertension, smoking status, alcohol intake and anthropometry [including body mass index (BMI), weight history and waist circumference].
It is common for people to present to exercise services with a chief complaint of T2D, obesity or metabolic syndrome but without a formal specialist review or diagnosis of MAFLD. In these instances, MAFLD may be suspected based on clinical presentation. Given the common underlying pathophysiology, if MAFLD is suspected based on the individual’s clinical history and risk factors (e.g. high waist circumference, T2D, low physical activity), depending on the scope and standards of the local healthcare systems, the exercise professional may facilitate referral to the individual’s primary care physician to establish a diagnosis and determine appropriate care pathways. Potential referral pathways and clinical care team partners are illustrated in Fig. 2.
1.4.2 Clinical Assessment and Management Priorities for the Exercise Professional
The management of MAFLD requires a multi-disciplinary approach. From the medical perspective, the overall management priorities for people with MAFLD are centred around two primary goals:
-
(i)
The resolution of MAFLD and/or prevention of liver disease progression
-
(ii)
The prevention of cardiovascular related morbidity and mortality
The forefront of management for most people with MAFLD is lifestyle therapy, including both dietary modification and regular exercise, with a primary goal of reducing hepatic steatosis, achieving adiposity reduction, managing cardiometabolic comorbidities and preventing lifestyle-related extra-hepatic cancers. Weight loss of 5–10% will result in clinically meaningful health improvements [24]; however, a number of health benefits are likely to be achieved irrespective of significant weight loss. Beyond body weight, a 30% reduction in hepatic steatosis as measured by magnetic-resonance imaging (MRI) is associated with histological improvement and resolution of steatohepatitis [25]. One challenge in assessing the efficacy of therapeutic interventions to improve liver histology is the limited availability of robust measures that can reliably and non-invasively identify clinically meaningful improvements in these parameters. Tools to quantify and monitor liver fat and liver fibro-inflammation are presently available in research settings only. A suite of tools based on common biochemical and anthropometric measures have been proposed as surrogates to imaging methods and liver biopsy [26]. However, these are validated as screening tools only and are not used routinely for assessing change in liver health status, meaning assessing waist and body weight change remains the primary strategy for longitudinal monitoring in response to treatment.
People with MAFLD have lower levels of cardiorespiratory fitness than the general population [27, 28]. Low cardiorespiratory fitness has been reported to be a potent risk factor for MAFLD and is inversely associated with steatohepatitis [29] and liver fibrosis [28], and may predict the degree of steatosis reduction possible with lifestyle intervention [30]. This may explain in part why people with MAFLD frequently report high levels of fatigue, low levels of energy and low exercise-related self-efficacy [31].
Dietary targets include improving diet quality with a focus on heart healthy eating patterns, that are predominantly plant based with abundant daily vegetables, fruit consumption, use of extra virgin olive oil, fish, seafood, legumes and nuts as preferred protein sources and selecting reduced fat dairy and wholegrain bread and cereal options. Reducing intake of alcohol, sugar-sweetened beverages, highly processed foods and processed red meats is also recommended. Practical resources are available elsewhere [32].
Regardless of the presentation or referral pathway, a risk assessment should be undertaken by an appropriately trained exercise professional [e.g. using the Australian Pre-Exercise Screening System (APSS) screening tool].
For the clinical exercise professional, assessment and management priorities for people with known or suspected MAFLD are suggested to include:
-
(i)
Cardiometabolic risk factors: Practitioners should assess waist circumference, BMI, blood pressure, family history of heart disease and diabetes, smoking status, alcohol use and physical inactivity. Further clinical assessment, requested by the treating physician, may also include blood lipids and lipoproteins, blood glucose and HbA1c. Scores such as the Atherosclerotic Cardiovascular Disease (ASCVD) score (score calculator freely available online https://www.mdcalc.com/calc/3398/ascvd-atherosclerotic-cardiovascular-disease-2013-risk-calculator-aha-acc) may be used to inform 10 year risk of heart disease or stroke. Management priorities should include reduction in central obesity (as waist circumference), blood pressure and sedentary time, and increasing physical activity. Reducing excess adiposity remains an important part of the clinical management of MAFLD, irrespective of BMI. However, weight loss and/or additional components of metabolic syndrome may also be targets for management depending on clinical presentation. Reductions of 3–5% body weight can improve the cardiometabolic profile [33], reduce liver steatosis [8] and achieve MAFLD remission in people with BMI < 25 kg/m2 [34]. Body weight reductions of ≥ 7–10% are recommended for improvements in histological features of MAFLD, especially in people with comorbid MAFLD and overweight/obesity [8, 35]. In people who achieved ≥ 10% weight loss, 90% had steatohepatitis resolution, 81% had fibrosis regression and all improved hepatic steatosis [36].
-
(ii)
Physical capacity: Assessment may include physical activity levels and sedentary behaviour, cardiorespiratory fitness, neuromuscular fitness and assessment of any musculoskeletal or orthopaedic limitations (including sarcopenia) that may impact physical and functional capacity. Whilst not necessarily a focus of research to date in MAFLD, it is highly likely that consideration of musculoskeletal health, its assessment and prescription are relevant for many people with MAFLD, when functional capacity and avoidance of muscle loss/sarcopenia are key considerations. Further clinical assessment to establish sarcopenia [37] and frailty via imaging and/or relevant clinical measures such as the short physical performance battery (SPPB) [38] may be clinically appropriate to consider based on individual presentation. While all these outcomes are important for people with MAFLD, priority for assessment and targets for management will depend on individual presentation and intervention targets. Identifying barriers to the uptake and maintenance of physical activity/exercise may also enable personalised exercise programmes (see Sect. 3.3).
-
(iii)
Comorbidities: Comorbidities such as metabolic syndrome, pre-diabetes, T2D, obesity, polycystic ovarian syndrome (PCOS), hypertension and depression/mental ill health should be identified, and the broader management of these conditions should be considered in the context of MAFLD management.
-
(iv)
Patient-important outcome measures: Practitioners should assess issues particularly relevant to MAFLD such as rating levels of fatigue, energy and exercise-related self-efficacy (e.g. via the Self-Efficacy for Exercise Scale [39]). This may also include sleep quality (e.g. via the Pittsburgh Sleep Quality Index [40]), low-mood, ability to undertake activities of daily living and health-related quality of life (e.g. via the Chronic Liver Disease Questionnaire – Non-Alcoholic Fatty Liver Disease version [41]) or other goals specific to the patient that exercise may address. Patient-important outcome measures could be monitored and assessed using visual analogue scales or the goal attainment scale[42].
-
(v)
Identification of need for referral onwards based on clinical opinion. Practitioners should ask about:
-
-
Diet (in accordance with national healthy eating guidelines) with referral to a dietitian if indicated. Referral is indicated if the patient’s needs extend beyond general healthy eating advice, if they have complications due to MAFLD (notably liver cirrhosis, see Sect. 4.3), have additional comorbidities, have specific nutritional composition questions and/or request extra specialist support.
