Abstract
Introduction
Patients undergoing video-assisted thoracoscopic lobectomy (VATL) often experience chronic postsurgical pain (CPSP). Postoperative pain can affect the recovery of postoperative lung function, prolong postoperative recovery time, and increase patient hospitalization expenses. Transcutaneous electrical acupoint stimulation (TEAS) is an alternative therapy based on acupuncture that has shown promise in postoperative recovery and pain management across various medical fields. However, research specifically focused on the improvement of CPSP after VATL is currently lacking. The purpose of this study is to evaluate whether TEAS can effectively reduce the severity and occurrence of chronic postsurgical pain in patients undergoing VATL. By investigating the potential benefits of TEAS in mitigating CPSP after VATL, this study aims to provide valuable clinical evidence to support the integration of TEAS into postoperative care protocols for patients undergoing VATL.
Methods
This study is a prospective, single-center, double-blinded, randomized controlled trial to be conducted at the 920th Hospital of Joint Logistics Support Force. Eighty patients undergoing VATL will be randomly divided into an experimental group (TEAS group) and a control group (sham group). The experimental group will receive TEAS at bilateral PC6, LI4, LR3, LU5, TE5, and LI11. The control group will not receive TEAS at the same acupoints. Both groups will receive TEAS or no TEAS before anesthesia induction and 1–7 days after surgery, with each session lasting 30 min.
Planned Outcomes
The primary outcome will be the incidence of CPSP at 3 months after surgery. Secondary outcomes will include the incidence of CPSP at 6 months after surgery, the numerical rating scale (NRS) scores at 3 and 6 months after surgery, as well as the NRS scores at 24, 48, and 72 h after surgery, remifentanil consumption during general anesthesia, demand for rescue analgesics, number and duration of indwelling chest tubes, incidence of postoperative nausea and vomiting, and changes of norepinephrine (NE), cortisol (Cor), tumor necrosis factor (TNF- α), and interleukin 6 (IL-6) in serum.
Trial Registration
ChiCTR2300069458. Registered on March 16, 2023.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Chronic postsurgical pain (CPSP) is a common complication after video-assisted thoracoscopic lobectomy (VATL). Postoperative pain seriously affects the patient’s recovery and increases the patient’s medical burden. However, there is still a lack of standard treatment to reduce postoperative pain after VATL. |
This trial aims to demonstrate that continuous preventive application of TEAS can reduce the severity and incidence of chronic postsurgical pain after VATL. |
What might be learned from the study? |
The results of this study may provide evidence-based medical evidence for the use of transcutaneous electrical acupoint stimulation to reduce postoperative pain after VATL and offer patients additional pain management options. |
The findings of this study hold great significance in terms of improving patient care, enhancing the medical system, and potentially reducing the social and economic burdens caused by CPSP. This can be beneficial not only for individual patients but also for healthcare systems and society as a whole. |
Introduction
In the 11th revision of the International Classification of Diseases, chronic postsurgical pain (CPSP) is defined as persistent or recurrent pain in the area of the surgery and lasts at least 3 months [1]. CPSP is a serious postoperative problem for patients, and opioid abuse is an important cause of this condition. CPSP, like other chronic pain, requires a comprehensive biological, psychological, and social treatment approach. In thoracic surgery, CPSP is a common complication with an incidence rate of up to 65% [2]. Research has shown that nearly a quarter of patients after thoracic surgery may experience CPSP, with one-third of them experiencing neuropathic components [3]. Currently, video-assisted thoracoscopic lobectomy (VATL) is considered the preferred surgical method for non-small cell lung cancer due to its advantages of less trauma, reduced bleeding, and decreased postoperative pain. It has gradually replaced traditional thoracotomy [4]. However, a meta-analysis in 2022 pointed out that the incidence of CPSP is still as high as 43.99% [5]. Pain following VATL may lead to patients being unwilling to cough and expectorate after surgery, which can affect the recovery of pulmonary function and even lead to atelectasis, pulmonary infection, and hypoxemia. Therefore, the incidence and severity of postoperative pain following VATL are directly related to the patient’s postoperative recovery. Reducing postoperative pain following VATL and improving the patient’s quality of life have significant clinical implications.
