Abstract
Objective
This study aimed to systematically review cost-effectiveness studies of newer antidiabetic medications.
Methods
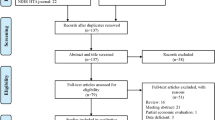
The PubMed/MEDLINE, EMBASE, CINAHL Plus, Cochrane Library–NHS Economic Evaluation Database (Wiley), Cochrane Library–Health Technology Assessment Database (Wiley), Cochrane Library–Database of Abstracts of Reviews of Effects (Wiley), and the Cost-Effectiveness Analysis Registry databases (from 1 January 2000 to 1 June 2018) were searched. The search strategies included the Medical Subject Heading (MeSH) term ‘economics’, and the MeSH entry terms ‘cost’, ‘cost effectiveness’, ‘value’, and ‘cost utility’, as well as all names for GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT2 inhibitors. Inclusion criteria included (1) cost-effectiveness studies of the newer antidiabetic medications, including sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and dipeptidyl peptidase-4 (DPP-4) inhibitors; and (2) full-text publications in English. Two reviewers independently screened the titles, abstracts, and full-text articles to select studies for data extraction. Discrepancies were resolved by discussion and consensus. The quality of reporting cost-effectiveness analyses was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guideline.
Results
Among 85 studies selected, 82 clearly stated the types of diabetes model used (e.g. CORE model), and 70 studied used validated diabetes models. Seventy-four (87%) studies were funded by pharmaceutical companies, and 72 (85%) studies were conducted from a payer’s perspective. Seventy-six (89%) studies presented were of good quality (20–24 CHEERS items), and nine were of moderate quality (14–19 items). Thirty studies compared newer antidiabetic medications with insulin, 3 studies compared newer antidiabetic medications with thiazolidinediones (TZDs), 15 studies compared newer antidiabetic medications with sulfonylureas, 40 studies compared new antidiabetic medications with alternative newer antidiabetic medication, and 9 studies compared other antidiabetic agents that were not included above. Newer antidiabetic medications were reported to be cost-effective in 26 of 30 (87%) studies compared with insulin, and 13 of 15 (87%) studies compared with sulfonylureas.
Conclusions
Most economic evaluations of antidiabetic medications have good reporting quality and use validated diabetes models. The newer antidiabetic medications in most of the reviewed studies were found to be cost effective, compared with insulin, TZDs, and sulfonylureas.

Similar content being viewed by others
Change history
04 September 2019
CORE diabetes model was used throughout the article for consistency.
References
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nature Rev Dis Prim. 2015;1:15019.
Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6–12.
Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–30.
World Health Organization. Global report on diabetes: World Health Organization; 2016.
Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928.
GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260–344.
Tuomilehto J, Schwarz PEH. Preventing Diabetes: Early Versus Late Preventive Interventions. Diabetes Care. 2016;39 Suppl 2):S115.
Gourgari E, Wilhelm EE, Hassanzadeh H, Aroda VR, Shoulson I. A comprehensive review of the FDA-approved labels of diabetes drugs: Indications, safety, and emerging cardiovascular safety data. J Diabetes Compl. 2017;31(12):1719–27.
van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, Ijzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41(8):1543.
Zhuo X, Zhang P, Kahn HS, Bardenheier BH, Li R, Gregg EW. Change in medical spending attributable to diabetes: national data from 1987 to 2011. Diabetes Care. 2015;38(4):581.
Smith-Spangler CM, Bhattacharya J, Goldhaber-Fiebert JD. Diabetes, its treatment, and catastrophic medical spending in 35 develo** countries. Diabetes Care. 2012;35(2):319–26.
Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied methods of cost-effectiveness analysis in healthcare. Oxford: Oxford University Press; 2011.
Shao H, Fonseca V, Stoecker C, Liu S, Shi L. Novel Risk Engine for Diabetes Progression and Mortality in USA: building, Relating, Assessing, and Validating Outcomes (BRAVO). Pharmacoeconomics. 2018;36(9):1125–34.
Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–26.
Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–59.
Geng J, Yu H, Mao Y, Zhang P, Chen Y. Cost effectiveness of dipeptidyl peptidase-4 inhibitors for type 2 diabetes. Pharmacoeconomics. 2015;33(6):581–97.
Permsuwan U, Dilokthornsakul P, Saokaew S, Thavorn K, Chaiyakunapruk N. Cost-effectiveness of dipeptidyl peptidase-4 inhibitor monotherapy in elderly type 2 diabetes patients in Thailand. ClinicoEcon Outcomes Res. 2016;8:521–9.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Effect Resour Alloc. 2013;11(1):6.
Ray JA, Boye KS, Yurgin N, Valentine WJ, Roze S, McKendrick J, et al. Exenatide versus insulin glargine in patients with type 2 diabetes in the UK: a model of long-term clinical and cost outcomes. Curr Med Res Opin. 2007;23(3):609–22.
Shaya FT, Sohn K, Lee S, Bleu-Laine R, Lim J, Casciano M. Clinical and economic evaluation of exenatide for formulary decisions. J Med Econ. 2007;10(4):529–37.
Minshall ME, Oglesby AK, Wintle ME, Valentine WJ, Roze S, Palmer AJ. Estimating the long-term cost-effectiveness of exenatide in the United States: an adjunctive treatment for type 2 diabetes mellitus. Value Health. 2008;11(1):22–33.
Brändle M, Erny-Albrecht KM, Goodall G, Spinas GA, Streit P, Valentine WJ. Exenatide versus insulin glargine: a cost-effectiveness evaluation in patients with Type 2 diabetes in Switzerland. Int J Clin Pharmacol Ther. 2009;47(8):501–15.
Mittendorf T, Smith-Palmer J, Timlin L, Happich M, Goodall G. Evaluation of exenatide vs. insulin glargine in type 2 diabetes: Cost-effectiveness analysis in the German setting. Diabetes Obes Metab. 2009;11(11):1068–79.
Sullivan SD, Alfonso-Cristancho R, Conner C, Hammer M, Blonde L. Long-term outcomes in patients with type 2 diabetes receiving glimepiride combined with liraglutide or rosiglitazone. Cardiovasc Diabetol. 2009;8:12.
