Abstract
Background and Objective
Gene therapies for sickle cell disease (SCD) may offer meaningful benefits for patients and society. This study evaluated the cost-effectiveness of lovotibeglogene autotemcel (lovo-cel), a one-time gene therapy administered via autologous hematopoietic stem cell transplantation, compared with common care for patients in the United States (US) with SCD aged ≥ 12 years with ≥ 4 vaso-occlusive events (VOEs) in the past 24 months.
Methods
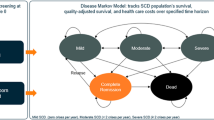
We developed a patient-level simulation model accounting for lovo-cel and SCD-related events, complications, and mortality over a lifetime time horizon. The pivotal phase 1/2 HGB-206 clinical trial (NCT02140554) served as the basis for lovo-cel efficacy and safety. Cost, quality-of-life, and other clinical data were sourced from HGB-206 data and the literature. Analyses were conducted from US societal and third-party payer perspectives. Uncertainty was assessed through probabilistic sensitivity analysis and extensive scenario analyses.
Results
Patients treated with lovo-cel were predicted to survive 23.84 years longer on average (standard deviation [SD], 12.80) versus common care (life expectancy, 62.24 versus 38.40 years), with associated discounted patient quality-adjusted life-year (QALY) gains of 10.20 (SD, 4.10) and direct costs avoided of $1,329,201 (SD, $1,346,446) per patient. Predicted societal benefits included discounted caregiver QALY losses avoided of 1.19 (SD, 1.38) and indirect costs avoided of $540,416 (SD, $262,353) per patient. Including lovo-cel costs ($3,282,009 [SD, $29,690] per patient) resulted in incremental cost-effectiveness ratios of $191,519 and $124,051 per QALY gained from third-party payer and societal perspectives, respectively. In scenario analyses, the predicted cost-effectiveness of lovo-cel also was sensitive to baseline age and VOE frequency and to the proportion of patients achieving and maintaining complete resolution of VOEs.
Conclusions
Our analysis of lovo-cel gene therapy compared with common care for patients in the US with SCD with recurrent VOEs estimated meaningful improvements in survival, quality of life, and other clinical outcomes accompanied by increased overall costs for the health care system and for broader society. The predicted economic value of lovo-cel gene therapy was influenced by uncertainty in long-term clinical effects and by positive spillover effects on patient productivity and caregiver burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
One-time gene therapies for sickle cell disease have the potential to deliver meaningful clinical and economic benefits for patients and society. We developed a lifetime economic model to predict the cost-effectiveness, or value for money, of lovo-cel gene therapy for patients with sickle cell disease and recurrent vaso-occlusive events in the United States using evidence from the pivotal HGB-206 clinical trial (NCT02140554) and the published literature. |
Our analysis predicted meaningful improvements in survival, quality of life, and other clinical outcomes for lovo-cel gene therapy compared with common care. The costs associated with lovo-cel were partially offset by predicted reductions in other health system and societal costs. |
Our findings highlight the contribution of positive spillover effects, such as patient productivity gains and reduced caregiver burden, to the potential economic value of lovo-cel gene therapy in the United States. The cost-effectiveness of lovo-cel was also found to be sensitive to patients’ baseline age and severity levels and to uncertainty in long-term effects. |
1 Introduction
Sickle cell disease (SCD) refers to a heterogeneous group of genetic abnormalities affecting hemoglobin, the oxygen-carrying protein in red blood cells (RBCs) [1]. The sickled RBCs characteristic of SCD are more rigid and adherent and have a shorter lifespan than normal RBCs, which impairs blood flow and oxygen delivery to the body and results in vessel obstruction and anemia [1, 2]. Sickle cell disease is characterized by frequent and debilitating vaso-occlusive events (VOEs) (e.g., pain crises), stroke, and multisystem organ damage and organ infarction [2], leading to a lifespan cut short by several decades [3, 4]. These events and complications result in broad impacts to quality of life, social functioning, and attainment of personal, educational, and financial goals [5, 6]. Life expectancy for the approximately 100,000 individuals living with SCD in the United States (US) today is estimated at 52.6 years [7, 8], although for those with hemoglobin SS (HbSS) or Sβ0-thalassemia (HbSβ0) genotypes, disease severity is much greater and life expectancy estimates are closer to 40 years [8, 9].
Although the adoption of clinical practice guidelines for SCD newborn screening, management, and treatment in recent decades has shifted the burden of disease and risk of early death from childhood into adulthood [10, 11], significant unmet need remains. For most individuals with SCD today, disease management involves lifelong use of acute and chronic therapies, including opioid and nonopioid pain management, hydration therapy, RBC transfusion, hydroxyurea (HU), and other newer disease-modifying therapies (l-glutamine, crizanlizumab, and voxelotor) [9, 12]. For the limited group of individuals with a suitable human leukocyte antigen (HLA)–matched sibling donor [13], allogeneic hematopoietic stem cell transplantation (HSCT) offers a potential cure [14].
