Abstract
Background and Objective
A phase I/II trial evaluated the safety, antitumor activity, and pharmacokinetics of avelumab (anti-PD-L1 antibody) in pediatric patients with refractory/relapsed solid tumors (NCT03451825). This study aimed to inform avelumab dose selection in pediatric populations using population pharmacokinetic modeling and simulations.
Methods
Patients aged < 18 years with refractory/relapsed solid tumors enrolled in phase I received avelumab 10 or 20 mg/kg intravenously every 2 weeks. A pediatric population pharmacokinetic model was developed via the frequentist prior approach.
Results
Pharmacokinetic parameters from 21 patients who received avelumab 10 mg/kg (n = 6) or 20 mg/kg (n = 15) were analyzed. Patients had a wide range of weights and ages (medians, 37.3 kg and 12 years). Exposures with 10-mg/kg dosing were lower vs adult dosing, particularly in patients weighing < 40 kg, whereas 20-mg/kg dosing achieved or exceeded adult exposures, irrespective of body weight. A two-compartment linear model with time-varying clearance using body weight as a covariate, with the frequentist prior approach, best described pediatric data. In this model, optimal overlap in exposure with adult data was achieved with 800 mg every 2 weeks for patients aged ≥ 12 years and weighing ≥ 40 kg, and 15 mg/kg every 2 weeks for patients aged < 12 years or weighing < 40 kg.
Conclusions
Based on exposure matching, the recommended doses for further avelumab studies, including combination studies, are 15 mg/kg every 2 weeks for pediatric patients aged < 12 years or weighing < 40 kg and the adult flat dose of 800 mg every 2 weeks for pediatric patients aged ≥ 12 years and weighing ≥ 40 kg.
Clinical Trial Registration
ClinicalTrials.gov NCT03451825.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this study, real-time pharmacokinetic analyses of avelumab exposure were used to inform dose decisions in this dose-escalation cohort. |
Subsequently, integration of adult model pharmacokinetic and pharmacodynamic data with pediatric data was used to support dose selection for future trials in pediatric patients. |
Recommended doses for further studies of avelumab in pediatric patients are 15 mg/kg every 2 weeks for patients aged < 12 years or weighing < 40 kg, and the adult flat dose of 800 mg every 2 weeks for patients aged ≥ 12 years and weighing ≥ 40 kg. |
1 Introduction
Several immune checkpoint inhibitors (ICIs) that target the PD-1/PD-L1 interaction have been approved as treatments for a wide range of adult cancers, but data in pediatric patients are limited. Recently, several phase I/II studies investigating ICI monotherapy in various pediatric cancers have shown that these agents were well tolerated, but antitumor activity was limited, except in patients with Hodgkin lymphoma [1,2,3]. Pediatric dose selection for ICIs, including atezolizumab (anti-PD-L1), pembrolizumab (anti-PD-1), and nivolumab (anti-PD-1), has been based on observed safety and tolerability profiles, similarity in estimated exposures to ranges seen with efficacious adult doses, and adult exposure–response and pharmacokinetic-pharmacodynamic (PK-PD) profiles [1,2,3,4]. Further studies to optimize ICI treatment for pediatric cancers are warranted, particularly combination approaches.
Avelumab is an anti-PD-L1 antibody approved in various countries as monotherapy for the treatment of adults and children aged ≥ 12 years with metastatic Merkel cell carcinoma and in combination with axitinib for adults with advanced renal cell carcinoma. Avelumab monotherapy is also approved for the treatment of patients with advanced urothelial carcinoma that has not progressed with first-line platinum-based chemotherapy (maintenance treatment) and for patients with disease progression following platinum-based chemotherapy. Initially, avelumab was approved with 10-mg/kg weight-based dosing administered every 2 weeks (q2w). Subsequently, approved dosing in the USA, Europe, and some other countries changed to an 800-mg flat dose q2w based on similar exposures and reduced variability compared with weight-based dosing in population PK modeling and simulations [5].
In a phase I/II trial, avelumab was administered to patients aged < 18 years with refractory or relapsed solid tumors at doses of 10 mg/kg q2w and 20 mg/kg q2w. In the phase I dose-escalation part, the safety profile of avelumab monotherapy was found to be consistent with that of previous adult studies in various solid tumors [6], and the maximum tolerated dose was not reached [7]. Clinical benefits in pediatric patients were limited [7], consistent with previous studies of ICI monotherapy in similar populations [1,2,3].