Lee WC, Conner C, Hammer M. Results of a model analysis of the cost-effectiveness of liraglutide versus exenatide added to metformin, glimepiride, or both for the treatment of type 2 diabetes in the United States. Clin Ther. 2010;32(10):1756–67.
Beaudet A, Palmer JL, Timlin L, Wilson B, Bruhn D, Boye KS, et al. Cost-utility of exenatide once weekly compared with insulin glargine in patients with type 2 diabetes in the UK. J Med Econ. 2011;14(3):357–66.
Goodall G, Costi M, Timlin L, Reviriego J, Sacristán JA, Smith-Palmer J, et al. Cost-effectiveness of exenatide versus insulin glargine in Spanish patients with obesity and type 2 diabetes mellitus. Endocrinologia y Nutricion. 2011;58(7):331–40.
Lee WC, Conner C, Hammer M. Cost-effectiveness of liraglutide versus rosiglitazone, both in combination with glimepiride in treatment of type 2 diabetes in the US. Curr Med Res Opin. 2011;27(5):897–906.
Valentine WJ, Palmer AJ, Lammert M, Langer J, Brändle M. Evaluating the long-term cost-effectiveness of liraglutide versus exenatide BID in patients with type 2 diabetes who fail to improve with oral antidiabetic agents. Clin Ther. 2011;33(11):1698–712.
Davies MJ, Chubb BD, Smith IC, Valentine WJ. Cost-utility analysis of liraglutide compared with sulphonylurea or sitagliptin, all as add-on to metformin monotherapy in Type 2 diabetes mellitus. Diabet Med. 2012;29(3):313–20.
Lee WC, Samyshkin Y, Langer J, Palmer JL. Long-term clinical and economic outcomes associated with liraglutide versus sitagliptin therapy when added to metformin in the treatment of type 2 diabetes: a CORE diabetes model analysis. J Med Econ. 2012;15(Suppl. 2):28–37.
Samyshkin Y, Guillermin AL, Best JH, Brunell SC, Lloyd A. Long-term cost-utility analysis of exenatide once weekly versus insulin glargine for the treatment of type 2 diabetes patients in the US. J Med Econ. 2012;15(Suppl. 2):6–13.
Fonseca T, Clegg J, Caputo G, Norrbacka K, Dilla T, Alvarez M. The cost-effectiveness of exenatide once weekly compared with exenatide twice daily and insulin glargine for the treatment of patients with type two diabetes and body mass index ≥ 30 kg/m2 in Spain. J Med Econ. 2013;16(7):926–38.
Raya PM, Pérez A, De Arellano AR, Briones T, Hunt B, Valentine WJ. Incretin therapy for type 2 diabetes in Spain: a cost-effectiveness analysis of liraglutide versus sitagliptin. Diabetes Ther. 2013;4(2):417–30.
Brown ST, Grima DG, Sauriol L. Cost-effectiveness of insulin glargine versus sitagliptin in insulin-naïve patients with type 2 diabetes mellitus. Clin Ther. 2014;36(11):1576–87.
Huetson P, Palmer JL, Levorsen A, Fournier M, Germe M, McLeod E. Cost-effectiveness of once daily GLP-1 receptor agonist lixisenatide compared to bolus insulin both in combination with basal insulin for the treatment of patients with type 2 diabetes in Norway. J Med Econ. 2015;18(8):573–85.
Pérez A, Mezquita Raya P, Ramírez de Arellano A, Briones T, Hunt B, Valentine WJ. Cost-effectiveness analysis of incretin therapy for type 2 diabetes in spain: 1.8 mg liraglutide versus sitagliptin. Diabetes Ther. 2015;6(1):61–74.
Dilla T, Alexiou D, Chatzitheofilou I, Ayyub R, Lowin J, Norrbacka K. The cost-effectiveness of dulaglutide versus liraglutide for the treatment of type 2 diabetes mellitus in Spain in patients with BMI ≥ 30 kg/m2. J Med Econ. 2017;20(5):443–52.
Gordon J, McEwan P, Hurst M, Puelles J. The cost-effectiveness of alogliptin versus sulfonylurea as add-on therapy to metformin in patients with uncontrolled type 2 diabetes mellitus. Diabetes Ther. 2016;7(4):825–45.
Roussel R, Martinez L, Vandebrouck T, Douik H, Emiel P, Guery M, et al. Evaluation of the long-Term cost-effectiveness of liraglutide therapy for patients with type 2 diabetes in France. J Med Econ. 2016;19(2):121–34.
Hunt B, Glah D, van der Vliet M. Modeling the long-term cost-effectiveness of ideglira in patients with type 2 diabetes who are failing to meet glycemic targets on basal insulin alone in the Netherlands. Diabetes Ther. 2017;8(4):753–65.
Hunt B, Kragh N, McConnachie CC, Valentine WJ, Rossi MC, Montagnoli R. Long-term cost-effectiveness of two GLP-1 receptor agonists for the treatment of type 2 diabetes mellitus in the italian setting: liraglutide versus lixisenatide. Clin Ther. 2017;39(7):1347–59.
Hunt B, Mocarski M, Valentine WJ, Langer J. Evaluation of the long-term cost-effectiveness of IDegLira versus liraglutide added to basal insulin for patients with type 2 diabetes failing to achieve glycemic control on basal insulin in the USA. J Med Econ. 2017;20(7):663–70.
Hunt B, Mocarski M, Valentine WJ, Langer J. IDegLira versus insulin glargine U100: a long-term cost-effectiveness analysis in the US setting. Diabetes Ther. 2017;8(3):531–44.
Hunt B, Vega-Hernandez G, Valentine WJ, Kragh N. Evaluation of the long-term cost-effectiveness of liraglutide vs lixisenatide for treatment of type 2 diabetes mellitus in the UK setting. Diabetes Obes Metab. 2017;19(6):842–9.
Hunt B, Ye Q, Valentine WJ, Ashley D. Evaluating the long-term cost-effectiveness of daily administered GLP-1 receptor agonists for the treatment of type 2 diabetes in the united kingdom. Diabetes Ther. 2017;8(1):129–47.