Lovotibeglogene autotemcel (Lyfgenia™, henceforth “lovo-cel”) is a one-time gene therapy approved by the US Food and Drug Administration (FDA) for the treatment of patients 12 years of age or older with SCD and a history of VOEs [15]. Lovo-cel uses autologous transplantation of hematopoietic stem cells (HSC) transduced with a BB305 lentiviral vector encoding a modified β-globin gene, which produces an anti-sickling hemoglobin (Hb), HbAT87Q. Patients’ HSCs are mobilized and collected via apheresis, transduced in the laboratory with the gene to produce HbAT87Q, and, after myeloablative conditioning, infused back to the patient. Lovo-cel has demonstrated sustained efficacy—as well as a safety profile consistent with autologous HSCT requiring myeloablative conditioning—across a clinical trial program that includes the completed phase 1/2 HGB-205 (NCT02151526) and phase 1/2 HGB-206 (NCT02140554) studies and the ongoing LTF-307 (NCT04628585) and phase 3 HGB-210 (NCT04293185) studies [16]. In the pivotal phase 1/2 HGB-206 primary efficacy analysis, which has follow-up data up to 61 months after transplantation, 87.5% of individuals achieved complete resolution (i.e., 100% reduction) of VOEs (VOE-CR) during months 6 through 18 after transplantation [15]. On secondary endpoints, sustained total Hb levels and HbAT87Q anti-sickling fractions were observed [17]. Because lovo-cel uses autologous HSCT and does not require an HLA-matched donor, its approval in the US could result in expanded access to potentially curative treatments for SCD.
Cell and gene therapies, such as lovo-cel, constitute both an increasing percentage of the total drug development pipeline as well as an increasing share of new drug approvals [18, 19]. These technologies hold great promise for patients, their care networks, and broader society, but they are also prompting important discussions on value, access, and affordability [20, 21]. Economic evaluation is a tool for priority setting of healthcare expenditures and is increasingly employed in the US setting to understand the value of novel therapeutics and to inform coverage and reimbursement decisions. This context has shaped a number of recent economic evaluations of the gene therapies for SCD in the US [22,23,24,73].
2.5 Model Validation
Good practice guidelines for cost-effectiveness model validation emphasize face validity (of the modeling approach and data source), internal validity (of the model programming and predicted outcomes), and external validity (compared with other published studies) [32, 74]. The face validity of our modeling approach is supported by the involvement of clinical and economic advisors and a patient representative in model development and by similar comprehensive economic evaluations for SCD in the literature [24, 29]. We verified the internal validity of the model through a third-person, comprehensive review of all model programming and through extreme value testing. Finally, we assessed the external validity of our model by comparing outcomes with similarly comprehensive cost-effectiveness analyses for SCD gene therapy in the literature (presented in the Discussion section).
3 Results
3.1 Base-Case Analysis
The predicted lifetime outcomes for patients treated with lovo-cel in comparison with common care are presented in Table 2. Patients entered the model with an average baseline age of 24.9 years and an average of 0.93 existing SCD complications. Patients remaining on common care were predicted to survive 13.47 years (SD 9.08; 95% CI 13.12–13.83), with an estimated 67.81 lifetime VOCs and 3.93 cumulative chronic complications per patient. In contrast, patients treated with lovo-cel were predicted to survive 37.32 years (SD 18.97; 95% CI 36.57–38.06), with an estimated 6.75 lifetime VOCs and 2.05 cumulative chronic complications per patient. The predicted increase in survival of 23.84 years (SD 12.80; 95% CI 23.34–24.34) for lovo-cel versus common care translated to the mean age at death increasing from 38.40 to 62.24 years and the proportion of patients surviving past age 65 years increasing from < 1% to nearly 50% (Fig. 3). Of the 6.75 average lifetime VOCs for patients treated with lovo-cel, 2.48 VOCs occurred during the first 6 months (spanning the engraftment period); the remaining VOCs occurred only in those without VOE-CR after the initial 6 months. The cumulative proportions of patients develo** specific chronic complications and the associated annualized incidence predicted for lovo-cel compared with common care are presented in Fig. 4. Predicted lifetime and annualized incidence estimates for acute events are presented in Fig. S2 in the Supplementary Information.
The improvements in survival, quality of life, and other clinical outcomes predicted for lovo-cel versus common care translated to predicted increases in patient QALYs (16.44 vs. 6.25) and reductions in lifetime direct medical costs associated with common care, events, and complications ($860,020 versus $2,189,221). Reductions in caregiver burden also were predicted in the form of fewer caregiver QALYs lost (1.19 versus 2.38) and unpaid caregiving avoided ($86,157 versus $172,090) per patient. The predicted increase in annual earnings for patients achieving VOE-CR after lovo-cel resulted in a predicted increase in lifetime earnings of $454,483 per patient. The total lovo-cel-related costs (acquisition, preparation and administration, monitoring, and fertility preservation) were estimated to be $3,282,009 per patient.
The cost-effectiveness results for the co-base-case third-party payer and societal perspectives are presented in Table 3. From a third-party payer perspective, the predicted per-patient increases in total direct medical costs of $1,952,808 (SD $1,339,849; 95% CI $1,900,286–$2,005,330) and in patient QALYs of 10.20 (SD 4.10; 95% CI 10.04–10.36) resulted in an incremental cost-effectiveness ratio of $191,519 per QALY gained. From a broader societal perspective, the predicted per-patient increases in total direct and indirect costs of $1,412,393 (SD $1,452,720; 95% CI $1,355,446–$1,469,339) and in total patient and caregiver QALYs of 11.39 (SD 4.91; 95% CI 11.19–11.58) resulted in an incremental cost-effectiveness ratio of $124,051 per QALY gained.