-
-
Emotional, social and cognitive functioning with referral to a psychologist or behavioural counsellor if indicated.
-
-
Medication use to determine whether the individual is taking medication (e.g. for blood pressure, T2D, cholesterol) and if not, referring to their primary care physician for medical review if indicated based on clinical assessment. Medications may also need modifying by the primary care physician as the patient starts regular exercise or significantly changes their exercise programme (e.g. to reduce antihypertensives, oral hypoglycaemics or insulin).
-
-
If MAFLD is suspected, referral back to the primary care physician to establish a pathway for diagnosis, assessment of disease severity and additional care pathways if required.
2 Section 2: Evidence for the Role of Exercise in the Management of MAFLD
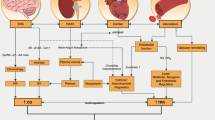
There is clear and consistent evidence that regular exercise is cardioprotective and has multiple benefits on musculoskeletal function and mental health, irrespective of weight loss [43]. The mechanisms by which exercise modulates hepatic steatosis have been detailed elsewhere [14, 44,45,46]. Briefly, putative mechanisms centre on altering the flux of free fatty acids (FFA) to and from the liver via changes to substrate metabolism within the muscle, adipose tissue and liver [11, 47, 48]. These may be mediated, in part, by improvements in peripheral insulin sensitivity and glucose uptake which alter liver signalling pathways [i.e. sterol regulatory element binding protein-1c (SREBP-1c) and carbohydrate response element binding protein (ChREBP)] and gene expression [e.g. lipogenic proteins fatty acid synthase (FAS) and acetyl-coenzyme A carboxylase (ACC)]. In rodents, exercise-mediated increases in mitochondrial enzymes (e.g. cytochrome c oxidase, citrate synthase and β-hydroxyacyldehydrogenase) and increases in mitochondrial content and oxidative capacity have been shown [49]. These improvements in liver mitochondrial content and function have been associated with increased β-oxidation, which may prevent the accumulation of metabolic by-products such as ceramides and diacylglycerides that contribute to insulin resistance (Fig. 3). A key signalling pathway for direct liver benefit may be the activation of the metabolic energy sensor adenosine monophosphate-activated protein kinase (AMPK). This pathway plays an important role in regulating metabolism (i.e. increasing fatty acid oxidation and glucose uptake). AMPK activity is reduced in obesity, diabetes and inflammatory states [50] and is increased acutely during and after exercise in mice [51], with indirect evidence suggesting that exercise modulates the AMPK/mTORC1 pathway in people with MAFLD [52]. A recent phase 2a pharmacological trial targeting AMPK activation observed reduction in liver fat and metabolic parameters in some people with MAFLD [53]. Extra-hepatic adaptations to exercise, notably exercise-mediated reductions in VAT, may also reduce the delivery of FFA and to the liver [11].
Putative mechanisms for the effects of exercise on reducing hepatic steatosis Solid lines indicate enhanced mechanisms (e.g. insulin sensitisation). Dashed lines indicate reduced mechanisms (e.g. reduced concentrations of plasma insulin and glucose, reduced de novo lipogenesis). VAT visceral adipose tissue, SAT subcutaneous adipose tissue, FFA free fatty acid, TG triglyceride, SREBP- 1c sterol regulatory element binding protein, ChREBP carbohydrate responsive element binding protein, DNL de novo lipogenesis, FAS fatty acid synthase, ACC acetyl-coenzyme A carboxylase, VLDL very low-density lipoprotein-cholesterol, AMPK adenosine monophosphate-activated protein kinase, SIRT1 sirtuin 1, IRS-1 insulin receptor substrate 1, PI3K phosphatidylinositol-3-kinase, GLUT4 glucose transporter type 4, G6P glucose 6-phosphate. Created with BioRender.com
2.1 Literature Search
A systematic online literature search for systematic reviews with meta-analyses was conducted (by SK) from database inception to June 2023 across seven electronic databases [PubMed, Cochrane Library, Embase (Ovid), CINAHL (Ebsco host), Web of Science and SPORTDiscus]. Search terms included keywords and Medical Subject Headings (MeSH) to find literature involving exercise and liver fat and/or MAFLD populations (see Online Resource 1 for the full list of search terms and specific database strategies). Included reviews were systematic reviews with meta-analyses that were in a MAFLD cohort and/or reported on a liver outcome (e.g. hepatic steatosis or liver biochemistries). The evidence generated by extraction (by SK and AS) and collation of literature via these searches was reviewed and evidence was graded (all authors). Consensus on the content and recommendations of the position statement was reached through an iterative process involving the multi-disciplinary authorship team. Decisions on how evidence-based guidelines could be best translated into clinical practice were developed via evidence review and authors’ professional experiences.
The majority of evidence was from early stage MAFLD, with limited data from people with metabolic-associated steatohepatitis and/or cirrhosis. Five overarching evidence statements were defined, and the strength of each evidence statement was graded based on the National Health and Medical Research Centre (NHMRC) guidelines (Table 1). The evidence grade reflects the degree of certainty based on the overall body of evidence that an effect or association is correct.
2.2 Evidence for the Benefits of Exercise on Hepatic Steatosis
As at June 2023, 25 systematic reviews with meta-analyses had examined the efficacy of exercise for reducing hepatic steatosis [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. Reviews predominantly included randomised controlled trials (RCTs) in adults [54, 55, 58, 59, 61, 62, 64, 67, 68, 70,71,72,73,74, 76,77,78,79,80,81], children or adolescents [59, 63], or both [66], with overweight or obesity [58, 66, 71] and/or confirmed MAFLD [54, 64, 65, 69, 72,73,74,75,76,77, 79,80,81,179]. Maintaining functional status and improving quality of life are key treatment goals in this patient group.
Data from people with broad-aetiology cirrhosis indicate that exercise training is safe and effective for lowering portal pressures [180, 181], with further exercise prescription guidance published (see Tandon et al. [158] for prescription recommendations). However, there is a dearth of evidence for the efficacy of exercise specifically in people with metabolic steatohepatitis-related cirrhosis. Data from small studies of patients with early stage or well-compensated cirrhosis (mixed aetiologies) have shown that supervised and home exercise programmes, combining aerobic and resistance exercise, are safe, with no apparent increased risk of variceal haemorrhage or encephalopathy; these programmes also led to increases in lean body mass, reduction in overall adiposity, improved mobility and a marginal reduction in hepatic venous pressure gradient [182,183,184,185].
Evidence to inform exercise interventions for people with decompensated cirrhosis is lacking. Low-intensity activity is likely safe and feasible for those with decompensated cirrhosis, but it is important to liaise closely with other members of the multi-disciplinary team (including physician evaluation) before initiating an exercise programme for all patients with cirrhosis. This is to ensure complications of end-stage liver disease are being appropriately managed for safety reasons. For example, if the patient is at high risk of varices or has had a previous variceal bleed, relevant prophylaxis must be in place before exercise is prescribed. For further information on clinical considerations regarding these complications and recommendations for exercise assessment and prescription for people with cirrhosis (see Tandon et al. [158].