At present, there are still significant variations in postoperative pain management strategies for thoracoscopic surgery. The 2022 guidelines for pain management after thoracoscopic surgery recommend the use of non-steroidal anti-inflammatory drugs, dexmedetomidine, paravertebral block, and erector muscle block to alleviate postoperative pain [6]. Opioid medications are recommended as rescue analgesics after surgery. However, these analgesics can lead to various side effects, including urinary retention, nausea, vomiting, and hyperalgesia. Therefore, there is an urgent need for a safer and more effective analgesic measure to reduce postoperative pain after VATL.
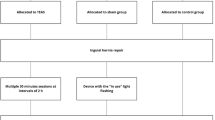
As an essential component of Chinese medicine treatment, acupuncture has been widely used in many fields for sedation and analgesia relief. Transcutaneous electrical acupuncture stimulation (TEAS) is a non-invasive adjunctive intervention based on acupuncture. It attaches an electrode sheet to the acupoint and connects the electroacupuncture apparatus to stimulate the acupoint, thus generating a dual effect of electrical and acupoint stimulation for disease prevention and treatment. TEAS combines modern transcutaneous electrical nerve stimulation with traditional Chinese acupuncture theory and has emerged as an important complementary and alternative therapy in clinical analgesia. Both acupuncture and TEAS have demonstrated their ability to reduce the consumption of opioids during general anesthesia [Sample Size According to the incidence of chronic pain after thoracoscopic surgery, which is as high as 43.99% [5], the sample size is calculated as a bilateral test and taken α with a value of 0.05, selecting a total of 64 people from each group of 32 as the total sample size can achieve a testing efficiency of 80% and an inter group difference of 30%. Considering a postoperative drop-out rate of 20%, a total of 80 cases will be selected as the sample size for this trial. Eighty patients who meet the eligibility criteria and sign the informed consent will be recruited at the 920th Hospital of Joint Logistics Support Force. We plan to enroll the first patient on March 20, 2023 and to end on March 20, 2024. All participants will be randomly divided into two groups: an experimental group (TEAS group) and a control group (sham group). The researchers’ screen subjects will be based on established standard treatment plans. Date collection will start from the collection of baseline data until the end of follow-up. Figure 1 shows the trial flow chart for the patient screening, treatment allocation, intervention, outcomes assessment and data analysis. Figure 2 provides an overview of the study conduct, review, description and interpretation. The schedule of trial enrollment, interventions, and assessments. TEAS transcutaneous electrical acupuncture stimulation, NRS numerical rating scale, CPSP chronic postsurgical pain, PONV postoperative nausea and vomiting, NE norepinephrine, Cor cortisol, TNF-α tumor necrosis factor, IL-6 interleukin 6, POD postoperative day The TEAS group will receive bilateral Hegu (LI4), Wai guan (TE5), Quchi (LI11), Neiguan (PC6), Chize (LU5), and Taichong (LR3) for electrical stimulation treatment (Fig. 3). LI4 belongs to the Hand Yangming Large Intestine Meridian, which is located at the midpoint of the radius of the second metacarpal bone on the back of the hand. TE5 belongs to the Hand Shaoyang Triple Jiao Classic, which is located in the posterior area of the forearm, 2 in. above the transverse line of the distal dorsal wrist, at the midpoint of the space between the ulna and radius. LI11 belongs to the Hand Yangming Large Intestine Meridian, which is located in the elbow area, at the midpoint depression of the line connecting the ulna and the external epicondyle of the humerus. PC6 belongs to the Hand Jueyin Pericardium Meridian, which is located in the anterior area of the forearm, 2 in. above the transverse line of the distal carpometacarpal side, between the palmaris longus tendon and the radial flexor carpi tendon. LU5 belongs to the Hand Taiyin Lung Meridian, which is located in the elbow area, on the transverse stripe of the elbow, in the concave radial margin of the biceps brachii tendon. LR3 belongs to the Foot Jueyin Liver Meridian, which is located on the dorsum of the foot, between the first and second metatarsals, in the anterior depression of the metatarsal junction. The electrode is attached to the skin surface of the patient’s acupoints and connected to the Huatuo Treatment Instrument (Suzhou Electronic Needle Therapy Instrument SDZ-II) through a wire. TEAS stimulation uses a dense-disperse frequency of 2/100 Hz alternating every 3 s. The waveform is a symmetric biphasic curve. The stimulation intensity will be set to the maximum tolerance level of each patient. TEAS or no TEAS will be performed 30 min before anesthesia induction and each of 7 days postoperatively, with each session lasting 30 min. In the control group, electrodes will be attached to the patients at the same acupoints, but the electrodes will be only connected to the device without electrical stimulation. All subjects will use the same anesthesia regimen. Anesthesia induction: midazolam 0.05 mg/kg, sufentanil 0.5 μg/kg, etomidate 0.2–0.6 mg/kg, rocuronium 0.6–0.9 mg/kg. Total intravenous anesthesia will be used for