Abstract
Background
Targeting glycolysis in cancer is an attractive approach for therapeutic intervention. 2-Deoxyglucose (2DG) is a synthetic glucose analog that inhibits glycolysis. However, its efficacy is limited by the systemic toxicity at high doses. Understanding the mechanism of 2DG resistance is important for further use of this drug in cancer treatment.
Methods
The expression of thioredoxin-1 (Trx-1) in colorectal cancer (CRC) cells treated with 2DG was detected by Western blotting. The effect of Trx-1 on the cytotoxicity of 2DG in CRC cells was examined in vitro and in vivo. The molecular mechanism involved in Trx-1-mediated activation of the SLC1A5 gene promoter activity was elucidated using in vitro models.
Results
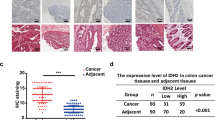
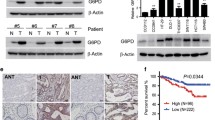
Inhibition glycolysis with 2DG increased the expression of Trx-1 in CRC cells. Overexpression of Trx-1 decreased the cytotoxicity of 2DG, whereas knockdown of Trx-1 by shRNA significantly increased the cytotoxicity of 2DG in CRC cells. The Trx-1 inhibitor PX-12 increased the cytotoxicity of 2DG on CRC cells both in vitro and in vivo. In addition, Trx-1 promoted SLC1A5 expression by increasing the promoter activity of the SLC1A5 gene by binding to SP1. We also found that the SLC1A5 expression was upregulated in CRC tissues, and inhibition of SLC1A5 significantly enhanced the inhibitory effect of 2DG on the growth of CRC cells in vitro and in vivo. Overexpression of SLC1A5 reduced the cytotoxicity of 2DG in combination with PX-12 treatment in CRC cells.
Conclusion
Our results demonstrate a novel adaptive mechanism of glycolytic inhibition in which Trx-1 increases GSH levels by regulating SLC1A5 to rescue cytotoxicity induced by 2DG in CRC cells. Inhibition of glycolysis in combination with inhibition of Trx-1 or SLC1A5 may be a promising strategy for the treatment of CRC.







Similar content being viewed by others
Data availability
The datasets analysed during the current research are available from the corresponding author on reasonable request.
References
I. Soerjomataram, J. Lortet-Tieulent, D.M. Parkin, J. Ferlay, C. Mathers, D. Forman, F. Bray, Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 380, 1840–1850 (2012). https://doi.org/10.1016/s0140-6736(12)60919-2
L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019). https://doi.org/10.3322/caac.21551
D. Hanahan, R.A. Weinberg, Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). https://doi.org/10.1016/j.cell.2011.02.013
S. Ganapathy-Kanniappan, J.F.H. Geschwind, Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol. Cancer 12, 152 (2013). https://doi.org/10.1186/1476-4598-12-152
S. Dey, N. Murmu, T. Mondal, I. Saha, S. Chatterjee, R. Manna, S. Haldar, S.K. Dash, T.R. Sarkar, B. Giri, Multifaceted entrancing role of glucose and its analogue, 2-deoxy-D-glucose in cancer cell proliferation, inflammation, and virus infection. Biomed. Pharmacother. 156, 113801 (2022). https://doi.org/10.1016/j.biopha.2022.113801
Y. Guan, W. Yao, H. Yu, Y. Feng, Y. Zhao, X. Zhan, Y. Wang, Chronic stress promotes colorectal cancer progression by enhancing glycolysis through beta2-AR/CREB1 signal pathway. Int. J. Biol. Sci. 19, 2006–2019 (2023). https://doi.org/10.7150/ijbs.79583
T. MaruYama, H. Miyazaki, Y.J. Lim, J. Gu, M. Ishikawa, T. Yoshida, W. Chen, Y. Owada, H. Shibata, Pyrolyzed deketene curcumin controls regulatory T cell generation and gastric cancer metabolism cooperate with 2-deoxy-d-glucose. Front. Immunol. 14, 1049713 (2023). https://doi.org/10.3389/fimmu.2023.1049713
M. Sivasubramanian, C.H. Chu, Y. Hsia, N.T. Chen, M.T. Cai, L.S. Tew, Y.C. Chuang, C.T. Chen, B. Aydogan, L.D. Liao, L.W. Lo, Illuminating and radiosensitizing tumors with 2DG-bound gold-based nanomedicine for targeted CT imaging and therapy. Nanomaterials (Basel) 13, 1790 (2023). https://doi.org/10.3390/nano13111790