At a WTP threshold of $150,000 per QALY gained, the NMB for lovo-cel compared with common care was predicted to be −$423,347 (95% CI −$488,805 to −$357,889) from a third-party payer perspective and $295,445 (95% CI $216,939–$373,950) from a societal perspective. At a higher WTP threshold of $200,000 per QALY gained, the NMB was predicted to be positive for both co-base-case perspectives. If incremental QALYs are valued at a WTP threshold of $150,000 per QALY gained (range $50,000–$200,000 per QALY gained) [37, 38], these results indicate that lovo-cel would be cost-effective at a one-time acquisition price of up to $2,676,653 (range $1,657,012–$3,186,473) from a third-party payer perspective and up to $3,395,445 (range $2,256,886–$3,964,724) from a societal perspective.
3.2 Heterogeneity and Uncertainty
The results for the base-case analysis indicated significant heterogeneity across patients in incremental costs and outcomes and in the resulting NMB estimates (Figs. S3 and S4 in the Supplementary Information for scatterplots across all patients and by age-specific subgroups). In particular, at a WTP threshold of $150,000 per QALY gained, 38.8% and 54.7% of patients were predicted to have a positive NMB from third-party payer and societal perspectives, respectively (Fig. S5 in the Supplementary Information).
In the PSA assessing the impact of joint parameter uncertainty, the proportions of iterations with a positive NMB at a WTP threshold of $150,000 per QALY gained were 27.8% and 79.4% for the co-base-case third-party payer and societal perspectives, respectively (Figs. 5 and S6 in the Supplementary Information). For the third-party payer perspective, the proportion of iterations with a positive NMB ranged from 1.8% to 64.8% over a WTP threshold range of $50,000 to $200,000 per QALY gained. Over this same WTP threshold range, the proportion of iterations with a positive NMB from a societal perspective ranged from 12.3% to 97.5%.
Table 4 presents the results from our targeted scenario and one-way sensitivity analyses. Among scenarios evaluating uncertainty regarding who receives lovo-cel, how well it works, and how long the effect lasts, the predicted cost-effectiveness of lovo-cel was most impacted by the age at treatment. Notably, the incremental cost-effectiveness ratios for lovo-cel compared with common care from both third-party payer and societal perspectives were over 40% lower than the base case for patients treated at ages 12–17 years and over 65% higher than the base case for patients treated at ages > 30 years. This variability reflects differences in baseline complication prevalence by age and our assumptions about the age-specific impacts of VOE-CR (Fig. 2). Similarly, relaxing the criteria for lovo-cel eligibility to include patients aged ≥ 12 years with any history of VOEs resulted in incremental cost-effectiveness ratios that were roughly 50% higher than the base case, primarily attributable to the lower VOC rates expected with common care (and thus avoided with lovo-cel).
Of the scenarios related to the lovo-cel treatment effect, uncertainty in the proportion of patients achieving VOE-CR had a greater impact on cost-effectiveness results than uncertainty in changes from baseline in total Hb or EQ-5D-3L utility values (Table 4). In general, variations in the proportion of patients achieving VOE-CR and the partial loss of treatment effect had a larger impact on the incremental cost-effectiveness ratios than variations in our assumptions around the age-specific impacts of VOE-CR. Excluding the partial reduction in VOEs for those without VOE-CR had a relatively limited impact on cost-effectiveness results. As anticipated, modeling the known Medicaid Prescription Drug Program rebate [60] (as a proxy for other potential payer-specific rebates) on the lovo-cel commercial list price had a significant impact on cost-effectiveness, lowering the incremental cost-effectiveness ratios by roughly 40–50% across third-party payer and societal perspectives. Variations in the direct costs associated with acute events and chronic complications had a larger impact on cost-effectiveness results than variations in disutilities associated with events and complications. Additional scenarios accounting for the potential impact of VOCs managed at home and related to limitations in the SCD caregiving literature had relatively limited impacts on the incremental cost-effectiveness ratios. Among scenarios considering alternative model settings, reductions in discount rates resulted in meaningfully lower incremental cost-effectiveness ratios, as expected. Although the inclusion of unrelated direct medical costs in years of additional survival for lovo-cel had minimal impact on cost-effectiveness results, the inclusion of consumption costs in years of additional survival offset some of the predicted productivity gains for patients treated with lovo-cel and thus increased the societal incremental cost-effectiveness ratio by nearly 30%.
4 Discussion
The emergence of one-time gene therapies heralds a new treatment paradigm for SCD with potentially meaningful benefits for patients, their families, the healthcare system, and broader society. This analysis of the cost-effectiveness of lovo-cel gene therapy compared with common care for patients in the US with SCD aged ≥ 12 years with recurrent VOEs is the first economic evaluation to leverage gene therapy clinical trial data with follow-up of up to 61 months after transplantation. Our base-case results estimate the potential economic value of lovo-cel from co-base-case third-party payer and societal perspectives, with predicted incremental cost-effectiveness ratios of $191,519 and $124,051 per QALY gained, respectively. This meaningful difference in the incremental cost-effectiveness ratio between the third-party payer and societal perspectives (falling on either side of the base-case WTP threshold of $150,000 per QALY gained) illustrates the contribution of positive spillover impacts, such as patient productivity gains and reduced caregiver burden, to the potential economic value of lovo-cel. Beyond the choice of perspective and WTP threshold, which can vary across stakeholders within the US, scenario analyses indicated that the predicted cost-effectiveness of lovo-cel was most sensitive to baseline age and VOE frequency. In particular, our predicted incremental cost-effectiveness ratios varied by 40% or greater for the youngest and oldest age subgroups and increased by roughly 50% for an alternative population with a lower historical burden of VOEs. Scenario analyses also illustrated the sensitivity of our cost-effectiveness results to the proportion of patients achieving and maintaining VOE-CR. Our findings also represent a comprehensive estimation of the broad potential clinical impact of lovo-cel that can facilitate open dialogue and shared decision-making among patients, clinicians, and families considering lovo-cel as a treatment option for SCD in the US.