anesthesia maintenance: dexmedetomidine hydrochloride 0.4 μg/kg/h, remifentanil 0.05–2 μg/kg/min, propofol 4–12 mg/kg/h, additional rocuronium as needed during surgery, and the bispectral index (BIS) maintained at 40–60. Postoperative patient-controlled intravenous analgesia (PCIA): 30 min before the end of surgery, the PCIA will be connected and the analgesic formula 2 μg/kg sufentanil plus saline to a total volume of 150 ml, 3 ml/bolus, 15-min interval. We will collect venous blood (5 ml) from patients before the first TEAS treatment and at the end of the operation, and then centrifuge and freeze the plasma supernatant for later testing. Patients’ vital signs will be constantly monitored during the study, and adverse events will be recorded if they encounter serious adverse reactions and will be promptly discontinued and given symptomatic treatment. The primary outcome of this study will be the incidence of CPSP at 3 months after surgery. At 3 months after surgery, the patients will be followed up using a telephonic questionnaire [14, 15]. The questionnaire is provided in Supplementary Material. The secondary outcomes will include the incidence of CPSP at 6 months after surgery, the NRS scores at 3 and 6 months after surgery, as well as the NRS scores at 24, 48, and 72 h after surgery. The NRS scores at 3 and 6 months after surgery will be assessed by the outcome evaluator using a telephone questionnaire (see Supplementary Material 1). The NRS scores at 24, 48, and 72 h after surgery will be evaluated by the outcome evaluator at the ward. To observe changes in serum stress factors, such as norepinephrine (NE), cortisol (Cor), and serum inflammatory factors, such as tumor necrosis factor (TNF- α) and interleukin 6 (IL-6). Secondary outcomes will also include remifentanil consumption during general anesthesia, the proportion of rescue analgesic demand at 24, 48, and 72 h after surgery, the duration of thoracic tube indwelling, the incidence of postoperative nausea and vomiting (PONV), and the number of thoracic tubes indwelling at 24, 48, and 72 h after surgery. We will perform all the analyses in an intention-to-treat population, which includes all patients who have undergone randomization and interventions. Descriptive statistics will be applied to present the characteristics of each group with mean ± standard deviation, median (interquartile), or the number of cases (%). Either analysis of variance or Chi-square test will be used to reveal the baseline balance between groups, depending on the characteristics of the variables. Repeated measures analysis of variance will be applied for repeated data, considering normality and homogeneity. A P value < 0.05 will be considered statistically significant. For missing data, analysis will be performed according to a worst-case scenario. In particular, patients lost to follow-up in the TEAS groups and the sham group will be considered with and without pain, respectively. All data will be analyzed using the statistical package for IBM SPSS Statistics V25.0. Data collection and management procedures will be developed by statisticians and principal investigators, and the database will be established. The data and safety monitoring committee (DMC) includes a expertise clinical doctor, a scientific research manager and a statistician. All the adverse events should be submitted to the DMC every 6 months. DMC has the right to decide whether to stop or modify the scheme. The scientific research management committee and the statistician will have access to all the data in the study. The researchers will obtain written informed consent from all participants recruited to the study before randomization. During the study, in order to ensure the privacy and safety of patients, we will use numerical numbers instead of subject names. The personal information of all subjects will be kept strictly confidential and will not be disclosed to any person or organization without authorization.Recruitment Strategies and Enrollment
Interventions
Outcome Assessment
Primary Outcome
Secondary Outcomes
Statistical Methods
Data Collection Methods and Study Monitoring
Informed Consent
Confidentiality
Discussion
The incidence of postoperative chronic pain not only slows down patients' postoperative recovery but also adds to their medical burden, leading to severe psychological issues. Additionally, postoperative chronic pain and neuropathic pain have a notable negative impact on the overall quality of life, resulting in a decrease in all SF-36 Health Survey domain scores [16]. Since its introduction into clinical practice, thoracoscopy has been widely adopted as an alternative to thoracotomy [17], primarily due to its ability to reduce postoperative acute pain. However, CPSP remains a significant complication, accounting for 43.99% [5]. Postoperative management of acute and chronic pain remains an important challenge for VATL. In the current postoperative analgesic strategies, it is recommended to retain opioid drugs as the main analgesic method [18]. The application of opioid drugs has to some extent alleviated postoperative acute and chronic pain, but it has also brought unavoidable side effects. Consequently, there is an urgent need for a safer and more effective clinical analgesic approach that can be applied to postoperative pain management following thoracoscopic surgery in clinical practice.