The objectives of this study were to integrate available PK data for avelumab obtained during the phase I dose-escalation part of this trial and develop a population PK model to characterize the dose–exposure relationship in pediatric patients with various solid tumors, with the aim of supporting dose selection for further clinical evaluation of avelumab in pediatric patients.
2 Methods
2.1 Study Design
Concentration–time data were obtained from patients enrolled in phase I of an open-label, single-arm, phase I/II trial investigating the dose, safety, antitumor activity, and pharmacokinetics of avelumab in pediatric patients with refractory or relapsed solid tumors (NCT03451825). Eligible patients were aged < 18 years (at first dose) and had a histologically or cytologically confirmed malignant solid tumor (including central nervous system tumors) or lymphoma, which had either progressed with standard therapy or for which no standard therapy existed. Other eligibility criteria included Lansky (age ≤ 16 years) or Karnofsky (age > 16 years) performance status of ≥ 50; estimated life expectancy > 3 months; adequate hematologic, hepatic, and renal function; and no prior therapy targeting a T-cell coregulatory protein. The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and the International Council on Harmonisation guidelines on Good Clinical Practice. The protocol was approved by the institutional review board or independent ethics committee of each center. All patients or legal representatives of patients provided written informed consent before enrollment.
Patients were enrolled in sequential cohorts of three to six patients. Avelumab was initially administered at 10 mg/kg by 1-h intravenous infusion q2w. Escalation to 20 mg/kg q2w was planned if exposure was not adequate compared with adult population PK-simulated values for median and distribution of exposure (Fig. 1 of the Electronic Supplementary Material [ESM]). As reported previously, dose-limiting toxicities were adverse events meeting defined criteria [7] that occurred during the dose-limiting toxicity observation period (first two cycles of treatment [28 days]).
Dose-normalized, noncompartmental-derived pharmacokinetic parameters following first infusion of avelumab (10- or 20-mg/kg dose), by body weight (< 40 vs ≥ 40 kg). A Maximum observed serum concentration (Cmax). B Area under the concentration–time curve (AUC0–τ). C Trough concentration (Ctrough). Boxes represent interquartile range, solid lines within the box are arithmetic means, whereas the dotted lines are medians. Individual values beyond the interquartile range tails are shown as outlying dots
2.2 PK Sampling and Analysis in Pediatric Study
Avelumab concentrations in serum were assessed in blood samples drawn before the first infusion (cycle 1); at the end of infusion; at 3 hours (patients weighing ≥ 10 kg) and 48–96 hours after infusion; before infusion in cycles 2, 3, 5, 7, 8, 13, and every six infusions thereafter; and at the end of infusion in cycles 3, 7, 13 (patients weighing ≥ 10 kg), and 19. Avelumab serum concentrations were analyzed by a validated sandwich immunoassay method using the Gyrolab™ platform. The lower limit of quantitation was 0.2 μg/mL. Pharmacokinetic parameters were calculated by a noncompartmental analysis (NCA) and estimated using previously developed, adult avelumab population PK models.
2.3 Simulations for Real-Time Analysis
Because no clinical, PK, or target occupancy (TO) data were available for avelumab in pediatric patients prior to this study, the starting and escalation doses were selected using simulations from previously developed, adult avelumab population PK models to identify the dosing regimen(s) that would provide exposures most similar to those in adults, with the assumption that similar exposures would result in similar effects between pediatric and adult patients. Because of the limited understanding of potential pediatric age-related changes in monoclonal antibody pharmacokinetics [8], simulations considered only baseline body weight as a covariate; age was not found to be a clinically relevant covariate in the adult population PK models. A simulated pediatric population was generated based on Centers for Disease Control and Prevention growth charts [9].