The approval of gene and other advanced therapies with high one-time acquisition costs and uncertain long-term benefits has increased interest in alternative payment models and performance-based managed entry agreements [75,76,77,78]. Whether focused at the individual patient or population-based level [76], these agreements are particularly relevant for SCD because the heterogeneity of the population eligible for treatment and the complexity of the disease make defining a simple, uniform, patient-level measure of treatment success impractical. As suggested by the impact on cost-effectiveness results of a rebate on the lovo-cel list price, population-level performance-based agreements negotiated between the lovo-cel manufacturer and US payers have the potential to further mitigate uncertainty about the long-term cost-effectiveness of lovo-cel.
This study contributes to the growing literature on the cost-effectiveness of advanced treatments for SCD, offering synergies and advantages relative to other recent work [79]. The breadth of SCD-related events and complications included in our analysis is comparable to other comprehensive analyses of gene therapies [22,23,24,25, 29], and there is general alignment on the importance of considering a societal perspective at least as a co-base case [23,24,25, 29]. The two recent economic evaluations of gene therapy for SCD most relevant for comparison with our analysis are the evaluation by the Institute for Clinical and Economic Review (ICER) [24] and the Model for Economic Analysis of Sickle Cell Cure (MEASURE), developed as part of the Cure Sickle Cell Initiative [23, 29]. The Hutchinson Institute Sickle Cell Disease Outcomes Research and Economics (HISCORE) model also developed as part of the Cure Sickle Cell Initiative considered a less severe SCD population and a narrower set of events and complications [22, 29].
Our analysis, the MEASURE analysis [29], and the ICER evaluation [24] all compared one-time gene therapy with common care (or the standard of care) in patients with SCD with recurrent VOEs, and all considered a comparably broad set of SCD-related events and complications. The MEASURE analysis used a patient-level simulation approach similar to ours, whereas the ICER evaluation used a Markov-based approach. However, the MEASURE analysis used event and complication risks from interconnected prediction models estimated simultaneously from a US claims database, while our analysis and the ICER evaluation relied primarily on risks from the published literature. All three analyses used VOE reduction as the primary lovo-cel efficacy endpoint, but each differed in how the VOE effect was extended to other events, complications, and mortality. Of note, our analysis and the ICER evaluation used a combination of literature-informed relationships and assumptions to reduce the risks of non-VOE events, complications, and mortality for those with VOE-CR to levels intended to approach general population risks, although the magnitude of the reductions differed between the two approaches. The ICER analysis did not consider partial reductions in VOEs for those without VOE-CR. In contrast, the MEASURE analysis assumed the risk of chronic complications was eliminated beyond 5 years after treatment, did not consider any reduction in non-VOE events other than those estimated indirectly from their statistical prediction models, and applied a curative mortality hazard ratio from patients with SCD treated with allogeneic HSCT. Although each took a societal perspective as at least a co-base case, there were differences in the specific outcomes considered (unpaid caregiving and productivity impacts in all three analyses, caregiver quality of life in our analysis and the MEASURE analysis only, and consumption costs in the MEASURE analysis only) and in the data sources used to inform the approaches.
Despite the similarities in the objectives of these three analyses, the structural differences in how common care outcomes were estimated and valued, how the lovo-cel treatment effect was implemented, and the specific outcomes included resulted in expected differences in model-predicted outcomes (see Table S17 in the Supplementary Information for a comparison). The survival predictions for common care from our analysis and the MEASURE analysis were quite similar (13.5 versus 13.4 years), but both were meaningfully shorter than the common care survival predictions for the ICER evaluation. The survival gains for gene therapy were larger in our analysis (23.8 years) than in the MEASURE analysis (17.4 years) or ICER evaluation (undiscounted outcomes not reported), likely stemming from our use of general population-anchored mortality risk reductions. The gains in patient QALYs for gene therapy versus common care mirrored the differences in survival predictions, although the fundamentally different approaches to quantifying caregiver QALYs (QALYs lost versus QALYs accrued) resulted in overall comparable total QALY gains in our analysis (11.39) and the MEASURE analysis (11.9). Our analysis predicted higher direct medical cost offsets, in part because our lifetime cost prediction for common care (~$2.1 million) was higher than the predictions from the MEASURE (~$1.2 million) and ICER (~$1.5 million) models. The higher prevalence of baseline chronic complications in the MEASURE model also may have limited opportunities for direct medical cost offsets, as gene therapy was not predicted in any model to reverse existing complications. Despite the MEASURE model including additional consumption costs during extended periods of survival associated with lovo-cel, they estimated much higher productivity gains than our model or the ICER evaluation, resulting in net productivity (productivity minus consumption) estimates that were larger than the productivity gains estimated in our model and the ICER evaluation (which both relied on the same educational pathways research to estimate productivity gains). Ultimately, the predictions from each of these models should be further validated against real-world patient outcomes as additional long-term data emerge on patients treated with lovo-cel and other gene therapies for SCD.