Kvapil M, Prázný M, Holik P, Rychna K, Hunt B. Cost-Effectiveness of IDegLira Versus Insulin Intensification Regimens for the Treatment of Adults with Type 2 Diabetes in the Czech Republic. Diabetes Ther. 2017;8(6):1331–47.
Mezquita-Raya P, Ramírez de Arellano A, Kragh N, Vega-Hernandez G, Pöhlmann J, Valentine WJ, et al. Liraglutide versus lixisenatide: long-term cost-effectiveness of GLP-1 receptor agonist therapy for the treatment of type 2 diabetes in Spain. Diabetes Ther. 2017;8(2):401–15.
Psota M, Psenkova MB, Racekova N, De Arellano AR, Vandebrouck T, Hunt B. Cost-effectiveness analysis of IDegLira versus basal-bolus insulin for patients with type 2 diabetes in the Slovak health system. ClinicoEcon Outcomes Res. 2017;9:749–62.
Vega-Hernandez G, Wojcik R, Schlueter M. Cost-Effectiveness of Liraglutide Versus Dapagliflozin for the Treatment of Patients with Type 2 Diabetes Mellitus in the UK. Diabetes Ther. 2017;8(3):513–30.
Basson M, Ntais D, Ayyub R, Wright D, Lowin J, Chartier F, et al. The cost-effectiveness of dulaglutide 1.5 mg versus exenatide QW for the treatment of patients with type 2 diabetes mellitus in France. Diabetes Ther. 2018;9(1):13–25.
Ishii H, Madin-Warburton M, Strizek A, Thornton-Jones L, Suzuki S. The cost-effectiveness of dulaglutide versus insulin glargine for the treatment of type 2 diabetes mellitus in Japan. J Med Econ. 2018;21(5):488–96.
Bruhn D, Martin AA, Tavares R, Hunt B, Pollock RF. Cost-utility of albiglutide versus insulin lispro, insulin glargine, and sitagliptin for the treatment of type 2 diabetes in the US. J Med Econ. 2016;19(7):672–83.
Davies MJ, Glah D, Chubb B, Konidaris G, McEwan P. Cost Effectiveness of IDegLira vs. Alternative basal insulin intensification therapies in patients with type 2 diabetes mellitus uncontrolled on basal insulin in a UK setting. PharmacoEconomics. 2016;34(9):953–66.
Bergenheim K, Williams SA, Bergeson JG, Stern L, Sriprasert M. US cost-effectiveness of saxagliptin in type 2 diabetes mellitus. Am J Pharm Benef. 2012;4(1):20–8.
Erhardt W, Bergenheim K, Duprat-Lomon I, McEwan P. Cost effectiveness of saxagliptin and metformin versus sulfonylurea and metformin in the treatment of type 2 diabetes mellitus in Germany: a cardiff diabetes model analysis. Clin Drug Investig. 2012;32(3):189–202.
Granström O, Bergenheim K, McEwan P, Sennfält K, Henriksson M. Cost-effectiveness of saxagliptin (Onglyza®) in type 2 diabetes in Sweden. Prim Care Diabetes. 2012;6(2):127–36.
Grzeszczak W, Czupryniak L, Kolasa K, Sciborski C, Lomon ID, McEwan P. The cost-effectiveness of saxagliptin versus NPH insulin when used in combination with other oral antidiabetes agents in the treatment of type 2 diabetes mellitus in Poland. Diabetes Technol Ther. 2012;14(1):65–73.
Elgart JF, Caporale JE, Gonzalez L, Aiello E, Waschbusch M, Gagliardino JJ. Treatment of type 2 diabetes with saxagliptin: a pharmacoeconomic evaluation in Argentina. Health Econ Rev. 2013;3(1):1–9.
Van Haalen HGM, Pompen M, Bergenheim K, McEwan P, Townsend R, Roudaut M. Cost effectiveness of adding dapagliflozin to insulin for the treatment of type 2 diabetes mellitus in the Netherlands. Clin Drug Investig. 2014;34(2):135–46.
Charokopou M, McEwan P, Lister S, Callan L, Bergenheim K, Tolley K, et al. Cost-effectiveness of dapagliflozin versus DPP-4 inhibitors as an add-on to metformin in the treatment of type 2 diabetes mellitus from a UK healthcare system perspective. BMC Health Serv Res. 2015;15:496.
Charokopou M, McEwan P, Lister S, Callan L, Bergenheim K, Tolley K, et al. The cost-effectiveness of dapagliflozin versus sulfonylurea as an add-on to metformin in the treatment of Type 2 diabetes mellitus. Diabet Med. 2015;32(7):890–8.
Deng J, Gu S, Shao H, Dong H, Zou D, Shi L. Cost-effectiveness analysis of exenatide twice daily (BID) vs insulin glargine once daily (QD) as add-on therapy in Chinese patients with Type 2 diabetes mellitus inadequately controlled by oral therapies. J Med Econ. 2015;18(11):974–89.
Gu S, Deng J, Shi L, Mu Y, Dong H. Cost-effectiveness of saxagliptin vs glimepiride as a second-line therapy added to metformin in Type 2 diabetes in China. J Med Econ. 2015;18(10):808–20.
Chuang LH, Verheggen BG, Charokopou M, Gibson D, Grandy S, Kartman B. Cost-effectiveness analysis of exenatide once-weekly versus dulaglutide, liraglutide, and lixisenatide for the treatment of type 2 diabetes mellitus: an analysis from the UK NHS perspective. J Med Econ. 2016;19(12):1127–34.
Gordon J, McEwan P, Sabale U, Kartman B, Wolffenbuttel BHR. The cost-effectiveness of exenatide twice daily (BID) vs insulin lispro three times daily (TID) as add-on therapy to titrated insulin glargine in patients with type 2 diabetes. J Med Econ. 2016;19(12):1167–74.
Gu S, Mu Y, Zhai S, Zeng Y, Zhen X, Dong H. Cost-Effectiveness of dapagliflozin versus acarbose as a monotherapy in type 2 diabetes in China. PLoS One. 2016;11(11):e0165629.