2.4 Develo** a Pediatric Population PK Model Based on Trial Data
Data were analyzed using the first-order conditional estimation with interaction method and NONMEM (version 7.3.0), PsN (version 4.4.8), and Pirana (version 2.9.2) software; pre-processing and post-processing were performed using R (version 3.5.1) and RStudio (version 1.0.153). The model structure was assumed to be equivalent to the previously published, adult avelumab population PK model (N = 1827) [10], i.e., a two-compartment linear model with time-varying clearance (CL), between-patient variability in CL, central volume of distribution (V1), and peripheral volume of distribution (V2), and combined additive and proportional residual unexplained variability. Because of the small number of pediatric PK samples, different modeling approaches that used the adult population PK model were investigated, including: (1) prediction of individual (maximum a posteriori empirical Bayes estimates) pediatric patient-specific parameters from the adult model without re-estimation of the model parameters, (2) re-estimation and prediction of model parameters using pediatric data only, (3) re-estimation and prediction using pooled pediatric and adult data, (4) re-estimation and prediction using pediatric data and the frequentist prior approach [11, 12], and (5) re-estimation and prediction of a reduced model (without time variance in CL) using pediatric data only. The final model performance was assessed using standard criteria, including parameter value plausibility, estimation precision, goodness-of-fit plots, and visual predictive checks.
Simulations were performed with the final pediatric population PK model to compare different dosing regimens (10, 15, and 20 mg/kg q2w for all pediatric patients, and 800 mg q2w for patients aged ≥ 12 years and weighing ≥ 40 kg). A simulated pediatric population was generated based on Centers for Disease Control and Prevention growth chart data [9], comprising 2000 patients, of which half were aged 2–12 years and the other half were aged 12–18 years. Simulated exposures (trough serum concentration [Ctrough], maximum serum concentration [Cmax], and area under the concentration–time curve [AUC] after a single dose and at steady state) were calculated and compared with simulated adult exposures with 10-mg/kg and 800-mg dosing. Finally, exposure distribution data were used to estimate the population fraction that would achieve a Ctrough considered representative of achieving maximal PD-L1 TO.
3 Results
3.1 Patients
In total, 21 pediatric patients were enrolled and all had data available for PK analyses. Patients had a wide range in body weights (13.4–78.7 kg; median, 37.3 kg) and ages (3–17 years; median 12 years). Patients received avelumab q2w at doses of 10 mg/kg (n = 6) or 20 mg/kg (n = 15).
3.2 Real-Time Analysis of PK Data During Dose Escalation
The avelumab starting dose of 10 mg/kg q2w was selected based on preliminary modeling and simulations using adult population PK models. Comparing simulated single-dose exposure with 10, 15, and 20-mg/kg q2w dosing in pediatric patients with adult exposure, the 10-mg/kg dose was found to be most appropriate with no indication to test a lower dose. To guide dose selection during the dose-escalation part of this trial, observed safety data [7] were considered along with three pediatric PK parameters: Ctrough, concentration–time profile, and AUC. Area under the concentration–time curve was derived by an NCA, but because pediatric PK was limited, AUC was also derived using the adult population PK models, which was impacted less by outliers compared with NCA-derived values.
More than two-thirds of Ctrough, concentration–time profile, and AUC data from patients in the 10-mg/kg cohort were below the 50th percentile of adult data, and more than one-third of pediatric data were below the 25th percentile. Noncompartmental analysis-derived and model-derived AUC data were consistent. Based on predefined exposure criteria (Fig. 1 of the ESM) and available safety data (reported previously [7]) and PK data in the 10-mg/kg cohort (Table 1 and Fig. 2 of the ESM), the avelumab dose was increased to 20 mg/kg in subsequent patients.
3.3 Observed PK Data at Both Dose Levels
Differences in the pharmacokinetics with 10-mg/kg and 20-mg/kg doses according to body weight and age, in addition to similarity to adult exposure, were assessed initially using observed Ctrough, Cmax, and NCA-derived AUCs during cycle 1 (Table 1; Fig. 1). Although no formal statistical analysis was performed because of the small sample size, a trend for lower exposure in pediatric patients weighing < 40 kg vs ≥ 40 kg was observed at both dose levels. Additionally, in pediatric patients weighing ≥ 40 kg, approximately dose-proportional increases in median and geometric mean Cmax, AUC, and Ctrough were observed with 20 vs 10-mg/kg dosing, whereas, in patients with a body weight < 40 kg, the increase in median and geometric mean AUC and Ctrough was more than dose proportional. Furthermore, median and geometric mean AUC and Ctrough in the 10-mg/kg cohort appeared lower than in adults with the approved 800-mg flat dose, particularly in patients weighing < 40 kg. In the 20-mg/kg cohort, median and geometric mean AUC and Ctrough were similar or higher than those in adults with the approved dose, irrespective of body weight. However, pediatric Cmax and AUC in the 20-mg/kg cohort were within the range of adult exposures with 20-mg/kg dosing. No association between age and exposure was observed (Fig. 3 of the ESM). Additionally, the single patient who had a dose-limiting toxicity at 20 mg/kg had a similar PK profile to other pediatric patients receiving the same dose.