Currently, TEAS has been demonstrated to effectively reduce opioid usage during surgery, alleviate intraoperative anxiety, and provide relief from postoperative pain [19]. In patients undergoing mastectomy, TEAS at combined acupoints before surgery was associated with reduced chronic pain at 6 months after surgery [15]. TEAS has been applied to patients undergoing abdominal surgery in the perioperative period, and the results showed that TEAS can promote the recovery of postoperative gastrointestinal function, which may be related to the brain–gut axis [20]. In cardiothoracic surgery, the application of TEAS primarily focused on its anti-inflammatory properties and its ability to alleviate postoperative acute pain. The study has confirmed that TEAS can reduce intubation reactions during thoracic surgery and aid in the postoperative recovery [21]. TEAS treatment was performed on patients undergoing pneumonectomy at acupoints PC6, LI4, Houxi (SI3), and Zhigou (TE6). The results of the study indicated that both TEAS and electroacupuncture have similar effects in regulating immune function. Both techniques were found to effectively inhibit the production of postoperative immune factors, leading to a reduction in immune suppression experienced by the patients [22]. Indeed, some studies have demonstrated that TEAS treatment can accelerate the recovery of early postoperative pulmonary function in patients undergoing VATL [23]. In the Chinese guidelines, PC6, LI4, LR3, and SI3 were recommended as pain relief points for thoracic surgery, and LI4, PC6, LR3, LU5, and TE5 were pain relief points after lobectomy [24, 25]. Based on consensus, PC6, LI4, LU5, TE5, LI11, and LR3 acupoints will be chosen for this research. PC6 belongs to the Hand Jueyin Pericardium Meridian, which mostly cures palpitations such as chest pain, tightness, tachycardia, and bradycardia. LI4 belongs to the Hand Yangming Large Intestine Meridian, which mostly addresses pain symptoms such as headache, toothache, redness, and swelling in the eyes. LU5 belongs to the Hand Taiyin Lung Meridian and is mostly used to treat lung ailments such as coughing, wheezing, and hemoptysis. TE5 belongs to the Hand Shaoyang Triple Jiao Classic and is commonly used to treat rib discomfort, headaches, upper-limb paralysis, and other symptoms. LI11 belongs to the Hand Yangming Large Intestine Meridian and is mostly used to treat gastrointestinal discomfort symptoms such as abdominal pain, diarrhea, and vomiting. LR3 belongs to the Foot Jueyin Liver Meridian and is used to alleviate symptoms such as chest pain, bloating, and hiccups.
While the effectiveness of TEAS on acute pain after VATL surgery has been confirmed, its impact on long-term chronic pain after surgery remains unclear. To address this, a prospective randomized controlled trial will be conducted to investigate the effects of TEAS on acute pain and chronic postsurgical pain (CPSP) following VATL. The study aims to evaluate the safety and effectiveness of TEAS in this context. The experimental results of this study have the potential to demonstrate the impact of TEAS on CPSP and its subsequent effects on the quality of life for patients undergoing VATL. Positive results indicating the safety and effectiveness of TEAS in managing CPSP would have significant implications. By providing an effective method for managing CPSP in patients, TEAS could contribute to improving their overall quality of life. Reduced postoperative pain and better pain management can positively impact patients’ physical and emotional well-being, enabling them to recover more smoothly and resume their daily activities sooner. Moreover, the successful implementation of TEAS as a safe and effective treatment option for CPSP could have broader implications for the medical system. It may lead to the integration of alternative therapies like acupuncture into standard postoperative care protocols, offering patients additional pain management options and potentially reducing reliance on pain medications with potential side effects. Therefore, by improving patient outcomes and reducing the burden of CPSP, TEAS has the potential to decrease social and economic costs associated with long-term chronic pain management. This can be beneficial not only for individual patients but also for healthcare systems and society as a whole.