Gu S, Zeng Y, Yu D, Hu X, Dong H. Cost-Effectiveness of saxagliptin versus acarbose as second-Line therapy in type 2 diabetes in China. PLoS One. 2016;11(11):e0167190.
Tzanetakos C, Tentolouris N, Kourlaba G, Maniadakis N. Cost-effectiveness of dapagliflozin as add-on to metformin for the treatment of type 2 diabetes mellitus in Greece. Clin Drug Investig. 2016;36(8):649–59.
Zhang X, Liu S, Li Y, Wang Y, Tian M, Liu G. Long-term effectiveness and cost-effectiveness of metformin combined with liraglutide or exenatide for type 2 diabetes mellitus based on the CORE diabetes model study. PLoS One. 2016;11(6):e0156393.
Gu S, Wang X, Qiao Q, Gao W, Wang J, Dong H. Cost-effectiveness of exenatide twice daily vs insulin glargine as add-on therapy to oral antidiabetic agents in patients with type 2 diabetes in China. Diabetes Obes Metab. 2017;19(12):1688–97.
Shao H, Zhai S, Zou D, Mir MU, Zawadzki NK, Shi Q, et al. Cost-effectiveness analysis of dapagliflozin versus glimepiride as monotherapy in a Chinese population with type 2 diabetes mellitus. Curr Med Res Opin. 2017;33(2):359–69.
Tzanetakos C, Bargiota A, Kourlaba G, Gourzoulidis G, Maniadakis N. Cost effectiveness of exenatide once weekly versus insulin glargine and liraglutide for the treatment of type 2 diabetes mellitus in Greece. Clin Drug Investig. 2018;38(1):67–77.
Woehl A, Evans M, Tetlow AP, McEwan P. Evaluation of the cost effectiveness of exenatide versus insulin glargine in patients with sub-optimally controlled Type 2 diabetes in the United Kingdom. Cardiovasc Diabetol. 2008;7:24.
Klarenbach S, Cameron C, Singh S, Ur E. Cost-effectiveness of second-line antihyperglycemic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. CMAJ. 2011;183(16):E1213–20.
Gao L, Zhao FL, Li SC. Cost-utility analysis of liraglutide versus glimepiride as add-on to metformin in type 2 diabetes patients in China. Int J Technol Assess Health Care. 2012;28(4):436–44.
Viriato D, Calado F, Gruenberger JB, Ong SH, Carvalho D, Silva-Nunes J, et al. Cost-effectiveness of metformin plus vildagliptin compared with metformin plus sulphonylurea for the treatment of patients with type 2 diabetes mellitus: a Portuguese healthcare system perspective. J Med Econ. 2014;17(7):499–507.
Kousoulakou H, Hatzikou M, Baroutsou V, Yfantopoulos J. Cost effectiveness of vildagliptin versus glimepiride as add-on treatment to metformin for the treatment of diabetes mellitus type 2 patients in Greece. Cost Effect Resour Alloc. 2017;15(1):19.
Kiadaliri AA, Gerdtham UG, Eliasson B, Carlsson KS. Cost-utility analysis of glucagon-like peptide-1 agonists compared with dipeptidyl peptidase-4 inhibitors or neutral protamine hagedorn basal insulin as add-on to metformin in type 2 diabetes in Sweden. Diabetes Ther. 2014;5(2):591–607.
Steen Carlsson K, Persson U. Cost-effectiveness of add-on treatments to metformin in a Swedish setting: liraglutide vs sulphonylurea or sitagplitin. J Med Econ. 2014;17(9):658–69.
Ericsson Å, Lundqvist A. Cost effectiveness of insulin degludec plus liraglutide (IDegLira) in a fixed combination for uncontrolled type 2 diabetes mellitus in Sweden. Appl Health Econ Health Policy. 2017;15(2):237–48.
Ericsson Å, Glah D, Lorenzi M, Jansen JP, Fridhammar A. Cost-effectiveness of liraglutide versus lixisenatide as add-on therapies to basal insulin in type 2 diabetes. PLoS One. 2018;13(2):e0191953.
Neslusan C, Teschemaker A, Johansen P, Willis M, Valencia-Mendoza A, Puig A. Cost-effectiveness of canagliflozin versus sitagliptin as add-on to metformin in patients with type 2 diabetes mellitus in Mexico. Value Health Reg Issues. 2015;8:8–19.
Sabapathy S, Neslusan C, Yoong K, Teschemaker A, Johansen P, Willis M. Cost-effectiveness of canagliflozin versus sitagliptin when added to metformin and sulfonylurea in type 2 diabetes in Canada. J Popul Ther Clin Pharmacol. 2016;23(2):e151–68.
Neslusan C, Teschemaker A, Willis M, Johansen P, Vo L. Cost-effectiveness analysis of canagliflozin 300 mg versus dapagliflozin 10 mg added to metformin in patients with type 2 diabetes in the United States. Diabetes Ther. 2018;9(2):565–81.
Gaebler JA, Soto-Campos G, Alperin P, Cohen M, Blickensderfer A, Wintle M, et al. Health and economic outcomes for exenatide once weekly, insulin, and pioglitazone therapies in the treatment of type 2 diabetes: a simulation analysis. Vasc Health Risk Manag. 2012;8(1):255–64.
Schwarz B, Gouveia M, Chen J, Nocea G, Jameson K, Cook J, et al. Cost-effectiveness of sitagliptin-based treatment regimens in European patients with type 2 diabetes and haemoglobin A1c above target on metformin monotherapy. Diabetes Obes Metab. 2008;10(Suppl 1):43–55.
Sinha A, Rajan M, Hoerger T, Pogach L. Costs and consequences associated with newer medications for glycemic control in type 2 diabetes. Diabetes Care. 2010;33(4):695–700.
Wang B, Roth JA, Nguyen H, Felber E, Furnback W, Garrison LP. The short-term cost-effectiveness of once-daily liraglutide versus once-weekly exenatide for the treatment of type 2 diabetes mellitus in the United States. PLoS One. 2015;10(4):e0121915.