F. Lu, D. Fang, S. Li, Z. Zhong, X. Jiang, Q. Qi, Y. Liu, W. Zhang, X. Xu, Y. Liu, W. Zhu, L. Jiang, Thioredoxin 1 supports colorectal cancer cell survival and promotes migration and invasion under glucose deprivation through interaction with G6PD. Int. J. Biol. Sci. 18, 5539–5553 (2022). https://doi.org/10.7150/ijbs.71809
S. Yang, J. Zhao, X. Cui, Q. Zhan, K. Yi, Q. Wang, M. **ao, Y. Tan, B. Hong, C. Fang, C. Kang, TCA-phospholipid-glycolysis targeted triple therapy effectively suppresses ATP production and tumor growth in glioblastoma. Theranostics 12, 7032–7050 (2022). https://doi.org/10.7150/thno.74197
S. Lampaki, G. Lazaridis, K. Zarogoulidis, I. Kioumis, A. Papaiwannou, K. Tsirgogianni, A. Karavergou, T. Tsiouda, V. Karavasilis, L. Yarmus, K. Darwiche, L. Freitag, A. Sakkas, A. Kantzeli, S. Baka, W. Hohenforst-Schmidt, P. Zarogoulidis, Defining the role of tyrosine kinase inhibitors in early stage non-small cell lung cancer. J. Cancer 6, 568–574 (2015). https://doi.org/10.7150/jca.11893
C. Laussel, S. Leon, Cellular toxicity of the metabolic inhibitor 2-deoxyglucose and associated resistance mechanisms. Biochem. Pharmacol. 182, 114213 (2020). https://doi.org/10.1016/j.bcp.2020.114213
F. Lin, P. Zhang, Z. Zuo, F. Wang, R. Bi, W. Shang, A. Wu, J. Ye, S. Li, X. Sun, J. Wu, L. Jiang, Thioredoxin-1 promotes colorectal cancer invasion and metastasis through crosstalk with S100P. Cancer Lett. 401, 1–10 (2017). https://doi.org/10.1016/j.canlet.2017.04.036
W. Shang, Z. **e, F. Lu, D. Fang, T. Tang, R. Bi, L. Chen, L. Jiang, Jiang, increased Thioredoxin-1 expression promotes cancer progression and predicts poor prognosis in patients with gastric cancer. Oxid. Med. Cell. Longev. 2019, 9291683 (2019). https://doi.org/10.1155/2019/9291683
S.J. Welsh, W.T. Bellamy, M.M. Briehl, G. Powis, The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 62, 5089–5095 (2002)
J. Muri, H. Thut, Q. Feng, M. Kopf, Thioredoxin-1 distinctly promotes NF-kappaB target DNA binding and NLRP3 inflammasome activation independently of Txnip. Elife 9, e53627 (2020). https://doi.org/10.7554/eLife.53627
Z. Zuo, P. Zhang, F. Lin, W. Shang, R. Bi, F. Lu, J. Wu, L. Jiang, Interplay between Trx-1 and S100P promotes colorectal cancer cell epithelial-mesenchymal transition by up-regulating S100A4 through AKT activation. J. Cell. Mol. Med. 22, 2430–2441 (2018). https://doi.org/10.1111/jcmm.13541
M. Chen, Y. Pan, H. Liu, F. Ning, Q. Lu, Y. Duan, X. Gan, S. Lu, H. Hou, M. Zhang, Y. Tian, G.E. Lash, Ezrin accelerates breast cancer liver metastasis through promoting furin-like convertase-mediated cleavage of Notch1. Cell. Oncol. (Dordr.) 46, 571–587 (2023). https://doi.org/10.1007/s13402-022-00761-x
K.L. Bloomfield, S.A. Osborne, D.D. Kennedy, F.M. Clarke, K.F. Tonissen, Thioredoxin-mediated redox control of the transcription factor Sp1 and regulation of the thioredoxin gene promoter. Gene 319, 107–116 (2003). https://doi.org/10.1016/s0378-1119(03)00799-6
S. Paul, S. Ghosh, S. Kumar, Tumor glycolysis, an essential sweet tooth of tumor cells. Semin. Cancer Biol. 86, 1216–1230 (2022). https://doi.org/10.1016/j.semcancer.2022.09.007
X. Zhong, X. He, Y. Wang, Z. Hu, H. Huang, S. Zhao, P. Wei, D. Li, Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications. J. Hematol. Oncol. 15, 160 (2022). https://doi.org/10.1186/s13045-022-01358-5
C.R. Kennedy, S.B. Tilkens, H. Guan, J.A. Garner, P.M. Or, A.M. Chan, Differential sensitivities of glioblastoma cell lines towards metabolic and signaling pathway inhibitions. Cancer Lett. 336, 299–306 (2013). https://doi.org/10.1016/j.canlet.2013.03.020