The results of this study are subject to the limitations typical of all economic evaluations. Our target population was aligned with the efficacy population in the HGB-206 clinical trial; however, the population treated with lovo-cel in real-world settings may differ from the trial population in terms of baseline age, SCD severity, and comorbidity profile. Additionally, while HGB-206 data up to 61 months after transplantation show a sustained efficacy response [17], longer-term data from ongoing clinical research (including the LTF-307 study) and real-world data are needed to confirm the lifetime impact of lovo-cel. As noted previously, our scenario analysis results illustrate the sensitivity of our cost-effectiveness estimates to uncertainty in both achieving and maintaining VOE-CR. Our analysis relied on simplifying assumptions related to treatment status over time in the common care arm, including the assumption that HU or chronic transfusion use remains constant and the exclusion of common care-specific adverse events (e.g., iron overload). These assumptions likely have counteractive impacts on the predicted cost-effectiveness of lovo-cel compared with common care, and thus we find them unlikely to meaningfully impact our findings.
This study also has several notable strengths. The HGB-206 trial data represent the longest available follow-up of a gene therapy for SCD. Our patient-level simulation methodology, which accounts for the heterogeneity of the SCD population and the interconnectedness of SCD events and complications, was developed collaboratively over several years through a deliberative process relying on the SCD literature, clinician expertise, and patient perspectives. Although assumptions remained necessary to extrapolate the lovo-cel treatment effect to long-term outcomes, our assumptions were tempered to reasonably reflect heterogeneity in patients’ age and comorbid status at the time of treatment. Additionally, we varied key lovo-cel treatment effect assumptions in scenario analyses to illustrate the impact of uncertainty on the predicted clinical and economic value of lovo-cel. Our scenario analyses also explored the impact of uncertainty stemming from gaps in the literature related to SCD caregiver burden and the broader societal costs of SCD. Given the difference between the predicted third-party payer and societal perspective results in our analysis, addressing these data gaps also will be important in estimating the true long-term societal impact of lovo-cel and other gene therapies for SCD.
Although cost-effectiveness analysis is an important tool for value-based decision-making, the traditional framework employed by health technology assessment bodies in the US and globally often fails to account for novel value elements (e.g., value of hope, equity) [39] that may be particularly relevant for one-time therapies with curative intent [20, 21]. Accurately assessing the societal value of new therapies is particularly important for SCD, where the potentially transformative clinical benefit of advanced therapies such as lovo-cel may result in far-reaching effects for a patient population and community that remain marginalized [26, 80]. Stakeholders and decision-makers are encouraged to consider the broad potential societal benefits of these novel therapies when making value-based decisions related to access and reimbursement.
In summary, our findings suggest that lovo-cel gene therapy has the potential to meaningfully improve the lifetime health and economic trajectories of patients with SCD with recurrent VOEs. While data from the ongoing lovo-cel clinical trial program and from real-world studies are required to verify long-term outcomes, our results provide insights for decision-makers and other stakeholders in the US regarding the potential patient, healthcare system, and societal value of lovo-cel as a gene therapy for SCD.
References
National Heart, Lung, and Blood Institute. Sickle cell disease. 2022. https://www.nhlbi.nih.gov/health/sickle-cell-disease. Accessed 26 Aug 2022.
Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood Rev. 2013;27(6):279–87.
Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease—life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–44.
Lubeck D, Agodoa I, Bhakta N, Danese M, Pappu K, Howard R, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2(11): e1915374.
Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Z, et al. Health-related quality of life in children with sickle cell disease: a report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatr Blood Cancer. 2010;55(3):485–94.
Dampier C, LeBeau P, Rhee S, Lieff S, Kesler K, Ballas S, et al. Health-related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. Am J Hematol. 2011;86(2):203–5.
Jiao B, Johnson KM, Ramsey SD, Bender MA, Devine B, Basu A. Long-term survival with sickle cell disease: a nationwide cohort study of Medicare and Medicaid beneficiaries. Blood Adv. 2023;7(13):3276–83.
Hassell KL. Population estimates of sickle cell disease in the US. Am J Prev Med. 2010;38(4 Suppl):S512–21.
Pecker LH, Lanzkron S. Sickle cell disease. Ann Intern Med. 2021;174(1):ITC1–16.
National Heart, Lung, and Blood Institute. A century of progress: milestones in sickle cell disease research and care. 2010. https://www.nhlbi.nih.gov/files/docs/public/blood/Tagged2NHLBISickleCellTimeline.pdf. Accessed 16 Dec 2021.
Centers for Disease Control and Prevention. Sickle cell disease clinical guidelines. 2021. https://www.cdc.gov/ncbddd/sicklecell/recommendations.html. Accessed 16 Dec 2021.
Kavanagh PL, Fasipe TA, Wun T. Sickle cell disease: a review. JAMA. 2022;328(1):57–68.
Sheth S, Licursi M, Bhatia M. Sickle cell disease: time for a closer look at treatment options? Br J Haematol. 2013;162(4):455–64.
Kanter J, Liem RI, Bernaudin F, Bolanos-Meade J, Fitzhugh CD, Hankins JS, et al. American Society of Hematology 2021 guidelines for sickle cell disease: stem cell transplantation. Blood Adv. 2021;5(18):3668–89.
bluebird bio. Lyfgenia (lovotibeglogene autotemcel) [package insert]. US Food and Drug Administration website. 2023. https://www.fda.gov/media/174610/download?attachment. Accessed 7 Feb 2024.
bluebird bio. bluebird bio Announces FDA Priority Review of the Biologics License Application for lovotibeglogene autotemcel (lovo-cel) for patients with sickle cell disease (SCD) 12 years and older with a history of vaso-occlusive events. 2023. https://www.businesswire.com/news/home/20230621695509/en/. Accessed 30 Aug 2023.