Cazarim MDS, da Cruz-Cazarim ELC, Baldoni ADO, dos Santos TBE, de Souza PG, Silva IDA, et al. Cost-effectiveness analysis of different dipeptidyl-peptidase 4 inhibitor drugs for treatment of type 2 diabetes mellitus. Diabetes Metab Syndrome Clin Res Rev. 2017;11:S859–65.
Drummond R, Malkin S, Du Preez M, Lee XY, Hunt B. The management of type 2 diabetes with fixed-ratio combination insulin degludec/liraglutide (IDegLira) versus basal-bolus therapy (insulin glargine U100 plus insulin aspart): a short-term cost-effectiveness analysis in the UK setting. Diabetes Obes Metab. 2018;20(10):2371–8.
Langer J, Hunt B, Valentine WJ. Evaluating the short-term cost-effectiveness of liraglutide versus sitagliptin in patients with type 2 diabetes failing metformin monotherapy in the United States. J Manag Care Pharm. 2013;19(3):237–46.
Ektare VU, Lopez JM, Martin SC, Patel DA, Rupnow MF, Botteman MF. Cost efficiency of canagliflozin versus sitagliptin for type 2 diabetes mellitus. Am J Manag Care. 2014;20(10):S204–15.
Hunt B, McConnachie CC, Gamble C, Dang-Tan T. Evaluating the short-term cost-effectiveness of liraglutide versus lixisenatide in patients with type 2 diabetes in the United States. J Med Econ. 2017;20(11):1117–20.
Hunt B, Mocarski M, Valentine WJ, Langer J. Evaluation of the short-term cost-effectiveness of IDegLira versus continued up-titration of insulin glargine U100 in patients with type 2 diabetes in the USA. Adv Ther. 2017;34(4):954–65.
Lasalvia P, Baquero L, Otálora-Esteban M, Castañeda-Cardona C, Rosselli D. Cost-effectiveness of dulaglutide compared with liraglutide and glargine in type 2 diabetes mellitus patients in Colombia. Value Health Reg Issues. 2017;14:35–40.
Chakravarty A, Rastogi M, Dhankhar P, Bell K, Bell KF. Comparison of costs and outcomes of dapagliflozin with other glucose-lowering therapy classes added to metformin using a short-term cost-effectiveness model in the US setting. J Med Econ. 2018;21(5):497–509.
Gourzoulidis G, Tzanetakos C, Ioannidis I, Tsapas A, Kourlaba G, Papageorgiou G, et al. Cost-effectiveness of empagliflozin for the treatment of patients with type 2 diabetes mellitus at increased cardiovascular risk in Greece. Clin Drug Investig. 2018;38(5):417–26.
Nguyen E, Coleman CI, Nair S, Weeda ER. Cost-utility of empagliflozin in patients with type 2 diabetes at high cardiovascular risk. J Diabetes Compl. 2018;32(2):210–5.
Sabale U, Ekman M, Granström O, Bergenheim K, McEwan P. Cost-effectiveness of dapagliflozin (Forxiga®) added to metformin compared with sulfonylurea added to metformin in type 2 diabetes in the Nordic countries. Prim Care Diabetes. 2015;9(1):39–47.
Teramachi H, Ohta H, Tachi T, Toyoshima M, Mizui T, Goto C, et al. Pharmacoeconomic analysis of DPP-4 inhibitors. Pharmazie. 2013;68(11):909–15.
DeKoven M, Lee WC, Bouchard J, Massoudi M, Langer J. Real-world cost-effectiveness: lower cost of treating patients to glycemic goal with liraglutide versus exenatide. Adv Ther. 2014;31(2):202–16.
Thornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract Res Clin Endocrinol Metab. 2009;23(4):479–86.
Kalra S, Baruah MP, Sahay RK, Unnikrishnan AG, Uppal S, Adetunji O. Glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes: past, present, and future. Indian J Endocrinol Metab. 2016;20(2):254.
Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5(2):355–66.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–43.
Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27–40.
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33.
Zhao Y, Campbell CR, Fonseca V, Shi L. Impact of hypoglycemia associated with antihyperglycemic medications on vascular risks in veterans with type 2 diabetes. Diabetes Care. 2012;35(5):1126–32.
Shi L, Shao H, Zhao Y, Thomas NA. Is hypoglycemia fear independently associated with health-related quality of life? Health Qual Life Outcomes. 2014;12:167.
Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559–69.
Palmer AJ, Si L, Tew M, Hua X, Willis MS, Asseburg C, et al. Computer modeling of diabetes and its transparency: a report on the eighth mount hood challenge. Value Health. 2018;21(6):724–31.
Claxton K, Sculpher M, McCabe C, Briggs A, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ. 2005;14(4):339–47.
Acknowledgements
Lizheng Shi and Dongzhe Hong formulated the study question. Dongzhe Hong also drafted the data abstraction form and the project proposal, and developed the search strategies for study selection; all other authors reviewed and revised the search strategies and the study materials. Dongzhe Hong and Minghuan Jiang screened the titles and abstracts, and extracted articles for full-text review. Hui Shao, Yingnan Zhao, Yan Li, and Lizheng Shi performed the critical appraisal of the studies. Dongzhe Hong, Lei Si, Minghuan Jiang, and Wai-kit Ming abstracted data from the selected articles, and Dongzhe Hong conducted the data synthesis. Dongzhe Hong also drafted the Methods and Introduction sections of the manuscript, and Lei Si drafted the Introduction, Discussion, and Conclusions sections. All authors reviewed and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
Lei Si is a recipient of the National Health and Medical Research Council Early Career Fellowship (GNT1139826). Yan Li was partly supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL141427. Dongzhe Hong, Minghuan Jiang, Hui Shao, Wai-kit Ming, Yingnan Zhao, and Lizheng Shi have no conflicts of interest to declare.
Additional information
Dongzhe Hong and Lei Si contribution as the first co-authors.
Minghuan Jiang and Hui Shao as the second co-authors.
Appendices
Appendix 1: Details of the search strategies for the identification of studies
(1) (GLP-1) AND (cost effectiveness OR cost utility OR economics OR value OR cost).
(2) (exenatide OR Byetta OR Bydureon OR liraglutide OR Victoza OR Saxenda OR lixisenatide OR Lyxumia OR albiglutide OR Tanzeum OR dulaglutide OR trulicity OR emaglutide OR Ozempic) AND (cost effectiveness OR cost utility OR economics OR value OR cost).