Z. Luo, J. Xu, J. Sun, H. Huang, Z. Zhang, W. Ma, Z. Wan, Y. Liu, A. Pardeshi, S. Li, Li, Co-delivery of 2-Deoxyglucose and a glutamine metabolism inhibitor V9302 via a prodrug micellar formulation for synergistic targeting of metabolism in cancer. Acta Biomater. 105, 239–252 (2020). https://doi.org/10.1016/j.actbio.2020.01.019
T. Zhang, X. Zhu, H. Wu, K. Jiang, G. Zhao, A. Shaukat, G. Deng, C. Qui, Targeting the ROS/PI3K/AKT/HIF-1alpha/HK2 axis of breast cancer cells: Combined administration of Polydatin and 2-Deoxy-d-glucose. J. Cell. Mol. Med. 23, 3711–3723 (2019). https://doi.org/10.1111/jcmm.14276
D. Zhang, Q. Fei, J. Li, C. Zhang, Y. Sun, C. Zhu, F. Wang, Y. Sun, Sun, 2-Deoxyglucose reverses the promoting effect of insulin on colorectal cancer cells in vitro. PLoS One 11, e0151115 (2016). https://doi.org/10.1371/journal.pone.0151115
S. Singh, S. Pandey, A.S. Chawla, A.N. Bhatt, B.G. Roy, D. Saluja, B.S. Dwarakanath, Dietary 2-deoxy-D-glucose impairs tumour growth and metastasis by inhibiting angiogenesis. Eur. J. Cancer 123, 11–24 (2019). https://doi.org/10.1016/j.ejca.2019.09.005
R. Pusapati, J. Settleman, TORquing metabolic reprogramming in cancer cells. Cell Cycle 15, 2387–2388 (2016). https://doi.org/10.1080/15384101.2016.1204850
L. Li, M.A. Fath, P.M. Scarbrough, W.H. Watson, D.R. Spitz, Combined inhibition of glycolysis, the pentose cycle, and thioredoxin metabolism selectively increases cytotoxicity and oxidative stress in human breast and prostate cancer. Redox Biol. 4, 127–135 (2015). https://doi.org/10.1016/j.redox.2014.12.001
R. Rashmi, X. Huang, J.M. Floberg, A.E. Elhammali, M.L. McCormick, G.J. Patti, D.R. Spitz, J.K. Schwarz, Radioresistant cervical cancers are sensitive to inhibition of glycolysis and redox metabolism. Cancer Res. 78, 1392–1403 (2018). https://doi.org/10.1158/0008-5472.Can-17-2367
H.C. May, J.J. Yu, M.N. Guentzel, J.P. Chambers, A.P. Cap, B.P. Arulanandam, Repurposing auranofin, ebselen, and PX-12 as antimicrobial agents targeting the thioredoxin system. Front. Microbiol. 9, 336 (2018). https://doi.org/10.3389/fmicb.2018.00336
F. Wang, F. Lin, P. Zhang, W. Ni, L. Bi, J. Wu, L. Jiang, Thioredoxin-1 inhibitor, 1-methylpropyl 2-imidazolyl disulfide, inhibits the growth, migration and invasion of colorectal cancer cell lines. Oncol. Rep. 33, 967–973 (2015). https://doi.org/10.3892/or.2014.3652
B.J. Altman, Z.E. Stine, C.V. Dang, From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016). https://doi.org/10.1038/nrc.2016.71
T. Hensley, A.T. Wasti, R.J. DeBerardinis, Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest. 123, 3678–3684 (2013). https://doi.org/10.1172/jci69600
C. Gorrini, I.S. Harris, T.W. Mak, Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 (2013). https://doi.org/10.1038/nrd4002
D.A. Scott, A.D. Richardson, F.V. Filipp, C.A. Knutzen, G.G. Chiang, Z.A. Ronai, A.L. Osterman, J.W. Smith, Comparative metabolic flux profiling of melanoma cell lines: beyond the Warburg effect. J. Biol. Chem. 286, 42626–42634 (2011). https://doi.org/10.1074/jbc.M111.282046
J. Jeon, S. Khelifa, B. Ratnikov, D.A. Scott, Y. Feng, F. Parisi, C. Ruller, E. Lau, H. Kim, L.M. Brill, T. Jiang, D.L. Rimm, R.D. Cardiff, G.B. Mills, J.W. Smith, A.L. Osterman, Y. Kluger, Z.A. Ronai, Regulation of glutamine carrier proteins by RNF5 determines breast cancer response to ER stress-inducing chemotherapies. Cancer Cell 27, 354–369 (2015). https://doi.org/10.1016/j.ccell.2015.02.006
N.P. Shanware, A.R. Mullen, R.J. DeBerardinis, R.T. Abraham, Glutamine: pleiotropic roles in tumor growth and stress resistance. J. Mol. Med. (Berl.) 89, 229–236 (2011). https://doi.org/10.1007/s00109-011-0731-9
M. van Geldermalsen, Q. Wang, R. Nagarajah, A.D. Marshall, A. Thoeng, D. Gao, W. Ritchie, Y. Feng, C.G. Bailey, N. Deng, K. Harvey, J.M. Beith, C.I. Selinger, S.A. O’Toole, J.E. RaskoJ, J. Holst, ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 35, 3201–3208 (2016). https://doi.org/10.1038/onc.2015.381