3.4 Pediatric Population PK Model
In total, 153 PK observations were available for model development. Pediatric trial data alone were not sufficiently informative to support estimation of parameters in the preestablished base model in adults. Additionally, when pediatric data were pooled with adult data, parameter estimates were shrinking toward the estimates from the adult model because of the larger adult data set. Subsequently, using pediatric data with the frequentist prior approach, in which estimated parameters that could not be adequately informed by pediatric data (change in CL over time, V2, and intercompartmental clearance) were informed by prior estimates from the adult model, was found to overcome the limitations of other approaches. The effect of body weight as a covariate was investigated with body weight normalized to the median value from enrolled pediatric patients (37 kg), the body weight effect on V2 and intercompartmental clearance was fixed to 1, and the effect on V1 and baseline CL was estimated. Thus, the final model was a two-compartment model with time-varying linear CL and body weight as a covariate. The parameters in the final model were estimated with satisfactory precision, with a relative standard error for fixed-effect and random-effect parameters of ≤ 13 and ≤ 48%, respectively. Visual predictive checks demonstrated good predictive performance of the final model (Fig. 4 of the ESM).
Table 2 shows parameter estimates in the final pediatric population PK model and corresponding estimates from the adult population PK model. Using the final model, baseline pediatric CL was estimated to be 0.0177 L/h (relative standard error, 7%) for a typical pediatric patient with a body weight of 37 kg (i.e., observed median). The CL value in a pediatric patient with a body weight of 71 kg (i.e., median weight in the adult model population [10]) was 0.0316 L/h, while the CL in a typical adult weighing 71 kg was 0.0274 L/h. The estimated V1 was 1.96 L (relative standard error, 3%) in a typical pediatric patient weighing 37 kg, and 2.98 L in a pediatric patient weighing 71 kg, which is expectedly lower than that seen in adults (3.15 L). Other pediatric parameters, which were informed by prior estimates, were similar to adult values, including a V2 of 0.91 L for both pediatric and adult patients, an intercompartmental clearance of 0.0323 L/h and 0.0299 L/h, respectively, and a slightly lower maximal decrease in CL in pediatric patients (2%). The effect of body weight was higher in pediatric patients vs adults, with an estimated power exponent of 0.892 vs 0.545 for CL and 0.631 vs 0.475 for V1, respectively.
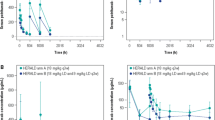
Individual concentrations, including Ctrough, were predicted well by the final model (Fig. 5 of the ESM), and model-predicted AUCs were within the range of simulated AUCs (Fig. 2A–C). The distribution of model-predicted baseline CL values was also within the simulated distribution, with no trend apparent for a higher baseline CL in the 10-mg/kg cohort (Fig. 2D).