Strengths and Limitations
Unlike traditional acupuncture, TEAS is a non-invasive treatment that greatly improves patient comfort and satisfaction. In addition, TEAS will reduce the demand for opioids after surgery, thereby reducing the side effects caused by opioids and reducing the medical burden of patients. This study will provide powerful and up-to-date evidence for clinicians and patients seeking innovative and effective methods to relieve pain after VATL.
However, it is important to acknowledge certain limitations of the study. Firstly, although the study is a double-blind design, there is a possibility that some patients may become aware of their assigned groups during the intervention process, which could potentially introduce biases and affect the accuracy of the results. Maintaining blinding throughout the study is crucial to minimize such issues. Secondly, ensuring timely and accurate postoperative follow-up is a crucial aspect of this study, considering the large number of observation time points involved. The ability to accurately track and assess patient outcomes following VATL is essential for evaluating the safety and effectiveness of TEAS in managing CPSP. Lastly, because this is a single-center trial with a limited sample size, the results could not be sufficiently representative. Later on, relevant investigations will be conducted in order to increase the sample size and achieve more precise results.
Ethics and Dissemination
The trial follows the principles of the Declaration of Helsinki. It has been reviewed and approved by the Ethics Committee of the 920th Hospital of Joint Logistics Support Force. The protocol complies with GCP principles. All recruited patients provide written informed consent to participate in the trial.
Conclusions
The findings of this study hold great significance in terms of improving patient care, enhancing the medical system, and potentially reducing social and economic burdens caused by CPSP.
Trial Status
This is version 1.0 of the protocol, dated August 29, 2022. Recruitment began on March 20, 2023. Recruitment is anticipated to end on March 20, 2024. This study is still in progress.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–7.
Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–46.
Peng Z, Li H, Zhang C, Qian X, Feng Z, Zhu S. A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on qualify of life. PLoS ONE. 2014;9(2): e90014.
Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–44.
Zhang Y, Zhou R, Hou B, et al. Incidence and risk factors for chronic postsurgical pain following video-assisted thoracoscopic surgery: a retrospective study. BMC Surg. 2022;22(1):76.
Feray S, Lubach J, Joshi GP, et al. PROSPECT guidelines for video-assisted thoracoscopic surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2022;77(3):311–25.
Wang H, **e Y, Zhang Q, et al. Transcutaneous electric acupoint stimulation reduces intra-operative remifentanil consumption and alleviates postoperative side-effects in patients undergoing sinusotomy: a prospective, randomized, placebo-controlled trial. Br J Anaesth. 2014;112(6):1075–82.
Lu Z, Dong H, Wang Q, **ong L. Perioperative acupuncture modulation: more than anaesthesia. Br J Anaesth. 2015;115(2):183–93.
Hershman DL, Unger JM, Greenlee H, et al. Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA. 2018;320(2):167–76.
Sun E, Dexter F, Macario A. Can an acute pain service be cost-effective? Anesth Analg. 2010;111(4):841–4.
Chen J, Zhang Y, Li X, et al. Efficacy of transcutaneous electrical acupoint stimulation combined with general anesthesia for sedation and postoperative analgesia in minimally invasive lung cancer surgery: a randomized, double-blind, placebo-controlled trial. Thorac Cancer. 2020;11(4):928–34.
Huang S, Peng W, Tian X, et al. Effects of transcutaneous electrical acupoint stimulation at different frequencies on perioperative anesthetic dosage, recovery, complications, and prognosis in video-assisted thoracic surgical lobectomy: a randomized, double-blinded, placebo-controlled trial. J Anesth. 2017;31(1):58–65.
Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–7.
Cho AR, Kwon JY, Kim KH, et al. The effects of anesthetics on chronic pain after breast cancer surgery. Anesth Analg. 2013;116(3):685–93.
Lu Z, Wang Q, Sun X, et al. Transcutaneous electrical acupoint stimulation before surgery reduces chronic pain after mastectomy: a randomized clinical trial. J Clin Anesth. 2021;74: 110453.
Fiorelli S, Cioffi L, Menna C, et al. Chronic pain after lung resection: risk factors, neuropathic pain, and quality of life. J Pain Symptom Manag. 2020;60:326–35. https://doi.org/10.1016/j.jpainsymman.2020.03.012.
Yang CJ, Kumar A, Deng JZ, et al. A national analysis of short-term outcomes and long-term survival following thoracoscopic versus open lobectomy for clinical stage II non-small-cell lung cancer. Ann Surg. 2021;273(3):595–605.