F. Huang, Y. Zhao, J. Zhao, S. Wu, Y. Jiang, H. MaT, T. Zhang, Upregulated SLC1A5 promotes cell growth and survival in colorectal cancer. Int. J. Clin. Exp. Pathol. 7, 6006–6014 (2014)
M. Hassanein, M.D. Hoeksema, M. Shiota, J. Qian, B.K. Harris, H. Chen, J.E. Clark, W.E. Alborn, R. Eisenberg, P.P. Massion, SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin. Cancer Res. 19, 560–570 (2013). https://doi.org/10.1158/1078-0432.Ccr-12-2334
L. Willems, N. Jacque, A. Jacquel, N. Neveux, T.T. Maciel, M. Lambert, A. Schmitt, L. Poulain, A.S. Green, M. Uzunov, O. Kosmider, I. Radford-Weiss, I.C. Moura, P. Auberger, N. Ifrah, V. Bardet, N. Chapuis, C. Lacombe, P. Mayeux, J. Tamburini, D. Bouscary, Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood 122, 3521–3532 (2013). https://doi.org/10.1182/blood-2013-03-493163
H. Lu, X. Li, Y. Lu, S. Qiu, Z. Fan, ASCT2 (SLC1A5) is an EGFR-associated protein that can be co-targeted by cetuximab to sensitize cancer cells to ROS-induced apoptosis. Cancer Lett. 381, 23–30 (2016). https://doi.org/10.1016/j.canlet.2016.07.020
R. Farina, L. Cappabianca, G. DeSantis, N. Di Ianni, P. Ruggeri, M. Ragone, S. Merolle, K.F. Tonissen, A. Gulino, A.R. Mackay, Thioredoxin stimulates MMP-9 expression, de-regulates the MMP-9/TIMP-1 equilibrium and promotes MMP-9 dependent invasion in human MDA-MB-231 breast cancer cells. FEBS Lett. 585, 3328–3336 (2011). https://doi.org/10.1016/j.febslet.2011.09.023
L. Console, M. Scalise, Z. Tarmakova, I.R. Coe, C. Indiveri, N-linked glycosylation of human SLC1A5 (ASCT2) transporter is critical for trafficking to membrane. Biochim. Biophys. Acta 1853, 1636–1645 (2015). https://doi.org/10.1016/j.bbamcr.2015.03.017
M. Scalise, L. Pochini, L. Console, M.A. Losso, C. Indiveri, The human SLC1A5 (ASCT2) amino acid transporter: From function to structure and role in cell biology. Front Cell Dev Biol 6, 96 (2018). https://doi.org/10.3389/fcell.2018.00096
F. Polet, R. Martherus, C. Corbet, A. Pinto, O. Feron, Inhibition of glucose metabolism prevents glycosylation of the glutamine transporter ASCT2 and promotes compensatory LAT1 upregulation in leukemia cells. Oncotarget 7, 46371–46383 (2016). https://doi.org/10.18632/oncotarget.10131
M. Scalise, L. Pochini, L. Console, M.A. Losso, C. Indiveri, The human SLC1A5 (ASCT2) amino acid transporter: From function to structure and role in cell biology. Front. Cell Dev. Biol. 6, 96 (2018). https://doi.org/10.3389/fcell.2018.00096
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82272652, 82172816), Zhejiang Provincial Natural Science Foundation of China (No. LY22H160021), Key funding for Wenzhou High-level Talent Innovation Technology Project.
Author information
Authors and Affiliations
Contributions
T.T., L.J., X.S.: Conception and design, investigation. T.T., D.F.: Methodology. T.T., D.F., Z.J., Z.Z.: Acquisition of data. B.Z.: Data curation, software analysis. T.T., L.J., Z.J.: Writing, review, and/or revision of manuscript. L.Y., L.J., X.S.: Study supervision, funding acquisition.
Corresponding authors
Ethics declarations
Ethics statement
All animal experiments in this study were performed under the institutional ethical guidelines approved by the Animal Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Competing interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, T., Fang, D., Ji, Z. et al. Inhibition of thioredoxin-1 enhances the toxicity of glycolysis inhibitor 2-deoxyglucose by downregulating SLC1A5 expression in colorectal cancer cells. Cell Oncol. 47, 607–621 (2024). https://doi.org/10.1007/s13402-023-00887-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00887-6