People with cirrhosis are hypercatabolic and may experience decreased appetite, early satiety and nutritional malabsorption. Nutritional assessment and optimisation should occur alongside the initiation of exercise to identify malnutrition, ensure that patients are meeting the appropriate protein and calorie requirements, and determine whether an increased energy intake is required due to becoming more physically active. Continued communication with the dietetic team should be maintained throughout [186].
5 Conclusions
MAFLD is a highly prevalent chronic liver condition characterised by hepatic steatosis that is strongly linked with both cardiometabolic and liver-related sequalea. MAFLD management centres on lifestyle therapy (exercise and diet) driven by a patient-centred multi-disciplinary team. The broader management goals should target preventing liver disease progression and reversing MAFLD and reducing cardiovascular morbidity and mortality. The exercise professional plays a central care role in MAFLD management. There is strong evidence that aerobic exercise reduces hepatic steatosis with modest benefits (2–4%) and can be demonstrated irrespective of weight loss. At least 135 min/week and up to 240 min/week of moderate intensity aerobic activity is recommended for hepatic steatosis reduction, with no further benefit from completing higher intensity exercise (including HIIT approaches) so long as this volume of aerobic activity is achieved. This exercise dose is likely to result in broader health benefits. There is no clear evidence for a benefit of resistance training on outcomes for MAFLD beyond its established effects on lean mass maintenance, blood glucose control and neuromuscular strength. Resistance training should be considered in addition to, and not instead of, aerobic exercise targets. Exercise management should encompass shared decision making and engage practical behavioural strategies that are cognisant of an individual’s local, cultural and socioeconomic circumstances, along with personal preferences, comorbidities and physical capacity.
Data availability
Not applicable.
References
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–9. https://doi.org/10.1016/j.jhep.2020.03.039. (published Online First: 2020/04/12).
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71. https://doi.org/10.1016/j.jhep.2018.09.014.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. https://doi.org/10.1002/hep.28431. (published Online First: 2015/12/29).
Adams LA, Roberts SK, Strasser SI, et al. Nonalcoholic fatty liver disease burden: Australia, 2019–2030. J Gastroenterol Hepatol. 2020;35(9):1628–35. https://doi.org/10.1111/jgh.15009. (published Online First: 2020/02/13).
Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. https://doi.org/10.1038/nrgastro.2017.109. (published Online First: 2017/09/21).
Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67(5):1726–36. https://doi.org/10.1002/hep.29546. (published Online First: 2017/09/25).
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. https://doi.org/10.1002/hep.29367.
Le MH, Yeo YH, Li X, et al. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20(12):2809-17.e28. https://doi.org/10.1016/j.cgh.2021.12.002.
Targher G. Concordance between MAFLD and NAFLD diagnostic criteria in “real-world” data. Liver Int. 2020;40(11):2879–80. https://doi.org/10.1111/liv.14623. (published Online First: 2020/08/02).
Gastaldelli A, Natali A, Vettor R, Corradini SG. Insulin resistance, adipose depots and gut: interactions and pathological implications. Dig Liver Dis. 2010;42(5):310–9. https://doi.org/10.1016/j.dld.2010.01.013.
Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–75. https://doi.org/10.1053/j.gastro.2008.01.075. (published Online First: 2008/03/22).
Yki-Järvinen H. Fat in the liver and insulin resistance. Ann Med. 2005;37(5):347–56. https://doi.org/10.1080/07853890510037383. (published Online First: 2005/09/24).
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–51. https://doi.org/10.1172/jci23621. (published Online First: 2005/05/03).
Hughes A, Dahmus J, Rivas G, et al. Exercise training reverses gut dysbiosis in patients with biopsy-proven nonalcoholic steatohepatitis: a proof of concept study. Clin Gastroenterol Hepatol. 2021;19(8):1723–5. https://doi.org/10.1016/j.cgh.2020.08.063. (published Online First: 2020/09/04).
Adams LA, Lymp JF, St. Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–21. https://doi.org/10.1053/j.gastro.2005.04.014.
Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57(4):1357–65. https://doi.org/10.1002/hep.26156. (published Online First: 2012/11/24).
Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–53. https://doi.org/10.1053/j.gastro.2011.06.061. (published Online First: 2011/07/06).
Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113(11):1649–59. https://doi.org/10.1038/s41395-018-0088-6.
Allen AM, Therneau TM, Ahmed OT, et al. Clinical course of non-alcoholic fatty liver disease and the implications for clinical trial design. J Hepatol. 2022. https://doi.org/10.1016/j.jhep.2022.07.004. (published Online First: 2022/07/18).
Younossi ZM, Loomba R, Rinella ME, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68(1):361–71. https://doi.org/10.1002/hep.29724.
Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport. 2010;13(5):496–502. https://doi.org/10.1016/j.jsams.2009.09.008.
Liguori G, Medicine ACoS. ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins; 2020.
EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64(6):1388–402. https://doi.org/10.1016/j.jhep.2015.11.004 (published Online First: 2016/04/12)
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71.
Stine JG, Munaganuru N, Barnard A, et al. Change in MRI-PDFF and histologic response in patients with nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19(11):2274–83. https://doi.org/10.1016/j.cgh.2020.08.061. (e5; Published Online First: 2020/09/04).
Dufour J-F, Anstee QM, Bugianesi E, et al. Current therapies and new developments in NASH. Gut. 2022;71(10):2123–34. https://doi.org/10.1136/gutjnl-2021-326874.
Argo CK, Stine JG, Henry ZH, et al. Physical deconditioning is the common denominator in both obese and overweight subjects with nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2018;48(3):290–9. https://doi.org/10.1111/apt.14803. (published Online First: 2018/05/26).
Canada JM, Abbate A, Collen R, et al. Relation of hepatic fibrosis in nonalcoholic fatty liver disease to left ventricular diastolic function and exercise tolerance. Am J Cardiol. 2019;123(3):466–73. https://doi.org/10.1016/j.amjcard.2018.10.027. (published Online First: 2018/12/07).
Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47(4):1158–66. https://doi.org/10.1002/hep.22137. (published Online First: 2008/02/13).
Kantartzis K, Thamer C, Peter A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58(9):1281. https://doi.org/10.1136/gut.2008.151977.
Doward LC, Balp MM, Twiss J, et al. Development of a patient-reported outcome measure for non-alcoholic steatohepatitis (NASH-CHECK): results of a qualitative study. Patient. 2021;14(5):533–43. https://doi.org/10.1007/s40271-020-00485-w. (published Online First: 2020/12/19).
Queensland Health Nutrition Education Mstarials Online. The Mediterranean Diet; Queensland Government: Brisbane, Australia, 2019.
Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79–104. https://doi.org/10.1002/hep.23623. (published Online First: 2010/06/26).