(3) (DPP-4) AND (cost effectiveness OR cost utility OR economics OR value OR cost).
(4) (Sitagliptin OR Januvia OR Vildagliptin OR Galvus OR Saxagliptin OR Onglyza OR Linagliptin OR Tradjenta OR Gemigliptin OR Zemiglo OR Anagliptin OR Suiny OR Teneligliptin OR Tenelia OR Alogliptin OR Nesina OR Vipidia OR Trelagliptin OR Zafatek OR Omarigliptin OR MK-3102 OR Evogliptin OR Suganon OR Gosogliptin OR SatRx) AND (cost effectiveness OR cost utility OR economics OR value OR cost).
(5) (SGLT2) AND (cost effectiveness OR cost utility OR economics OR value OR cost).
(6) (Dapagliflozin OR Farxiga OR Empagliflozin OR Jardiance OR Canagliflozin OR Invokana OR Ipragliflozin OR ASP-1941 OR Suglat OR Tofogliflozin OR Apleway OR Deberza OR Ertugliflozin OR Steglatro) AND (cost effectiveness OR cost utility OR economics OR value OR cost).
Appendix 2: Summary of sensitivity analyses in the included studies
References | Comparison | Probabilistic sensitivity analysis (Y/N) | Cost-effectiveness acceptability curve (Y/N) | Sensitive factors 1 | Sensitive factors 2 | Sensitive factors 3 | Sensitive factors 4 | Sensitive factors 5 |
|---|---|---|---|---|---|---|---|---|
Ray et al., 2007 [20] | Exenatide vs. insulin | N | Y | Exenatide price | HbA1c change | Insulin dose | Time horizon | |
Shaya et al., 2007 [21] | Exenatide vs. placebo; exenatide vs. no additional treatment | N | N | HbA1c change | Discount rate | |||
Minshall et al., 2008 [22] | Exenatide vs. no additional treatment beyond metformin and/or a sulfonylurea | Y | Y | Time horizon | HbA1c change | BMI change | Lipid change | |
Schwarz et al., 2008 [88] | Sitagliptin vs. rosiglitazone; sitagliptin vs. sulfonylurea | N | N | Lipid change | SBP change | Congestive heart failure risk | Sitagliptin efficacy | SBP change |
Woehl et al., 2008 [75] | Exenatide vs. insulin glargine in three scenarios | N | N | Hypoglycemic events | Coefficient of durability of sitagliptin | |||
Brandle et al., 2009 [23] | Exenatide vs. insulin glargine | Y | Y | Time horizon | Discount rate | Cost of exenatide | Complication costs | |
Mittendorf et al., 2009 [24] | Exenatide vs. insulin glargine | Y | Y | Baseline BMI | Baseline HbA1c | Time horizon | HbA1c effect sustainability | |
Sullivan et al., 2009 [25] | Liraglutide vs. rosiglitazone | N | N | HbA1c change | ||||
Lee et al., 2010 [26] | Liraglutide vs. exenatide | N | N | Discount rate | Time horizon | HbA1c reduction | Disutility for BMI | |
Sinha et al., 2010 [89] | Exenatide vs. sitagliptin; Sitagliptin vs. glyburide | N | N | Disutility for injectable medication | Disutility for injectable medication, and cost of medication | Utility for weight change | Third-line therapy change | |
Beaudet et al., 2011 [27] | Exenatide vs. insulin glargine | Y | Y | Time horizon | Cost of complications | Discount rate | Exenatide price | |
Goodall et al., 2011 [28] | Exenatide vs. insulin glargine | Y | Y | Time horizon | HbA1c effect sustainability | Discount rate | HbA1c reduction | |
Klarenbach et al., 2011 [76] | DPP-4 vs. no additional treatment | N | Y | Clinical effects of HbA1c, weight, hypoglycemia | Treatment intensification | Price of DPP-4 inhibitors | Cost of complications | |
Lee et al., 2011 [29] | liraglutide vs. rosiglitazone | N | N | Discount rate | Time horizon | HbA1c reduction | (Dis)utility for BMI change | |
Valentine et al., 2011 [30] | Liraglutide vs. exenatide | N | Y | Time horizon | Discount rate | HbA1c and minor hypoglycemia benefits only | HbA1c reduction | |
Bergenheim et al., 2012 [56] | Saxagliptin vs. sulfonylurea | Y | N | Cost and disutility for weight gain and hypoglycemic event | Cost and disutility for hypoglycemic event | HbA1c effect sustainability, cost and disutility for hypoglycemic event | HbA1c effect sustainability, cost and disutility for weight gain and hypoglycemic event | |
Davies et al., 2012 [31] | Liraglutide vs. glimepiride; liraglutide vs. sitagliptin | N | Y | Treatment duration | Discount rate | Weight gain | BMI disutility | |
Erhardt et al., 2012 [57] | Saxagliptin vs. sulfonylurea | Y | Y | Model entry on second-line treatment | Weight change | BMI disutility | Discount rate | |
Gaebler et al., 2012 [87] | Exenatide vs. insulin | N | N | Time horizon | ||||
Gao et al., 2012 [77] | Liraglutide vs. glimepiride | N | N | Time horizon | Discount rate | Complication costs | Initial utility score: use UKPDS utilities | |
Granstrōm et al., 2012 [58] | Saxagliptin vs. sulfonylurea; | Y | Y | Hypoglycemia rates | Cost of hypoglycemia | BMI disutility | Sustainability of weight change | |
Grzeszczak et al., 2012 [59] | Saxagliptin vs. insulin | Y | Y | Model entry on second-line treatment | Baseline HbA1c | Weight change | Discount rate | |
Lee et al., 2012 [32] | Liraglutide vs. sitagliptin | Y | Y | Time horizon | ||||
Samyshkin et al., 2012 [33] | Exenatide vs. insulin glargine | Y | N | Time horizon | HbA1c reduction | BMI change | Convenience of less frequent administration of exenatide | |
Elgart et al., 2013 [60] | Saxagliptin vs. sulfonylureas | Y | Y | HbA1c values | age | Cost values | Utility values | |
Fonseca et al., 2013 [34] | Exenatide once weekly vs. exenatide twice daily; exenatide once weekly vs. insulin glargine | Y | Y | Time horizon | 95% upper CI for HbA1c | (Dis)utility for BMI/nausea | ||