Simulations with the pediatric population pharmacokinetic model vs observations after a single dose of avelumab at 10 or 20 mg/kg every 2 weeks (q2w). A Serum concentration. B Trough concentration (Ctrough). C Area under the concentration–time curve (AUC). D Clearance (CL). In A pink dots are individual observations, and the shaded blue areas represent predicted concentrations at each dose. In B–D, boxes represent interquartile range; horizontal lines within the box are medians; whiskers extend to the most extreme point, which is no more than 1.5 times the length of the box away from the box; pink dots are observations; and blue dots are observations that are outside 1.5 times the length of the box away from the box
3.5 Simulations at Various Dose Regimens Using the Final Pediatric Population PK Model
Flat dosing is generally preferred because of greater convenience and higher compliance [5], and a flat dose of avelumab (800 mg q2w) has been approved in the USA for the treatment of adults and children aged ≥ 12 years with metastatic Merkel cell carcinoma, whose body weight is generally within adult ranges. For younger children weighing < 40 kg, however, flat dosing is not typically recommended because body weight may have a significant impact on pharmacokinetics [13]. Therefore, we simulated dosing with 10 mg/kg, 15 mg/kg, 20 mg/kg, and 800 mg q2w using the pediatric population PK model in two pediatric age and weight subpopulations: ≥ 12 years and ≥ 40 kg, and < 12 years or < 40 kg, based on US Centers for Disease Control and Prevention weight-for-age data, and compared with adult exposures at the approved 800-mg q2w dose. Based on single-dose and steady-state simulations (Fig. 3A, B, Fig. 6 of the ESM and Tables 1 and 2 of the ESM), optimal exposure was achieved with the 800-mg flat dose for children aged ≥ 12 years and weighing ≥ 40 kg, and with 15-mg/kg dosing for children aged < 12 years or weighing < 40 kg, with geometric mean exposures of ≥ 90% of adult exposures. Simulations also predicted an acceptable exposure overlap with 15-mg/kg dosing in children in the lowest body weight category (10–20 kg; Fig. 3C, D). Consistent with NCA-derived results, the pediatric geometric mean exposures for the selected doses were predicted to be below both pediatric and adult exposures achieved with 20-mg/kg dosing (Tables 1 and 2 of the ESM).
Simulations of A trough concentration (Ctrough) and B area under the concentration–time curve (AUC) in subgroups defined by age and body weight and C Ctrough, and D AUC in subgroups defined by body weight after a single dose of avelumab at different doses in pediatric patients. Boxes represent interquartile range; horizontal lines are medians; whiskers extend to the most extreme point, which is no more than 1.5 times the length of the box away from the box; points are observations that are outside 1.5 times the length of the box away from the box. The pink dashed line represents the median for adult 800-mg data. For C and D, ages ranged from 2 to 9 years for the 10–20 kg 15-mg/kg subgroup, 3–12 years for the 20–30 kg 15-mg/kg subgroup, and 5–15 years for the 30–40 kg 15-mg/kg subgroup
3.6 Simulations of the Population Fraction That Achieves Ctrough Associated with Maximal PD-L1 TO
In PK-PD evaluations in a phase I study in adults and in ex vivo concentration-PD profiles, an avelumab serum concentration of > 1 μg/mL resulted in full TO in peripheral blood mononuclear cells [14, 15]. Although the concentration of avelumab required for maximum tumor tissue saturation is unknown, based on data from other ICIs and monoclonal antibodies, tissue penetration of ≈ 10–50% is expected [4, 14, 16]. Thus, a serum concentration of 2–10 μg/mL was expected to achieve maximal PD-L1 TO within tumors, and a target Ctrough of ≥ 6 μg/mL (i.e., median of 2–10 μg/mL) was chosen for further simulations. Using the final pediatric population PK model, selected pediatric dosing regimens for avelumab (800 mg and 15 mg/kg q2w) were expected to maintain maximal tumor TO in ≥ 95% of pediatric patients both after a single dose and at steady state (Fig. 4).
Simulations of A concentration–time profiles and B population fractions that achieved trough concentration (Ctrough) associated with maximal PD-L1 target occupancy with avelumab recommended doses after a single dose. Lines are medians, shaded areas are 90% prediction intervals, and dashed horizontal lines are target concentrations of 6 µg/mL
4 Discussion
Pediatric dose selection based on exposure matching with adult data is a widely accepted practice, as exemplified by studies with other ICIs [1,2,3,4]. Although data on PD-L1 expression in pediatric tumors are limited, exposures required for PD-L1 blockade are assumed to be similar between adults and children based on the consistent function of PD-L1 in normal immune regulation [17] and similar ICI exposures seen in adults with tumor types that have varying PD-L1 expression [10]. In this dose-escalation study, exposure with avelumab 10 mg/kg q2w (initially approved weight-based dose in adults) was found to be lower than adult population-PK-simulated values, particularly in pediatric patients weighing < 40 kg. This conclusion was based on consideration of three PK parameters: Ctrough, which was the most commonly collected measure and is thought to be associated with ICI efficacy [5]; concentration–time profile, which used all available pediatric PK data and was therefore potentially less impacted by outliers; and AUC, which is thought to be associated with the safety and efficacy of ICIs [4, 5, 18]. Subsequently, the pediatric dose was escalated to 20 mg/kg, which achieved or exceeded exposures seen with adult dosing irrespective of body weight.