Wong VW-S, Wong GL-H, Chan RS-M, et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018;69(6):1349–56. https://doi.org/10.1016/j.jhep.2018.08.011.
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. https://doi.org/10.1016/j.jhep.2015.11.004.
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–78. https://doi.org/10.1053/j.gastro.2015.04.005. (e5; quiz e14-5; published Online First: 2015/04/14).
Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(3):1611–44. https://doi.org/10.1002/hep.32049.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-94. https://doi.org/10.1093/geronj/49.2.m85.
Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise scale. Nurs Res. 2000;49(3):154–9. https://doi.org/10.1097/00006199-200005000-00007.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4.
Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37(8):1209–18. https://doi.org/10.1111/liv.13391. (published Online First: 20170313).
Bovend’Eerdt TJ, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: a practical guide. Clin Rehabil. 2009;23(4):352–61. https://doi.org/10.1177/0269215508101741. (published Online First: 2009/02/25).
Thyfault JP, Bergouignan A. Exercise and metabolic health: beyond skeletal muscle. Diabetologia. 2020;63(8):1464–74. https://doi.org/10.1007/s00125-020-05177-6.
Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, Azarbayjani MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur J Sport Sci. 2019;19(7):994–1003. https://doi.org/10.1080/17461391.2019.1571114.
Brouwers B, Hesselink MK, Schrauwen P, Schrauwen-Hinderling VB. Effects of exercise training on intrahepatic lipid content in humans. Diabetologia. 2016;59(10):2068–79. https://doi.org/10.1007/s00125-016-4037-x. (published Online First: 2016/07/10).
Heinle JW, DiJoseph K, Sabag A, et al. Exercise is medicine for nonalcoholic fatty liver disease: exploration of putative mechanisms. Nutrients. 2023. https://doi.org/10.3390/nu15112452.
Solomon TP, Haus JM, Marchetti CM, Stanley WC, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2009;297(2):E552–9. https://doi.org/10.1152/ajpendo.00220.2009. (published Online First: 2009/06/18).
Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr. 2000;72(2 Suppl):558s-s563. https://doi.org/10.1093/ajcn/72.2.558S. (published Online First: 2000/08/02).
Rector RS, Thyfault JP, Morris RT, et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G619–26. https://doi.org/10.1152/ajpgi.00428.2007. (published Online First: 2008/01/05).
Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311(4):E730–40. https://doi.org/10.1152/ajpendo.00225.2016. (published Online First: 2016/09/01).
Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418(2):261–75. https://doi.org/10.1042/BJ20082055.
Stine JG, Xu D, Schmitz K, Sciamanna C, Kimball SR. Exercise attenuates ribosomal protein six phosphorylation in fatty liver disease. Dig Dis Sci. 2020;65(11):3238–43. https://doi.org/10.1007/s10620-020-06226-1. (published Online First: 2020/04/03).
Cusi K, Alkhouri N, Harrison SA, et al. Efficacy and safety of PXL770, a direct AMP kinase activator, for the treatment of non-alcoholic fatty liver disease (STAMP-NAFLD): a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Gastroenterol Hepatol. 2021;6(11):889–902. https://doi.org/10.1016/s2468-1253(21)00300-9.
Babu AF, Csader S, Lok J, et al. Positive effects of exercise intervention without weight loss and dietary changes in NAFLD-related clinical parameters: a systematic review and meta-analysis. Nutrients. 2021. https://doi.org/10.3390/nu13093135. (published Online First: 2021/09/29).
Baker CJ, Martinez-Huenchullan SF, D’Souza M, et al. Effect of exercise on hepatic steatosis: Are benefits seen without dietary intervention? A systematic review and meta-analysis. J Diabetes. 2021;13(1):63–77. https://doi.org/10.1111/1753-0407.13086.
Battista F, Ermolao A, van Baak MA, et al. Effect of exercise on cardiometabolic health of adults with overweight or obesity: focus on blood pressure, insulin resistance, and intrahepatic fat-A systematic review and meta-analysis. Obes Rev. 2021;22(Suppl 4): e13269. https://doi.org/10.1111/obr.13269.
González-Ruiz K, Ramírez-Vélez R, Correa-Bautista JE, Peterson MD, García-Hermoso A. The effects of exercise on abdominal fat and liver enzymes in pediatric obesity: a systematic review and meta-analysis. Child Obes. 2017;13(4):272–82. https://doi.org/10.1089/chi.2017.0027. (published Online First: 2017/03/23).
Hens W, Taeyman J, Cornelis J, Gielen J, Van Gaal L, Vissers D. The effect of lifestyle interventions on excess ectopic fat deposition measured by noninvasive techniques in overweight and obese adults: a systematic review and meta-analysis. J Phys Act Health. 2016;13(6):671–94. https://doi.org/10.1123/jpah.2015-0560. (published Online First: 2015/12/24).
Hens W, Vissers D, Hansen D, et al. The effect of diet or exercise on ectopic adiposity in children and adolescents with obesity: a systematic review and meta-analysis. Obes Rev. 2017;18(11):1310–22. https://doi.org/10.1111/obr.12577.
Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism. 2017;68:119–32. https://doi.org/10.1016/j.metabol.2016.12.006.
Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57(1):157–66. https://doi.org/10.1016/j.jhep.2012.02.023. (published Online First: 2012/03/15).
Khalafi M, Symonds ME. The impact of high intensity interval training on liver fat content in overweight or obese adults: a meta-analysis. Physiol Behav. 2021;236: 113416. https://doi.org/10.1016/j.physbeh.2021.113416. (published Online First: 2021/04/07).
Medrano M, Cadenas-Sanchez C, Álvarez-Bueno C, et al. Evidence-based exercise recommendations to reduce hepatic fat content in youth—a systematic review and meta-analysis. Prog Cardiovasc Dis. 2018;61(2):222–31. https://doi.org/10.1016/j.pcad.2018.01.013. (published Online First: 2018/02/17).
Mohammad Rahimi GR, Attarzadeh Hosseini SR. Effect of aerobic exercise alone or in conjunction with diet on liver function, insulin resistance and lipids in non-alcoholic fatty liver disease. Biol Res Nurs. 2022;24(2):259–76. https://doi.org/10.1177/10998004211068026. (published Online First: 2022/02/09).
Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55(4):885–904. https://doi.org/10.1007/s00125-011-2446-4. (published Online First: 2012/01/27).
Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin Gastroenterol Hepatol. 2016;14(10):1398–411. https://doi.org/10.1016/j.cgh.2016.04.036. (published Online First: 2016/05/08).
Sabag A, Barr L, Armour M, et al. The effect of high-intensity interval training versus moderate-intensity continuous training on liver fat: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2021. https://doi.org/10.1210/clinem/dgab795. (published Online First: 2021/11/02).
Sabag A, Way KL, Keating SE, et al. Exercise and ectopic fat in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2017;43(3):195–210. https://doi.org/10.1016/j.diabet.2016.12.006. (published Online First: 2017/02/07).