Kanter J, Thompson AA, Kwiatkowski JL, Parikh S, Mapara M, Rifkin-Zenenberg S, et al. 1051 Efficacy, safety, and health-related quality of life (HRQOL) in patients with sickle cell disease (SCD) who have received lovotibeglogene autotemcel (lovo-cel) gene therapy: up to 60 months of follow-up. Presented at the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, San Diego, California. 2023. https://ash.confex.com/ash/2023/webprogram/Paper174229.html. Accessed 7 Feb 2024.
Young CM, Quinn C, Trusheim MR. Durable cell and gene therapy potential patient and financial impact: US projections of product approvals, patients treated, and product revenues. Drug Discov Today. 2022;27(1):17–30.
Senior M. Fresh from the biotech pipeline: fewer approvals, but biologics gain share. Nat Biotechnol. 2023;41(2):174–82.
Garrison LP Jr, Jiao B, Dabbous O. Gene therapy may not be as expensive as people think: challenges in assessing the value of single and short-term therapies. J Manag Care Spec Pharm. 2021;27(5):674–81.
Qiu T, Pochopien M, Liang S, Saal G, Paterak E, Janik J, Toumi M. Gene therapy evidence generation and economic analysis: pragmatic considerations to facilitate fit-for-purpose health technology assessment. Front Public Health. 2022;10: 773629.
Winn A, Basu A, Ramsey SD. A framework for a health economic evaluation model for patients with sickle cell disease to estimate the value of new treatments in the United States of America. Pharmacoecon Open. 2023;7(2):313–20.
Johnson KM, Jiao B, Bender MA, Ramsey SD, Devine B, Basu A. Development of a conceptual model for evaluating new non-curative and curative therapies for sickle cell disease. PLoS One. 2022;17(4): e0267448.
Beaudoin F, Thokala P, Nikitin D, Campbell J, Spackman E, McKenna A, et al. Gene therapies for sickle cell disease: effectiveness and value; draft evidence report. Institute for Clinical and Economic Review. 2023. https://icer.org/wp-content/uploads/2023/04/SCD_FOR-PUBLICATION.pdf. Accessed 2 Aug 2023.
Udeze C, **e Y, Ogunsile FJ, Mujumdar U, Yang H, Chen X, et al. EE173 Economic evaluation of exagamglogene autotemcel (EXA-CEL) gene-edited therapy in patients with sickle cell disease with recurrent vaso-occlusive crises. Value Health. 2023;26(6):S91.
Goshua G, Calhoun C, Ito S, James LP, Luviano A, Krishnamurti L, Pandya A. Distributional cost-effectiveness of equity-enhancing gene therapy in sickle cell disease in the United States. Ann Intern Med. 2023;176(6):779–87.
Salcedo J, Bulovic J, Young CM. Cost-effectiveness of a hypothetical cell or gene therapy cure for sickle cell disease. Sci Rep. 2021;11(1):10838.
DeMartino P, Haag MB, Hersh AR, Caughey AB, Roth JA. A budget impact analysis of gene therapy for sickle cell disease: the Medicaid perspective. JAMA Pediatr. 2021;175(6):617–23.
Basu A, Winn AN, Johnson KM, Jiao B, Devine B, Hankins JS, et al. Gene therapy versus common care for eligible individuals with sickle cell disease in the United States: a cost-effectiveness analysis. Ann Intern Med. 2024;177:155–64.
Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–1. Med Decis Mak. 2012;32(5):667–77.
Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M, Force I-SMGRPT. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—2. Value Health. 2012;15(6):804–11.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, Force I-SMGRPT. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—7. Value Health. 2012;15(6):843–50.
Purser M, Gallagher M, Mladsi D, Weber JM, Andemariam B, Kaye JA, Chawla A. PBI20 evaluation of published models in sickle cell disease against key criteria for an economic model for a potentially curative one-time treatment. Value Health. 2020;23(2):S414.
ClinicalTrials.gov. NCT02140554. 20 April 2020. https://clinicaltrials.gov/ct2/show/NCT02140554. Accessed 1 June 2021.
Kanter J, Walters MC, Krishnamurti L, Mapara MY, Kwiatkowski JL, Rifkin-Zenenberg S, et al. Biologic and clinical efficacy of LentiGlobin for sickle cell disease. N Engl J Med. 2022;386(7):617–28.
Migotsky M, Beestrum M, Badawy SM. Recent advances in sickle-cell disease therapies: a review of voxelotor, crizanlizumab, and l-glutamine. Pharmacy (Basel). 2022;10(5):123.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
Institute for Clinical and Economic Review. 2020–2023 value assessment framework. 2020. https://icer-review.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf. Accessed 29 Oct 2020.
Lakdawalla DN, Doshi JA, Garrison LP Jr, Phelps CE, Basu A, Danzon PM. Defining elements of value in health care-a health economics approach: an ISPOR Special Task Force Report [3]. Value Health. 2018;21(2):131–9.
Shah NR, Bhor M, Latremouille-Viau D, Kumar Sharma V, Puckrein GA, Gagnon-Sanschagrin P, et al. Vaso-occlusive crises and costs of sickle cell disease in patients with commercial, Medicaid, and Medicare insurance—the perspective of private and public payers. J Med Econ. 2020;23(11):1345–55.
McClish DK, Smith WR, Levenson JL, Aisiku IP, Roberts JD, Roseff SD, Bovbjerg VE. Comorbidity, pain, utilization, and psychosocial outcomes in older versus younger sickle cell adults: the PiSCES project. Biomed Res Int. 2017;2017:1–10.