Schug SA, Palmer GM, Scott DA, Halliwell R, Trinca J. Acute pain management: scientific evidence, fourth edition, 2015. Med J Aust. 2016;204(8):315–7.
Sun K, **ng T, Zhang F, et al. Perioperative transcutaneous electrical acupoint stimulation for postoperative pain relief following laparoscopic surgery: a randomized controlled trial. Clin J Pain. 2017;33(4):340–7.
Li W, Gao C, An L, Ji Y, Xue F, Du Y. Perioperative transcutaneous electrical acupoint stimulation for improving postoperative gastrointestinal function: a randomized controlled trial. J Integr Med. 2021;19(3):211–8.
Yu ZY, Zhang YY, Zhang H, et al. Effects of transcutaneous electrical acupoint stimulation on stress response during intubation and extubation in patients undergoing video-assisted thoracoscopic surgery: a prospective, randomized controlled trial. Evid Based Complement Altern Med. 2021. https://doi.org/10.1155/2021/1098915.
Fan WC, Ma W, Zhao C, Tong QY, Shen WD. Influence of acupuncture-drug compound anesthesia with different frequency electroacupuncture on immune function in patients undergoing pneumonectomy. Chin Acupunct Moxib. 2012;32:8 (in Chinese).
Peng WP, Huang S, Feng Y, Liang HS. Effects of transcutaneous electrical acupoint stimulation undergoing video-assisted thoracoscopic pneumonectomy. Chin J Anesthesiol. 2014;34:4 (in Chinese).
Chinese Society of Anesthesiology Task Force on Perioperative Application of Acupoint Stimulation. Consensus on perioperative application of acupoint stimulation. Chin J Anesthesiol. 2017;37:10 (in Chinese).
Chinese Society of Integrative Anesthesiology, Gansu Provincial Society of Integrative Anesthesiology. Clinical practice guidelines for acupoint stimulation as an adjuvant treatment of postoperative pain (2021). Chin J Anesthesiol. 2021;41:10 (in Chinese).
Acknowledgements
We thank all study participants for their involvement in the study, and all the principal investigators and clinical staff participating in the trial for their efforts. In particular, we would also like to thank the patients who will participate in this trial.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This work is supported by the key projects [2022]250, the Reserve Talents for Academic and Technical Leaders of Middle-aged and Young People in Yunnan Province (202305AC160069), Yunnan Provincial Ten Thousand Talents Program ‘Young Top Talent Project’ (YNWR-QNBJ-2019-184). The Rapid Service Fee was funded by two projects, the key projects [2022]250 and the Reserve Talents for Academic and Technical Leaders of Middle-aged and Young People in Yunnan Province (202305AC160069).
Author information
Authors and Affiliations
Contributions
Na Li is the general supervisor for this research, is a grant holder and has been responsible for the development of the protocol, implementation of the trial and drafting the protocol manuscript. Shuang Chen, Ying Ding, **aoming Zhang, Xue Zhang, Jiajia **ang, Yiling Deng, and **ngran Tao all contributed to the design and development of the study protocol. Shuang Chen and Ying Ding prepared the initial draft of the manuscript. Wenke Cai, Zhigui Li, and Fanyi Kong were responsible for designing statistical procedures and contributed to protocol development. Jiayu Chen provided clinical expertise and also contributed to protocol development and is a grant holder. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Shuang Chen, Ying Ding, **aoming Zhang, Xue Zhang, Jiajia **ang, Yiling Deng, **ngran Tao, Wenke Cai, Zhigui Li, Jiayu Chen, Fanyi Kong, and Na Li declare that they have no financial or other conflict of interest.
Ethical Approval
The trial follows the principles of the Declaration of Helsinki. The study has been approved by the Ethics Committee of the 920th Hospital of Joint Logistics Support Force, approval No. of the Ethic Committee: 2022-106(k)-01. The researchers will follow GCP principles and the approved protocol to implement clinical research and protect the health and rights of each patient. All participants will sign an informed consent before starting the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, S., Ding, Y., Zhang, X. et al. Efficacy of Transcutaneous Electrical Acupoint Stimulation on Chronic Postsurgical Pain After Video-Assisted Thoracoscopic Lobectomy: Study Protocol for a Prospective Randomized Controlled Trial. Pain Ther 13, 269–280 (2024). https://doi.org/10.1007/s40122-024-00580-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00580-y