Sargeant JA, Gray LJ, Bodicoat DH, et al. The effect of exercise training on intrahepatic triglyceride and hepatic insulin sensitivity: a systematic review and meta-analysis. Obes Rev. 2018;19(10):1446–59. https://doi.org/10.1111/obr.12719. (published Online First: 2018/08/10).
Słomko J, Zalewska M, Niemiro W, et al. Evidence-based aerobic exercise training in metabolic-associated fatty liver disease: systematic review with meta-analysis. J Clin Med. 2021. https://doi.org/10.3390/jcm10081659. (published Online First: 2021/05/01).
Smart NA, King N, McFarlane JR, Graham PL, Dieberg G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: a systematic review and meta-analysis. Br J Sports Med. 2018;52(13):834–43. https://doi.org/10.1136/bjsports-2016-096197. (published Online First: 2016/06/19).
Fernández T, Viñuela M, Vidal C, Barrera F. Lifestyle changes in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. PLoS One. 2022;17(2): e0263931. https://doi.org/10.1371/journal.pone.0263931.
Chai XN, Zhou BQ, Ning N, et al. Effects of lifestyle intervention on adults with metabolic associated fatty liver disease: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1081096. https://doi.org/10.3389/fendo.2023.1081096.
Fu L, Zhang W, Ao Y, Zheng Z, Hu H. Efficacy of aerobic and resistance exercises in improving visceral adipose in patients with non-alcoholic fatty liver: a meta-analysis of randomized controlled trials. Z Gastroenterol. 2022;60(11):1644–58. https://doi.org/10.1055/a-1742-4257.
Houttu V, Bouts J, Vali Y, et al. Does aerobic exercise reduce NASH and liver fibrosis in patients with non-alcoholic fatty liver disease? A systematic literature review and meta-analysis. Front Endocrinol. 2022. https://doi.org/10.3389/fendo.2022.1032164.
Nam H, Yoo JJ, Cho Y, et al. Effect of exercise-based interventions in nonalcoholic fatty liver disease: a systematic review with meta-analysis. Dig Liver Dis. 2023. https://doi.org/10.1016/j.dld.2022.12.013.
Stine JG, DiJoseph K, Pattison Z, et al. Exercise training is associated with treatment response in liver fat content by magnetic resonance imaging independent of clinically significant body weight loss in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Am J Gastroenterol. 2022. https://doi.org/10.14309/ajg.0000000000002098.
Golabi P, Locklear CT, Austin P, et al. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: systematic review. World J Gastroenterol. 2016;22(27):6318–27. https://doi.org/10.3748/wjg.v22.i27.6318. (publishedOnlineFirst:2016/07/29).
Ghaffari M, Sadeghiyan S, Faramarzi M, Moghaddam M, Baghurst T. The effect of aerobic exercise on metabolic parameters of patients with non-alcoholic fatty liver disease: systematic review and meta-analysis. J Sports Med Phys Fitness. 2023;63(1):178–87. https://doi.org/10.23736/s0022-4707.22.13801-6.
Hejazi K, Hackett D. Effect of exercise on liver function and insulin resistance markers in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2023. https://doi.org/10.3390/jcm12083011.
Hong F, Liu Y, Lebaka VR, et al. Effect of exercise training on serum transaminases in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Front Physiol. 2022;13: 894044. https://doi.org/10.3389/fphys.2022.894044.
Liu Y, **e W, Li J, Ossowski Z. Effects of aerobic exercise on metabolic indicators and physical performance in adult NAFLD patients: a systematic review and network meta-analysis. Medicine. 2023;102(14): e33147. https://doi.org/10.1097/md.0000000000033147.
Sabag A, Barr L, Armour M, et al. The effect of high-intensity interval training vs moderate-intensity continuous training on liver fat: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107(3):862–81. https://doi.org/10.1210/clinem/dgab795. (published Online First: 2021/11/02).
Bril F, Sanyal A, Cusi K. Metabolic syndrome and its association with nonalcoholic steatohepatitis. Clin Liver Dis. 2023;27(2):187–210. https://doi.org/10.1016/j.cld.2023.01.002.
Houttu V, Csader S, Nieuwdorp M, Holleboom AG, Schwab U. Dietary interventions in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr. 2021;8: 716783. https://doi.org/10.3389/fnut.2021.716783. (published Online First: 2021/08/10).
Haigh L, Kirk C, El Gendy K, et al. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Clin Nutr. 2022;41(9):1913–31. https://doi.org/10.1016/j.clnu.2022.06.037.
Hashida R, Kawaguchi T, Bekki M, et al. Aerobic vs resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66(1):142–52. https://doi.org/10.1016/j.jhep.2016.08.023. (published Online First: 2016/10/23).
Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50(4):1105–12. https://doi.org/10.1002/hep.23129. (published Online First: 2009/07/29).
Keating SE, Hackett DA, Parker HM, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63(1):174–82. https://doi.org/10.1016/j.jhep.2015.02.022. (published Online First: 2015/04/13).
Zhang HJ, He J, Pan LL, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med. 2016;176(8):1074–82. https://doi.org/10.1001/jamainternmed.2016.3202. (published Online First: 2016/07/06).
Bacchi E, Negri C, Targher G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology. 2013;58(4):1287–95. https://doi.org/10.1002/hep.26393. (published Online First: 2013/03/19).
Keating SE, Hackett DA, Parker HM, et al. Effect of resistance training on liver fat and visceral adiposity in adults with obesity: a randomized controlled trial. Hepatol Res. 2017;47(7):622–31.
Slentz CA, Bateman LA, Willis LH, et al. Effects of aerobic vs resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011;301(5):1033–9. https://doi.org/10.1152/ajpendo.00291.2011. (published Online First: 2011/08/19).
Takahashi A, Abe K, Usami K, et al. Simple resistance exercise helps patients with non-alcoholic fatty liver disease. Int J Sports Med. 2015;36(10):848–52. https://doi.org/10.1055/s-0035-1549853. (published Online First: 2015/06/20).
Zelber-Sagi S, Buch A, Yeshua H, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol. 2014;20(15):4382–92. https://doi.org/10.3748/wjg.v20.i15.4382. (published Online First: 2014/04/26).
Damor K, Mittal K, Bhalla AS, et al. Effect of progressive resistance exercise training on hepatic fat in Asian Indians with non-alcoholic fatty liver disease. Br J Med Med Res. 2014;4(1):114–24.
Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012;61(11):2787–95. https://doi.org/10.2337/db12-0214. (published Online First: 2012/07/04).
Lee S, Deldin AR, White D, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;305(10):E1222–9. https://doi.org/10.1152/ajpendo.00285.2013. (published Online First: 2013/09/21).
Van Der Heijden G-J, Wang ZJ, Chu Z, et al. Strength exercise improves muscle mass and hepatic insulin sensitivity in obese youth. Med Sci Sports Exerc. 2010;42(11):1973–80. https://doi.org/10.1249/MSS.0b013e3181df16d9.
Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60(9):1278–83. https://doi.org/10.1136/gut.2011.242073. (published Online First: 2011/06/29).
Hickman IJ, Byrne NM, Croci I, et al. A pilot randomised study of the metabolic and histological effects of exercise in non-alcoholic steatohepatitis. J Diabet Metab. 2013;4(8):1–10.
Shamsoddini A, Sobhani V, Ghamar Chehreh ME, Alavian SM, Zaree A. Effect of aerobic and resistance exercise training on liver enzymes and hepatic fat in Iranian men with nonalcoholic fatty liver disease. Hepat Mon. 2015;15(10): e31434. https://doi.org/10.5812/hepatmon.31434. (published Online First: 2015/11/21).
Houghton D, Thoma C, Hallsworth K, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15(1):96-102.e3. https://doi.org/10.1016/j.cgh.2016.07.031. (published Online First: 2016/10/21).
Shojaee-Moradie F, Cuthbertson DJ, Barrett M, et al. Exercise training reduces liver fat and increases rates of vldl clearance but not VLDL production in NAFLD. J Clin Endocrinol Metab. 2016;101(11):4219–28. https://doi.org/10.1210/jc.2016-2353. (published Online First: 2016/11/05).
Sabag A, Little JP, Johnson NA. Low-volume high-intensity interval training for cardiometabolic health. J Physiol. 2022;600(5):1013–26. https://doi.org/10.1113/JP281210.
Motiani KK, Savolainen AM, Toivanen J, et al. Effects of short-term sprint interval and moderate-intensity continuous training on liver fat content, lipoprotein profile, and substrate uptake: a randomized trial. J Appl Physiol. 2019;126(6):1756–68. https://doi.org/10.1152/japplphysiol.00900.2018. (published Online First: 2019/04/19).
Oh S, Shida T, Sawai A, et al. Acceleration training for managing nonalcoholic fatty liver disease: a pilot study. Ther Clin Risk Manag. 2014;10:925–36. https://doi.org/10.2147/tcrm.S68322. (published Online First: 2014/11/19).
Oh S, Oshida N, Someya N, et al. Whole-body vibration for patients with nonalcoholic fatty liver disease: a 6-month prospective study. Physiol Rep. 2019;7(9): e14062. https://doi.org/10.14814/phy2.14062.
Çevik Saldiran T, Mutluay FK, Yağci İ, Yilmaz Y. Impact of aerobic training with and without whole-body vibration training on metabolic features and quality of life in non-alcoholic fatty liver disease patients. Ann Endocrinol. 2020;81(5):493–9. https://doi.org/10.1016/j.ando.2020.05.003.
Iwanaga S, Hashida R, Takano Y, et al. Hybrid training system improves insulin resistance in patients with nonalcoholic fatty liver disease: a randomized controlled pilot study. Tohoku J Exp Med. 2020;252(1):23–32. https://doi.org/10.1620/tjem.252.23. (published Online First: 2020/08/31).
Kawaguchi T, Shiba N, Maeda T, et al. Hybrid training of voluntary and electrical muscle contractions reduces steatosis, insulin resistance, and IL-6 levels in patients with NAFLD: a pilot study. J Gastroenterol. 2011;46(6):746–57. https://doi.org/10.1007/s00535-011-0378-x. (published Online First: 2011/02/23).
Oh S, Maruyama T, Eguchi K, et al. Therapeutic effect of hybrid training of voluntary and electrical muscle contractions in middle-aged obese women with nonalcoholic fatty liver disease: a pilot trial. Ther Clin Risk Manag. 2015;11:371–80. https://doi.org/10.2147/TCRM.S75109.
Keymasi Z, Sadeghi A, Pourrazi H. Effect of pilates training on hepatic fat content and liver enzymes in men with non-alcoholic fatty liver disease in Qazvin. J Shahrekord Univ Med Sci. 2020;22(1):22–8. https://doi.org/10.34172/jsums.2020.05.
Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106(3):460–8. https://doi.org/10.1038/ajg.2010.488. (quiz 69; published Online First: 2011/01/06).
Sherry AP, Willis SA, Yates T, et al. Physical activity is inversely associated with hepatic fibro-inflammation: a population-based cohort study using UK Biobank data. JHEP Rep. 2023;5(1): 100622. https://doi.org/10.1016/j.jhepr.2022.100622.
Eckard C, Cole R, Lockwood J, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. 2013;6(4):249–59. https://doi.org/10.1177/1756283X13484078.
O’Gorman P, Naimimohasses S, Monaghan A, et al. Improvement in histological endpoints of MAFLD following a 12-week aerobic exercise intervention. Aliment Pharmacol Ther. 2020;52(8):1387–98. https://doi.org/10.1111/apt.15989.
Carobene A, Braga F, Roraas T, Sandberg S, Bartlett WA. A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase. Clin Chem Lab Med. 2013;51(10):1997–2007. https://doi.org/10.1515/cclm-2013-0096. (published Online First: 2013/09/28).
Ma X, Liu S, Zhang J, et al. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: a systematic review and meta-analysis. BMC Gastroenterol. 2020;20(1):10. https://doi.org/10.1186/s12876-020-1165-z.
Wang S-T, Zheng J, Peng H-W, et al. Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2020;20(1):66. https://doi.org/10.1186/s12876-020-01204-3.
**ong Y, Peng Q, Cao C, Xu Z, Zhang B. Effect of different exercise methods on non-alcoholic fatty liver disease: a meta-analysis and meta-regression. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18063242. (published Online First: 2021/04/04).
Zhou BJ, Huang G, Wang W, et al. Intervention effects of four exercise modalities on nonalcoholic fatty liver disease: a systematic review and Bayesian network meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(24):7687–97. https://doi.org/10.26355/eurrev_202112_27615. (published Online First: 2022/01/05).
Zou TT, Zhang C, Zhou YF, et al. Lifestyle interventions for patients with nonalcoholic fatty liver disease: a network meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(7):747–55. https://doi.org/10.1097/meg.0000000000001135. (published Online First: 2018/04/24).
Gao Y, Lu J, Liu X, et al. Effect of long-term exercise on liver lipid metabolism in Chinese patients with NAFLD: a systematic review and meta-analysis. Front Physiol. 2021;12: 748517. https://doi.org/10.3389/fphys.2021.748517. (published Online First: 2021/12/10).
Ye YCL, Yang X. The efficacy of resistance training for non-alcoholic fatty liver disease: a meta-anlaysis of randomized controlled trials. Int J Clin Exp Med. 2019;12(2):13188–95.
Yu X, Wang Y, Lai J, Song T, Duan J. Comparative efficacy of exercise training processes in improving nonalcoholic fatty liver disease: a systematic review and meta-analysis. Ir J Med Sci. 2022. https://doi.org/10.1007/s11845-022-02988-x. (published Online First: 2022/04/03).