Paramore C, Kong A, Minegishi S, Shi W. Characterizing a population with severe manifestations of sickle cell disease using US real-world evidence. Blood. 2018;132(suppl 1):4811.
Wilson-Frederick SM, Hulihan M, Blaz J, Young BM, Center for Medicare and Medicare Services. Prevalence of sickle cell disease among Medicare fee-for-service beneficiaries, age 18–75 years, in 2016. 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/Downloads/Data-Highlight-15-Sickle-Cell-Disease.pdf. Accessed 16 Aug 2021.
Naik RP, Streiff MB, Haywood C Jr, Nelson JA, Lanzkron S. Venous thromboembolism in adults with sickle cell disease: a serious and under-recognized complication. Am J Med. 2013;126(5):443–9.
Manwani D, Burnett AL, Paulose J, Yen GP, Burton T, Anderson A, et al. Treatment patterns and burden of complications associated with sickle cell disease: a US retrospective claims analysis. EJHaem. 2022;3(4):1135–44.
Dong O, Purser M, Herring W, Mladsi D, Gallagher M, Chawla A, et al. Relationships among sickle cell disease complications and their implications for cost-effectiveness modeling for therapies with curative intent. Poster presented at the ISPOR 2023 Conference; May 7, 2023. Boston, MA. [abstract]. Value Health. 2023;26(6):S115–6.
Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease: rates and risk factors. N Engl J Med. 1991;325(1):11–6.
National Institute for Health and Care Excellence. Clinical guideline 143, sickle cell acute painful episode (appendix F). 2012. https://www.nice.org.uk/guidance/cg143/evidence/appendix-f-full-health-economic-report-pdf-186634334. Accessed 26 Aug 2022.
Maitra P, Caughey M, Robinson L, Desai PC, Jones S, Nouraie M, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica. 2017;102(4):626–36.
Arias E, Xu J. United States life tables, 2019. Natl Vital Stat Rep. 2022;70(19):1–59.
Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine. 2005;84(6):363–76.
Darbari DS, Wang Z, Kwak M, Hildesheim M, Nichols J, Allen D, et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS One. 2013;8(11): e79923.
Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol. 2014;89(5):530–5.
Brunson A, Lei A, Rosenberg AS, White RH, Keegan T, Wun T. Increased incidence of VTE in sickle cell disease patients: risk factors, recurrence and impact on mortality. Br J Haematol. 2017;178(2):319–26.
Chaturvedi S, Ghafuri DL, Jordan N, Kassim A, Rodeghier M, DeBaun MR. Clustering of end-organ disease and earlier mortality in adults with sickle cell disease: a retrospective-prospective cohort study. Am J Hematol. 2018;93(9):1153–60.
Walters MC, Tisdale JF, Mapara MY, Krishnamurti L, Kwiatkowski JL, Aygun B, et al. Sustained improvements in patient-reported quality of life up to 24 months post-treatment with LentiGlobin for sickle cell disease (bb1111) gene therapy. Blood. 2021;138(1):7.
Kansal AR, Reifsnider OS, Brand SB, Hawkins N, Coughlan A, Li S, et al. Economic evaluation of betibeglogene autotemcel (Beti-cel) gene addition therapy in transfusion-dependent beta-thalassemia. J Mark Access Health Policy. 2021;9(1):1922028.
Matza LS, Paramore LC, Stewart KD, Karn H, Jobanputra M, Dietz AC. Health state utilities associated with treatment for transfusion-dependent beta-thalassemia. Eur J Health Econ. 2020;21(3):397–407.
Red Book Online. IBM Micromedex [database online]. Truven Health Analytics/IBM Watson Health 2024. https://www.micromedexsolutions.com/home/dispatch/CS/63420F/PFActionId/pf.HomePage/ssl/true. Accessed 9 Feb 2024.
Dolan R. Understanding the Medicaid prescription drug rebate program. Kaiser Family Foundation (KFF); 2019. https://www.kff.org/medicaid/issue-brief/understanding-the-medicaid-prescription-drug-rebate-program/. Accessed 7 Feb 2024.
Campbell A, Cong Z, Agodoa I, Song X, Martinez DJ, Black D, et al. The economic burden of end-organ damage among Medicaid patients with sickle cell disease in the United States: a population-based longitudinal claims study. J Manag Care Spec Pharm. 2020;26(9):1121–9.
Gallagher ME, Chawla A, Brady BL, Badawy SM. Heterogeneity of the long-term economic burden of severe sickle cell disease: a 5-year longitudinal analysis. J Med Econ. 2022;25(1):1140–8.
Graf M, Tuly R, Gallagher M, Sullivan J, Jena AB. Value of a cure for sickle cell disease in reducing economic disparities. Am J Hematol. 2022;97(8):E289–91.
Jiao B, Basu A, Ramsey S, Roth J, Bender MA, Quach D, Devine B. Health state utilities for sickle cell disease: a catalog prepared from a systematic review. Value Health. 2022;25(2):276–87.
Barcelos G, Besser M, O’Flaherty ED, O’Sullivan SB, Bourke S, Oluboyede Y, et al. PCR116 quality of life of and economic burden on caregivers of individuals with sickle cell disease in the UK and France: a cross-sectional survey. Value Health. 2022;25(12):S413.
Basu A, Carlson J, Veenstra D. Health years in total: a new health objective function for cost-effectiveness analysis. Value Health. 2020;23(1):96–103.