Langer et al., 2013 [93] | Liraglutide vs. sitagliptin | Y | N | Discount rate | ||||
Raya et al., 2013 [35] | Liraglutide vs. sitagliptin | N | Y | Time horizon | Discount rates | Over- or underestimating the unit costs of diabetes complications | ||
Teramachi et al., 2013 [102] | Sitagliptin 100 mg vs. sitagliptin 50 mg; vildagliptin vs. sitagliptin; alogliptin vs. sitagliptin | N | N | The standard deviation of HbA1c | Price of each drug | |||
Brown et al., 2014 [36] | Sitagliptin vs. insulin glargine | Y | Y | Changes to the number of testing strips | ||||
DeKoven et al., 2014 [103] | Liraglutide vs. exenatide | N | N | |||||
Ektare et al., 2014 [94] | Canagliflozin vs. sitagliptin | Y | N | Drug costs | Treatment discontinuations | BMI | LDL-c | Discount rates |
Kiadaliri et al., 2014 [80] | GLP-1 vs. DPP-4; GLP-1 vs. NPH insulin; DPP-4 vs. NPH insulin | Y | N | Drug costs | Treatment discontinuations | BMI | LDL-c | HbA1c reduction |
Steen Carlsson et al., 2014 [81] | Liraglutide vs. sulfonylurea; liraglutide vs. sitagliptin | Y | Y | Frequency of hypoglycemia | Baseline BMI | HbA1c at which the second-line treatment was initiated | Cost of glucose monitoring strips | |
Van Haalen et al., 2014 [61] | Dapagliflozin vs. placebo | Y | Y | Uncertainty around the QALY point estimate | Price of test strips | |||
Viriato et al., 2014 [78] | Vildagliptin vs. sulfonylurea | Y | Y | Coefficient of treatment failure | Drug costs | HbA1c | Discount rates | Risk of congestive heart failure |
Charokopou et al., 2015 [62] | Dapagliflozin vs. DPP-4 | Y | Y | HbA1c change from baseline | Weight change from baseline | BMI utility values | Total non-drug costs | Risk of congestive heart failure |
Charokopou et al., 2015 [63] | Dapagliflozin vs. sulfonylurea | Y | Y | Change in HbA1c from baseline | Change in weight from baseline | Disutility for minor hypoglycemia | ||
Deng et al., 2015 [64] | Exenatide vs. insulin glargine | Y | Y | HbA1c level at baseline | Health utilities decrement | BMI at baseline | Disutility for minor hypoglycemia | |
Gu et al., 2015 [65] | Saxagliptin vs. glimepiride | Y | Y | Utility | HbA1c | BMI | Age | Disutility for minor hypoglycemia |
Huetson et al., 2015 [37] | Lixisenatide vs. basal-bolus insulin | Y | N | Discount rates | Reductions in HbA1c | Application of code-2 disutility for excess BMI | Disutility for minor hypoglycemia | |
Neslusan et al., 2015 [84] | Canagliflozin vs. sitagliptin | N | Y | QALY gains for canagliflozin | Time horizon | Disutility for overweight/obesity | Disutility for minor hypoglycemia | |
Pérez et al., 2015 [38] | Liraglutide vs. sitagliptin | N | N | Time horizon | Disutility for minor hypoglycemia | |||
Sabale et al., 2015 [101] | Dapagliflozin vs. sulfonylurea | Y | Y | Assumptions on body weight progression over time | Utility weights associated with this change | |||
Wang et al., 2015 [90] | Liraglutide vs. exenatide | Y | Y | the cost of liraglutide and exenatide | the liraglutide and exenatide AE rates | the cost of treating gastrointestinal AEs | the effectiveness of liraglutide and exenatide in patients who completed the full treatment duration | |
Bruhn et al., 2016 [54] | Albiglutide vs. insulin lispro; albiglutide vs. sitagliptin | Y | Y | Time horizon | Discount rate | Complication costs | Us ethnic breakdown | hypoglycemia disutility |
Chuang et al., 2016 [66] | Exenatide vs. dulaglutide; exenatide vs. liraglutide; exenatide vs. lixisenatide | Y | Y | HbA1c reduction | Weight loss | Treatment discontinuation due to AE | Nausea as an AE | |
Davies et al., 2016 [55] | IDegLira vs. basal insulin + liraglutide; IDegLira vs. insulin glargine + IAsp; IDegLira vs. uptitrated insulin glargine | Y | Y | Alternative time horizon | Alternative discount rate | Alternative treatment switching | Alternative BMI progression | Hypoglycemia disutility |
Gordon et al., 2016 [40] | Metformin + alogliptin vs. metformin + sulfonylurea | Y | Y | Time horizon | Costs | Utility | Discount rate | Age at model entry |
Gordon et al., 2016 [67] | Exenatide vs. lispro | Y | Y | Time horizon | Discount rate | Complication utility | Complication costs | |
Gu et al., 2016 [68] | Dapagliflozin vs. acarbose | Y | Y | Utility | Age | HbA1c | Ethnicity | |
Gu et al., 2016 [69] | Saxagliptin vs. acarbose | Y | Y | Utility | Age | HbA1c | Ethnicity | |
Permsuwan et al., 2016 [17] | Saxagliptin vs. metformin; sitagliptin vs. metformin; vildagliptin vs. metformin; saxagliptin vs. sulfonylurea; sitagliptin vs. sulfonylurea; vildagliptin vssulfonylurea | Y | Y | HbA1c change of DPP-4 inhibitors | Discount rate | Risk of severe hypoglycemia of DPP-4 inhibitors | Cost of sexagliptin | HbA1c reduction |
Roussel et al., 2016 [41] | Liraglutide vs. sitagliptin; liraglutide vs. glimepiride | Y | Y | Time horizon | Discount rate | Alternative costs of complications | HbA1c difference | Rule of treatment intensification |
Sabapathy et al., 2016 [85] | Canagliflozin vs. sitagliptin | Y | Y | Discount rate | Time horizon | eGFR drift | Alternative treatment thresholds | Insulin dose |
Tzanetakos et al., 2016 [70] | Dapagliflozin vs. sulfonylurea; dapagliflozin vs. DPP-4 | Y | Y | the assumptions around the HbA1c switching threshold | the utility weights applied to BMI progression | utilities t2 dm complications | Discount rate | Insulin dose |