Loomba R, Sanyal AJ, Kowdley KV, et al. Factors associated with histologic response in adult patients with nonalcoholic steatohepatitis. Gastroenterology. 2019;156(1):88-95.e5. https://doi.org/10.1053/j.gastro.2018.09.021. (published Online First: 2018/09/18).
Shaw KA, Gennat HC, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006. https://doi.org/10.1002/14651858.CD003817.pub3.
Oppert JM, Bellicha A, van Baak MA, et al. Exercise training in the management of overweight and obesity in adults: zynthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes Rev. 2021;22(4): e13273. https://doi.org/10.1111/obr.13273. (published Online First: 2021/06/03).
Armstrong A, Jungbluth Rodriguez K, Sabag A, et al. Effect of aerobic exercise on waist circumference in adults with overweight or obesity: a systematic review and meta-analysis. Obes Rev. 2022;23(8): e13446. https://doi.org/10.1111/obr.13446.
Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13(1):68–91. https://doi.org/10.1111/j.1467-789X.2011.00931.x. (published Online First: 2011/09/29).
Keating SE, Johnson NA, Mielke GI, Coombes JS. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–64. https://doi.org/10.1111/obr.12536. (published Online First: 2017/05/18).
Kuk JL, Lee S, Heymsfield SB, Ross R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr. 2005;81(6):1330–4. https://doi.org/10.1093/ajcn/81.6.1330. (published Online First: 2005/06/09).
Gonzalez A, Valero-Breton M, Huerta-Salgado C, Achiardi O, Simon F, Cabello-Verrugio C. Impact of exercise training on the sarcopenia criteria in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Transl Myol. 2021. https://doi.org/10.4081/ejtm.2021.9630.
Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond). 2016;130(2):93–104. https://doi.org/10.1042/cs20150447. (published Online First: 2015/10/02).
Pugh CJ, Spring VS, Kemp GJ, et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. 2014;307(9):H1298–306. https://doi.org/10.1152/ajpheart.00306.2014. (published Online First: 2014/09/07).
Shojaee-Moradie F, Baynes K, Pentecost C, et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia. 2007;50(2):404–13.
Abdelbasset WK, Tantawy SA, Kamel DM, et al. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: a comparative randomized controlled trial. Medicine. 2020;99(10): e19471. https://doi.org/10.1097/md.0000000000019471. (published Online First: 2020/03/10).
Stine JG, Schreibman IR, Faust AJ, et al. NASHFit: a randomized controlled trial of an exercise training program to reduce clotting risk in patients with NASH. Hepatology. 2022;76(1):172–85. https://doi.org/10.1002/hep.32274.
Hallsworth K, Thoma C, Hollingsworth KG, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci. 2015;129(12):1097–105. https://doi.org/10.1042/cs20150308. (published Online First: 2015/08/13).
Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity—a randomized trial. Metabolism. 2018;78:128–40. https://doi.org/10.1016/j.metabol.2017.08.012. (published Online First: 2017/09/25).
Keating SE, Croci I, Wallen MP, et al. High-intensity interval training is safe, feasible and efficacious in nonalcoholic steatohepatitis: a randomized controlled trial. Dig Dis Sci. 2023;68(5):2123–39. https://doi.org/10.1007/s10620-022-07779-z.
Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–402. https://doi.org/10.1249/mss.0000000000002117.
O’Donoghue G, Blake C, Cunningham C, Lennon O, Perrotta C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes Rev. 2021;22(2): e13137. https://doi.org/10.1111/obr.13137.
Way KL, Hackett DA, Baker MK, Johnson NA. The effect of regular exercise on insulin sensitivity in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab J. 2016;40(4):253–71. https://doi.org/10.4093/dmj.2016.40.4.253.
Leung FP, Yung LM, Laher I, Yao X, Chen ZY, Huang Y. Exercise, vascular wall and cardiovascular diseases. Sports Med. 2008;38(12):1009–24. https://doi.org/10.2165/00007256-200838120-00005.
Boulé NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia. 2003;46(8):1071–81.
Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72. https://doi.org/10.1111/sms.12581. (published Online First: 2015/11/26).
Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. https://doi.org/10.1001/jama.2009.681.
Cheng S, Ge J, Zhao C, et al. Effect of aerobic exercise and diet on liver fat in pre-diabetic patients with non-alcoholic-fatty-liver-disease: a randomized controlled trial. Sci Rep. 2017;7(1):15952. https://doi.org/10.1038/s41598-017-16159-x. (published Online First: 2017/11/23).
Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55(6):1738–45. https://doi.org/10.1002/hep.25548. (published Online First: 2012/01/04).
Sabag A, Way KL, Sultana RN, et al. The effect of a novel low-volume aerobic exercise intervention on liver fat in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2020;43(10):2371–8. https://doi.org/10.2337/dc19-2523. (published Online First: 2020/08/01).
Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48(16):1227–34. https://doi.org/10.1136/bjsports-2013-092576.
Sultana RN, Sabag A, Keating SE, Johnson NA. The effect of low-volume high-intensity interval training on body composition and cardiorespiratory fitness: a systematic review and meta-analysis. Sports Med. 2019;49(11):1687–721.
Taylor JL, Holland DJ, Spathis JG, et al. Guidelines for the delivery and monitoring of high intensity interval training in clinical populations. Prog Cardiovasc Dis. 2019;62(2):140–6. https://doi.org/10.1016/j.pcad.2019.01.004.
Hordern MD, Dunstan DW, Prins JB, Baker MK, Singh MA, Coombes JS. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. J Sci Med Sport. 2012;15(1):25–31. https://doi.org/10.1016/j.jsams.2011.04.005. (published Online First: 20110528).
Kanaley JA, Colberg SR, Corcoran MH, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American College of Sports Medicine. Med Sci Sports Exerc. 2022;54(2):353–68.
Tandon P, Ismond KP, Riess K, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol. 2018;69(5):1164–77. https://doi.org/10.1016/j.jhep.2018.06.017.
Dennis A, Kelly MD, Fernandes C, et al. Correlations between MRI biomarkers PDFF and cT1 with histopathological features of non-alcoholic steatohepatitis. Front Endocrinol. 2020;11: 575843. https://doi.org/10.3389/fendo.2020.575843.
Pugh CJ, Sprung VS, Jones H, et al. Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int J Obes. 2016;40(12):1927–30. https://doi.org/10.1038/ijo.2016.123. (published Online First: 2016/10/19).
Keating SECI, Wallen MP, Cox ER, Coombes JS, Burton NW, et al. High-intensity interval training for the management of nonalcoholic steatohepatitis: participant experiences and perspectives. J Clin Transl Hepatol. 2023. https://doi.org/10.14218/JCTH.2022.00091S. (Published online: Apr 21, 2023).
Carels RA, Cacciapaglia HM, Douglass OM, Rydin S, O’Brien WH. The early identification of poor treatment outcome in a women’s weight loss program. Eat Behav. 2003;4(3):265–82. https://doi.org/10.1016/s1471-0153(03)00029-1. (published Online First: 2004/03/06).