The reduction in observed Ctrough for avelumab 10-mg/kg q2w dosing in pediatric patients compared with the approved 800-mg q2w flat dose in adults appeared to be relatively large, particularly in patients weighing < 40 kg (≈ 50% reduction), and an apparent more than dose-proportional increase in AUC and Ctrough was observed between the 10 and 20-mg/kg doses. These findings, albeit based on limited data and PK sampling, suggest that the contribution of target-mediated drug disposition to avelumab clearance in pediatric patients weighing < 40 kg with 10-mg/kg dosing could not be ruled out. This observation was also consistent with PK and PD data in adults, which showed maximal PD-L1 TO in all patients and a similar half-life with 10 and 20-mg/kg q2w dosing, but incomplete PD-L1 TO in some patients and a shorter half-life with 3-mg/kg q2w dosing [15]. Additionally, based on available adult exposure-efficacy profiles for avelumab in various tumor types, a decrease in the probability of response with a 50% reduction in Ctrough could not be ruled out [5, 19, 20]. Therefore, it was concluded that for patients weighing < 40 kg, the avelumab dose should be higher than the body weight-based equivalent of the adult dose.
In a previous study, atezolizumab was administered to pediatric patients at a dose of 15 mg/kg every 3 weeks, which is the body weight-based equivalent to the adult 1200-mg flat dose [2, 4]. Similar to our findings, atezolizumab exposures in pediatric patients were lower than those in young adults (aged 18–29 years) receiving the 1200-mg dose [4] (20% lower geometric mean Ctrough). However, this reduction was not considered clinically meaningful because all patients achieved the target serum concentration (6 μg/mL) [2], and in adult patients, no clinically meaningful trend in exposure efficacy was seen [21]. Thus, the pediatric dose selected for atezolizumab was 15 mg/kg every 3 weeks [4].
Because avelumab 800 mg q2w is the approved dose for pediatric patients aged ≥ 12 years with metastatic Merkel cell carcinoma, we evaluated two body weight/age categories: patients aged ≥ 12 years and weighing ≥ 40 kg and patients aged < 12 years or weighing < 40 kg. A previously published, two-compartment, adult population PK model for avelumab was used as the basis for pediatric modeling [10]. Owing to the small sample size and the lack of association between age and exposure in pediatric patients, only body weight was included as a covariate. The effect of body weight was found to be higher in pediatric patients than in adults, with estimated power exponents for effects on CL and V1 of 0.891 and 0.635, respectively, compared with 0.545 and 0.475 in adults. The estimate for CL is similar to that reported previously for atezolizumab in pediatric patients [4], and suggests that weight-based dosing should be considered in children with a weight outside of the adult range (i.e., < 40 kg).
A two-compartment model with time-varying linear CL and body weight as a covariate best described the pediatric data. A target-mediated drug disposition component was not investigated because of the small data set and limited PK sampling; however, overall model performance without a target-mediated drug disposition was acceptable. However, it was noted that observed Ctrough values with 10-mg/kg dosing in patients aged < 12 years or weighing < 40 kg were below the population-simulated median, although individual Ctrough were predicted well by the model, and model-predicted individual AUCs were within the simulated distribution. Additionally, there was no evidence of higher baseline CL in patients with 10- vs 20-mg/kg dosing; therefore, it was not considered likely that poor disease status or cancer-related cachexia contributed to lower-than-expected exposures in this dose cohort.