Campbell JD, Whittington MD, Pearson SD. An alternative measure of health for value assessment: the equal value life-year. Pharmacoeconomics. 2023;41(10):1175–82.
York Health Economics Consortium. Net Monetary Benefit. 2024. https://yhec.co.uk/glossary/net-monetary-benefit/. Accessed 16 Feb 2024.
Lakdawalla DN, Phelps CE. Health technology assessment with diminishing returns to health: the generalized risk-adjusted cost-effectiveness (GRACE) approach. Value Health. 2021;24(2):244–9.
Towse A, Fenwick E. Uncertainty and cures: discontinuation, irreversibility, and outcomes-based payments: what is different about a one-off treatment? Value Health. 2019;22(6):677–83.
Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, Force I-SMGRPT. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—6. Value Health. 2012;15(6):835–42.
Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Moller J, Force I-SMGRPT. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—4. Value Health. 2012;15(6):821–7.
Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61.
Garrison LP, Pezalla E, Towse A, Yang H, Faust E, Wu EQ, et al. Hemophilia gene therapy value assessment: methodological challenges and recommendations. Value Health. 2021;24(11):1628–33.
Wenzl M, Chapman S. Performance-based managed entry agreements for new medicines in OECD countries and EU member states. 2019. https://www.oecd-ilibrary.org/content/paper/6e5e4c0f-en. Accessed 8 Feb 2024.
Wong CH, Li D, Wang N, Gruber J, Conti RM, Lo AW. Estimating the financial impact of gene therapy in the US. 2021. http://www.nber.org/papers/w28628. Accessed Apr 2021.
Besley S, Henderson N, Towse A, Cole A. Health technology assessment of gene therapies: are our methods fit for purpose? OHE Consulting Report, London: Office of Health Economics. 2022. www.ohe.org/publications/health-technology-assessment-gene-therapies-are-our-methods-fit-purpose. Accessed 9 Feb 2024.
Jiao B, Basu A, Roth J, Bender M, Rovira I, Clemons T, et al. The use of cost-effectiveness analysis in sickle cell disease: a critical review of the literature. Pharmacoeconomics. 2021;39(11):1225–41.
Linthicum M, Jalowsky M, Valentine A, Ahmed R, Bright J, Chapman R. Finding equity in value racial and health equity implications of U.S. HTA processes 2 November 2022. https://sickcells.org/wp-content/uploads/2022/10/IVI_Sick-Cells_Equity-in-Value_2022.pdf. Accessed 31 Aug 2023.
Acknowledgments
We extend our deepest gratitude to the investigators, patients, and families who participated in or otherwise contributed to the HGB-206 study, thereby making this analysis possible. This study was conducted by RTI Health Solutions in collaboration with bluebird bio, clinical advisors, and a patient representative. Editorial and graphical design assistance was provided by John Forbes, Jonathan Pattishall, Jason Mathes, and Denise Lingenfelser of RTI Health Solutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by bluebird bio.
Conflict of interest
William L. Herring, Deirdre Mladsi, and Olivia M. Dong are full-time employees of RTI Health Solutions, a nonprofit research organization that received funding for the conduct of this study pursuant to a contract with bluebird bio. Their compensation is unconnected to the studies on which they work. Meghan E. Gallagher, Anjulika Chawla, Jennifer W. Leiding, and Clark Paramore are employees and shareholders of bluebird bio. Nirmish Shah reports research funding from Pfizer and AkiraBio and honorarium or consultancy fees from Novo Nordisk, Pfizer, Vertex, Emmaus, and Alexion. KC Morse reports consultancy fees from bluebird bio. Lixin Zhang is a statistician consultant at bluebird bio, business owner of Solution-Stat Inc., and contractor at Cytel and GlobalTeq Consulting Inc. Biree Andemariam reports research funding from American Society of Hematology, Connecticut Department of Public Health, Hemanext, Health Resources and Services Administration, Imara, Novartis, Novo Nordisk, Patient-Centered Outcomes Research Institute, and Pfizer and honorarium or consultancy fees from Afimmune, Agios, bluebird bio, CVS/Accordant, Emmaus, Forma Therapeutics, GlaxoSmithKline, Global Blood Therapeutics, Hemanext, Novartis, Novo Nordisk, Protagonist Therapeutics, and Vertex.
Availability of data and material
Appropriately deidentified patient-level data sets and supporting documents from the HGB-206 clinical trial (NCT02140554) may be shared following attainment of applicable marketing approvals and consistent with criteria established by bluebird bio and/or industry best practices to maintain the privacy of study participants. For more information, please contact datasharing@bluebirdbio.com. All other data relevant to the study were obtained from the published literature or other publicly available sources and have been presented in the article and the Supplementary Information.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Authors’ contributions
William L. Herring, Deirdre Mladsi, Meghan E. Gallagher, Anjulika Chawla, Biree Andemariam, Clark Paramore, Nirmish Shah, and KC Morse contributed to the conception and design of the study. William L. Herring contributed to programming the model. Olivia M. Dong, William L. Herring, and Meghan E. Gallagher contributed to drafting the manuscript. All authors contributed to the acquisition of data or analysis and interpretation of data, revising the manuscript, and final approval of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Herring, W.L., Gallagher, M.E., Shah, N. et al. Cost-Effectiveness of Lovotibeglogene Autotemcel (Lovo-Cel) Gene Therapy for Patients with Sickle Cell Disease and Recurrent Vaso-Occlusive Events in the United States. PharmacoEconomics 42, 693–714 (2024). https://doi.org/10.1007/s40273-024-01385-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-024-01385-9