Johnson NA, Sultana RN, Brown WJ, Bauman AE, Gill T. Physical activity in the management of obesity in adults: a position statement from Exercise and Sport Science Australia. J Sci Med Sport. 2021;24(12):1245–54. https://doi.org/10.1016/j.jsams.2021.07.009. (published Online First: 2021/09/18).
Stine JG, Soriano C, Schreibman I, et al. Breaking down barriers to physical activity in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2021;66(10):3604–11.
Jang Y, Lee JY, Kim SU, Kim B. A qualitative study of self-management experiences in people with non-alcoholic fatty liver disease. Nurs Open. 2021;8(6):3135–42.
Hallsworth K, Dombrowski SU, McPherson S, Anstee QM, Avery L. Using the theoretical domains framework to identify barriers and enabling factors to implementation of guidance for the diagnosis and management of nonalcoholic fatty liver disease: a qualitative study. Transl Behav Med. 2019;10(4):1016–30.
Tincopa MA, Wong J, Fetters M, Lok AS. Patient disease knowledge, attitudes and behaviours related to non-alcoholic fatty liver disease: a qualitative study. BMJ Open Gastroenterol. 2021;8(1): e000634.
Avery L, Exley C, McPherson S, Trenell MI, Anstee QM, Hallsworth K. Lifestyle behavior change in patients with nonalcoholic fatty liver disease: a qualitative study of clinical practice. Clin Gastroenterol Hepatol. 2017. https://doi.org/10.1016/j.cgh.2017.06.011.
Frith J, Day CP, Robinson L, Elliott C, Jones DE, Newton JL. Potential strategies to improve uptake of exercise interventions in non-alcoholic fatty liver disease. J Hepatol. 2010;52(1):112–6.
O’Gorman P, Monaghan A, McGrath M, Naimimohasses S, Gormley J, Norris S. Determinants of physical activity engagement in patients with nonalcoholic fatty liver disease: the need for an individualized approach to lifestyle interventions. Phys Ther. 2021. https://doi.org/10.1093/ptj/pzaa195.
Gerber LH, Weinstein AA, Mehta R, Younossi ZM. Importance of fatigue and its measurement in chronic liver disease. World J Gastroenterol. 2019;25(28):3669–83.
Glass O, Liu D, Bechard E, et al. Perceptions of exercise and its challenges in patients with nonalcoholic fatty liver disease: a survey-based study. Hepatol Commun. 2022;6(2):334–44.
Choi JM, Chung GE, Kang SJ, et al. Association between anxiety and depression and nonalcoholic fatty liver disease. Front Med. 2021;7:585618–718. https://doi.org/10.3389/fmed.2020.585618.
Sayiner M, Stepanova M, Pham H, Noor B, Walters M, Younossi ZM. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1): e000106. https://doi.org/10.1136/bmjgast-2016-000106. (published Online First: 2016/09/21).
Golabi P, Otgonsuren M, Cable R, et al. Non-alcoholic Fatty Liver Disease (NAFLD) is associated with impairment of health related quality of life (HRQOL). Health Qual Life Outcomes. 2016;14:18. https://doi.org/10.1186/s12955-016-0420-z. (published Online First: 2016/02/11).
Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282(17):1659–64. https://doi.org/10.1001/jama.282.17.1659. (published Online First: 1999/11/30).
Croci I, Byrne NM, Choquette S, et al. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut. 2013;62(11):1625–33. https://doi.org/10.1136/gutjnl-2012-302789. (published Online First: 2012/10/19).
Francque S, Vonghia L. Pharmacological treatment for non-alcoholic fatty liver disease. Adv Ther. 2019;36(5):1052–74. https://doi.org/10.1007/s12325-019-00898-6.
Spengler EK, O’Leary JG, Te HS, et al. Liver Transplantation in the obese cirrhotic patient. Transplantation. 2017;101(10):2288–96.
Berzigotti A, Albillos A, Villanueva C, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65(4):1293–305. https://doi.org/10.1002/hep.28992. (published Online First: 2016/12/21).
Duarte-Rojo A, Bloomer PM, Rogers RJ, et al. Introducing EL-FIT (Exercise and Liver FITness): a smartphone app to prehabilitate and monitor liver transplant candidates. Liver Transpl. 2021;27(4):502–12. https://doi.org/10.1002/lt.25950. (published Online First: 2020/11/25).
Macías-Rodríguez RU, Ruiz-Margáin A, Román-Calleja BM, et al. Exercise prescription in patients with cirrhosis: recommendations for clinical practice. Rev Gastroenterol Mex (Engl Ed). 2019;84(3):326–43. https://doi.org/10.1016/j.rgmx.2019.02.011. (English, Spanish).
Wallen MP, Skinner TL, Pavey TG, Hall A, Macdonald GA, Coombes JS. Safety, adherence and efficacy of exercise training in solid-organ transplant candidates: a systematic review. Transplant Rev. 2016;30(4):218–26.
Williams FR, Vallance A, Faulkner T, et al. Home-based exercise in patients awaiting liver transplantation: a feasibility study. Liver Transpl. 2019;25(7):995–1006.
Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, et al. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol. 2016;7(7): e180.
Berzigotti A, Saran U, Dufour JF. Physical activity and liver diseases. Hepatology. 2016;63(3):1026–40. https://doi.org/10.1002/hep.28132.
Acknowledgements
The authors would like to acknowledge the advice and input of the following experts: Robert Stanton (ESSA); ESSA Position Statement Committee and Reviewers; ESSA Research Committee and Reviewers; Professor Jeff Coombes for mentorship; Juliette Grosvenor, librarian for the School of Human Movement and Nutrition Sciences, The University of Queensland, for assistance with develo** and testing the search strategy.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No sources of funding were used to assist in the preparation of this article.
Author information
Authors and Affiliations
Contributions
Conceptualisation: SEK, NAJ; Search: SEK; Title, abstract & full text screening: SEK, AS; Drafting: SEK, AS, NAJ, KH; Evidence grading: SEK, AS, NAJ, KH; Medical input: JGS, GAM, JG; Dietetic input: IH; Critical review and editing: All authors. All authors read and approved the final version.
Corresponding author
Ethics declarations
Conflicts of Interest
SEK has received funding for unrelated work by Diabetes Australia, Exercise and Sports Science Australia and NHMRC. JGS receives or has received research support from Astra Zeneca, Galectin, Noom, Inc., Novo Nordisk and Zydus. AS, KH, IJH, GAM, JG and NAJ have nil to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keating, S.E., Sabag, A., Hallsworth, K. et al. Exercise in the Management of Metabolic-Associated Fatty Liver Disease (MAFLD) in Adults: A Position Statement from Exercise and Sport Science Australia. Sports Med 53, 2347–2371 (2023). https://doi.org/10.1007/s40279-023-01918-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-023-01918-w