Using the final model, the optimal overlap in exposure distributions between adult and pediatric subgroups was achieved with the 800-mg flat dose for patients aged ≥ 12 years and weighing ≥ 40 kg and with 15-mg/kg dosing for patients aged < 12 years or weighing < 40 kg, which resulted in geometric mean exposures of ≥ 90% compared with adult data. The model also predicted an acceptable exposure distribution overlap in patients in the lowest body weight category (10–20 kg) with 15-mg/kg dosing. Geometric mean exposures for these doses are predicted to be below adult exposures resulting from 20-mg/kg dosing, which had a manageable safety and tolerability profile in adults with various solid tumors [15]. Additionally, exposure-safety modeling in adults found a relatively weak association between exposure to avelumab and immune-related adverse events but no increased probability of infusion-related reactions with increased avelumab exposure [5]. Last, based on previous estimations of drug serum concentrations required for maximal PD-L1 TO within tumors, it was estimated that ≥ 95% of pediatric patients would achieve maximal TO using the dosing regimens selected. The target serum concentration for a maximal PD effect in tumors chosen for this study (6 μg/mL) was also consistent with target concentrations of atezolizumab [16], which were based on preclinical and clinical data. However, the target serum concentration was defined based on the assumption that tissue penetration was 10–50%, which should be treated with caution because tissue penetration of monoclonal antibodies is highly variable and may be different in children compared with adults [13].
5 Conclusions
Recommended doses for further studies of avelumab in pediatric patients, including combination studies, are 15 mg/kg q2w for children aged < 12 years or weighing < 40 kg, and the approved adult flat dose of 800 mg q2w for children aged ≥ 12 years and weighing ≥ 40 kg. These doses are expected to provide exposures similar to those occurring in adults. Future trials of avelumab in pediatric patients will investigate these recommended doses and will include PK monitoring to confirm exposures.
References
Geoerger B, Kang HJ, Yalon-Oren M, Marshall LV, Vezina C, Pappo A, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020;21(1):121–33. https://doi.org/10.1016/S1470-2045(19)30671-0.
Geoerger B, Zwaan CM, Marshall LV, Michon J, Bourdeaut F, Casanova M, et al. Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): a multicentre phase 1–2 study. Lancet Oncol. 2020;21(1):134–44. https://doi.org/10.1016/S1470-2045(19)30693-X.
Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020;21(4):541–50. https://doi.org/10.1016/S1470-2045(20)30023-1.
Shemesh CS, Chanu P, Jamsen K, Wada R, Rossato G, Donaldson F, et al. Population pharmacokinetics, exposure-safety, and immunogenicity of atezolizumab in pediatric and young adult patients with cancer. J Immunother Cancer. 2019;7(1):314. https://doi.org/10.1186/s40425-019-0791-x.
Novakovic AM, Wilkins JJ, Dai H, Wade JR, Neuteboom B, Brar S, et al. Changing body weight-based dosing to a flat dose for avelumab in metastatic Merkel cell and advanced urothelial carcinoma. Clin Pharmacol Ther. 2020;107(3):588–96. https://doi.org/10.1002/cpt.1645.
Kelly K, Infante JR, Taylor MH, Patel MR, Wong DJ, Iannotti N, et al. Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN Solid Tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer. 2018;124(9):2010–7. https://doi.org/10.1002/cncr.31293.
Loeb DM, Lee JW, Morgenstern DA, Samson Y, Uyttebroeck A, Lyu CJ, et al. Avelumab in paediatric patients with refractory or relapsed solid tumours: dose-escalation results from an open-label, single-arm, phase 1/2 trial. Cancer Immunol Immunother. 2022. https://doi.org/10.1007/s00262-022-03159-8.
Edlund H, Melin J, Parra-Guillen ZP, Kloft C. Pharmacokinetics and pharmacokinetic-pharmacodynamic relationships of monoclonal antibodies in children. Clin Pharmacokinet. 2015;54(1):35–80. https://doi.org/10.1007/s40262-014-0208-4.
Centers for Disease Control and Prevention: National Center for Health Statistics. Clinical growth charts. 2017. Available from: https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed 27 Oct 2021.
Wilkins JJ, Brockhaus B, Dai H, Vugmeyster Y, White JT, Brar S, et al. Time-varying clearance and impact of disease state on the pharmacokinetics of avelumab in Merkel cell carcinoma and urothelial carcinoma. CPT Pharmacometrics Syst Pharmacol. 2019;8(6):415–27. https://doi.org/10.1002/psp4.12406.
Gisleskog PO, Karlsson MO, Beal SL. Use of prior information to stabilize a population data analysis. J Pharmacokinet Pharmacodyn. 2002;29(5–6):473–505. https://doi.org/10.1023/a:1022972420004.
Chan Kwong AH-XP, Calvier EA, Fabre D, Gattacceca F, Khier S. Prior information for population pharmacokinetic and pharmacokinetic/pharmacodynamic analysis: overview and guidance with a focus on the NONMEM PRIOR subroutine. J Pharmacokinet Pharmacodyn. 2020;47(5):431–46. https://doi.org/10.1007/s10928-020-09695-z.