Zhang et al., 2016 [71] | Liraglutide vs. exenatide | Y | Y | Discount rates | Simulated treatment period | |||
Cazarim et al., 2017 [91] | Linagliptin vs. none; linagliptin + metformin vs. none; sitagliptin vs. none; sitagliptin + metformin vs. none; vildagliptin vs. none; vildagliptin + metformin vs. none; saxagliptin vs. none | N | N | NA | ||||
Dilla et al., 2017 [39] | dulaglutide vs. liraglutide | Y | Y | Treatment effects | Treatment duration | Economics | Disutility exclusions | Discount rate |
Ericsson et al., 2017 [82] | IDegLira vs. insulin glargine; IDegLira vs. NPH insulin; IDegLira vs. insulin aspart + insulin glargine; IDegLira vs. insulin aspart + NPH insulin; IDegLira vs. liraglutide + insulin glargine; IDegLira vs. liraglutide + NPH insulin | Y | Y | Absolute treatment effect on the HbA1c level | Annual absolute drift of the HbA1c level, HbA1c | Initial absolute treatment effects on other biomarker levels | Absolute drift of the other biomarker levels | |
Gu et al., 2017 [72] | Exenatide vs. insulin glargine | Y | N | HbA1c threshold value for therapy | BMI-associated disutility | BMI-related prescription costs | Incidence, costs and disutility of the AEs | |
Hunt et al., 2017 [42] | Exenatide vs. insulin glargine | Y | Y | Time horizon | Upper and lower limits of HbA1c change | HbA1c progression | BMI progression | |
Hunt et al., 2017 [43] | Liraglutide vs. lixisenatide | Y | Y | Time horizon | Discount rates | HbA1c difference | Blood pressure difference | Lipid difference |
Hunt et al., 2017 [95] | Liraglutide vs. lixisenatide | N | N | NA | ||||
Hunt et al., 2017 [44] | IDegLira vs. liraglutide | Y | N | No influential parameters identified could influence base-case results | ||||
Hunt et al., 2017 [45] | IDegLira vs. insulin glargine | Y | N | Time horizon | HbA1c difference abolished | Cost of neutral protamine Hagedorn | ||
Hunt et al., 2017 [96] | IDegLira vs. insulin glargine | N | N | NA | ||||
Hunt et al., 2017 [46] | Liraglutide vs. lixisenatide | Y | N | Time horizon | HbA1c difference abolished | |||
Hunt et al., 2017 [47] | Liraglutide vs. exenatide; liraglutide vs. lixisenatide | N | N | No influential parameters identified could influence the base-case results | ||||
Kousoulakou et al., 2017 [79] | Vildagliptin vs. glimepiride | N | N | No influential parameters identified could influence the base-case results | ||||
Kvapil et al., 2017 [48] | IDegLira vs. basal insulin | N | N | No influential parameters identified could influence the base-case results | ||||
Lasalvia et al., 2017 [97] | Dulaglutide vs. liraglutide; dulaglutide vs. glargine | Y | N | Non-daily injection utility | Monthly dulaglutide cost | Percentage of patients achieving < 7% HbA1c with glargine | Glucometries per month | Blood pressure |
Mezquita-Raya et al., 2017 [49] | Liraglutide vs. lixisenatide | Y | Y | HbA1c difference abolished | Blood pressure | |||
Psota et al., 2017 [50] | IDegLira vs. basal bolus | Y | Y | No influential parameters identified could influence the base-case results | Blood pressure | |||
Shao et al., 2017 [73] | Dapagliflozin vs. glimepiride | Y | Y | No influential parameters identified could influence the base-case results | ||||
Vega-Hernandez et al., 2017 [51] | Liraglutide (dual therapy) vs. dapagliflozin (dual therapy); liraglutide (triple therapy) vs. dapagliflozin (triple therapy) | Y | Y | Treatment switch year | Time horizon | |||
Basson et al., 2018 [52] | Dulaglutide vs. exenatide | Y | Y | No influential parameters identified could influence the base-case results | ||||
Chakravarty et al., 2018 [98] | Dapagliflozin vs. DPP-4; dapagliflozin vs. GLP-1 | Y | Y | No influential parameters identified could influence the base-case results | ||||
Drummond et al., 2018 [92] | IDegLira vs. basal-bolus | N | N | NA | ||||
Ericsson et al., 2018 [83] | Liraglutide vs. lixisenatide | Y | Y | Between-treatment difference in HbA1c abolished | ||||
Gourzoulidis et al., 2018 [99] | Empagliflozin vs. standard of care (background glucose-lowering therapy) | Y | Y | Time horizon | ||||
Ishii et al., 2018 [53] | Dulaglutide vs. insulin glargine | Y | Y | No influential parameters identified could influence the base-case results | ||||
Neslusan et al., 2018 [86] | Canagliflozin vs. dapagliflozin | Y | Y | No influential parameters identified could influence the base-case results | ||||
Nguyen et al., 2018 [100] | Empagliflozin vs. standard treatment | Y | N | Cost of empagliflozin | Death rate of standard treatment | |||
Tzanetakos et al., 2018 [74] | Exenatide vs. insulin glargine; exenatide vs. liraglutide | Y | Y | No influential parameters identified could influence the base-case results |
Rights and permissions
About this article
Cite this article
Hong, D., Si, L., Jiang, M. et al. Cost Effectiveness of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors, Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists, and Dipeptidyl Peptidase-4 (DPP-4) Inhibitors: A Systematic Review. PharmacoEconomics 37, 777–818 (2019). https://doi.org/10.1007/s40273-019-00774-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-019-00774-9