Temrikar ZH, Suryawanshi S, Meibohm B. Pharmacokinetics and clinical pharmacology of monoclonal antibodies in pediatric patients. Paediatr Drugs. 2020;22(2):199–216. https://doi.org/10.1007/s40272-020-00382-7.
Heery CR, O’Sullivan Coyne GH, Marte JL, Singh H, Cordes LM, Madan RA, et al. Pharmacokinetic profile and receptor occupancy of avelumab (MSB0010718C), an anti-PD-L1 monoclonal antibody, in a phase I, open-label, dose escalation trial in patients with advanced solid tumors. J Clin Oncol. 2015;33(15 Suppl.):Abstract 3055. https://doi.org/10.1200/jco.2015.33.15_suppl.3055.
Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18(5):587–98. https://doi.org/10.1016/S1470-2045(17)30239-5.
Deng R, Bumbaca D, Pastuskovas CV, Boswell CA, West D, Cowan KJ, et al. Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. MAbs. 2016;8(3):593–603. https://doi.org/10.1080/19420862.2015.1136043.
Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. https://doi.org/10.1111/j.1600-065X.2010.00923.x.
Netterberg I, Li CC, Molinero L, Budha N, Sukumaran S, Stroh M, et al. A PK/PD analysis of circulating biomarkers and their relationship to tumor response in atezolizumab-treated non-small cell lung cancer patients. Clin Pharmacol Ther. 2019;105(2):486–95. https://doi.org/10.1002/cpt.1198.
Vugmeyster Y, Manitz J, **ong J, Rosen G, Sharma V, Novakovic A, et al. Exposure–response analysis of avelumab in patients with advanced urothelial carcinoma via a full-model approach. J Pharmacokinet Pharmacodyn. 2018;45(Suppl 1):S129 (abstract W-088).
Gulley JL, Spigel DR, Kelly K, Aisner J, Chand VK, Koenig A, et al. Exposure-response and PD-L1 expression analysis of second-line avelumab in patients with advanced NSCLC: data from the JAVELIN Solid Tumor trial. J Clin Oncol. 2017;35(15 Suppl.):abstract 9086. https://doi.org/10.1200/JCO.2017.35.15_suppl.9086.
Morrissey KM, Marchand M, Patel H, Zhang R, Wu B, Chan HP, et al. Alternative dosing regimens for atezolizumab: an example of model-informed drug development in the postmarketing setting. Cancer Chemother Pharmacol. 2019;84(6):1257–67. https://doi.org/10.1007/s00280-019-03954-8.
Acknowledgements
The authors thank the patients and their families, investigators, coinvestigators, and the study teams at each of the participating centers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Merck (CrossRef Funder ID: 10.13039/100009945), as part of an alliance between Merck and Pfizer. Medical writing support was provided by ClinicalThinking and was funded by Merck and Pfizer.
Conflict of interest
YV is an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA. AMG and PR are employees of Merck Healthcare KGaA, Darmstadt, Germany. MR was an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA at the time of the study, and holds stock in Bristol Myers Squibb. BB and AK are employees of Merck Healthcare KGaA, Darmstadt, Germany and hold stock in Merck. HD was an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA at the time of the study.
Ethics approval
The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and the International Council on Harmonisation guidelines on Good Clinical Practice. The protocol was approved by the institutional review board or independent ethics committee of each center.
Consent to participate
All legal representatives of patients or patients provided written informed consent before enrollment.
Consent for Publication
Not applicable.
Availability of data and material
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data sharing portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
Code availability
Not applicable.
Author contributions
All authors participated in the interpretation of the study results, and in the drafting, critical revision, and approval of the final version of the manuscript. All authors designed and performed the research and analyzed the data.
Additional information
Mary Ruisi and Haiqing Dai: Affiliation at the time of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Vugmeyster, Y., Grisic, AM., Brockhaus, B. et al. Avelumab Dose Selection for Clinical Studies in Pediatric Patients with Solid Tumors. Clin Pharmacokinet 61, 985–995 (2022). https://doi.org/10.1007/s40262-022-01111-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01111-8