Abstract
Recent genome-wide association studies of Huntington’s disease (HD) primarily highlighted genes involved in DNA damage repair mechanisms as modifiers of age at onset and disease severity, consistent with evidence that more DNA repair genes are being implicated in late age–onset neurodegenerative diseases. This provides an exciting opportunity to advance therapeutic development in HD, as these pathways have already been under intense investigation in cancer research. Also emerging are the roles of other polyglutamine disease proteins in DNA damage repair mechanisms. A potential universal trigger of oxidative DNA damage shared in these late age–onset diseases is the increase of reactive oxygen species (ROS) in human aging, defining an age-related mechanism that has defied other hypotheses of neurodegeneration. We discuss the potential commonality of DNA damage repair pathways in HD and other neurodegenerative diseases. Potential targets for therapy that may prove beneficial across many of these diseases are also identified, defining nodes in the ataxia telangiectasia-mutated (ATM) complex, mismatch repair, and poly ADP-ribose polymerases (PARPs).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
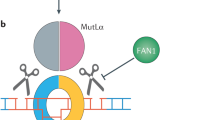
Huntington’s disease (HD) was precisely defined in 1993 when the huntingtin gene was cloned and the disease-causing CAG nucleotide expansion was defined, showing an inverse correlation between expansion length and age at onset [1]. The problem of HD was daunting, as the gene encoded one of the largest non-membrane proteins in the human proteome at 3144 amino acids or 350 kDa. Over the next 25 years, the normal function of huntingtin also eluded strict definition. Cell biology and biochemical studies revealed the protein to have no enzymatic activity and no conserved functional motifs, with the exception that proteins with polyglutamine tracts were often transcriptionally active and huntingtin contained HEAT repeats common to several protein scaffolds [2, 3]. Interactome studies showed huntingtin at the heart of several complexes [4] and it has been localized to the nucleus [5], endoplasmic reticulum [ The mismatch repair (MMR) pathway is of particular interest because of its influence on somatic expansion of CAG repeats, which can undergo progressive length increases over time, particularly in the brain [46]. Somatic expansion of huntingtin is associated with earlier age of HD onset [47] and more severe symptoms [48]. In mouse models, expansion is prevented upon deletion of MMR genes, mutS homologs 2 and 3 (msh2 and msh3), mutL homolog 1 (mlh1), or PMS1 homolog 2 (pms2) [49,50,51,52]. These proteins normally work in concert to repair insertion-deletion loops in the DNA, but they also drive disease-associated repeat expansions [53]. The initial evidence from mouse models has since been bolstered by human data, clearly implicating the MMR pathway in disease progression. Although the chromosomal region bearing MLH1 was identified by GWAS, but did not initially reach genome-wide significance [17], a subsequent SNP genoty** study confirmed a modifier haplotype at the MLH1 locus [21]. The genomic region bearing MSH3 was identified by a second GWAS with more in-depth measures of disease progression [18], and PMS2 was among the SNP locations independently confirmed by genoty** [20]. Disease-associated SNPs near the MLH1, MSH3, PMS2, and PMS1 loci have recently been confirmed and the importance of uninterrupted CAG repeats within the huntingtin gene itself, which also impacts somatic instability, is now clear [19, 23]. PMS2 and MSH3 have also been implicated in SCA1 and myotonic dystrophy, respectively [20, 22]. Modifier genes are summarized in Table 1. Although it is likely that MMR proteins influence pathology via somatic expansion, this may not be the sole mechanism, as tissue specificity of somatic expansion correlates with striatal neurodegeneration in HD, but not in SCAs [54], despite ubiquitous transcription of the CAG-containing proteins. It is possible that MMR proteins also contribute to disease modification through other DNA repair factors, including TP53 [55], breast cancer associated-1 (BRCA1) [56], and ATM [57]. The MMR machinery has also been implicated in the repair of several types of DNA damage [58,59,60], including ICLs [61]. In the patient population, pathology is likely to be caused by a combination of these mechanisms. The fact that MMR proteins, and scaffolds such as huntingtin and ATM are common components of many DNA repair complexes [91]. The relevance of PARylation to HD has yet to be determined, but from GWAS we may have a hint that ties hyper-PARylation to the ATM/huntingtin complex. Another major lead in HD GWAS is the ribonucleoside-diphosphate reductase subunit M2B, or RRM2B, also known as P53R2. RRM2B is a critical ribonucleotide salvager which nets a severe fatal pediatric disorder called mitochondrial DNA depletion syndrome (MDDS) that affects muscle, the brain, and the respiratory tract when null [92]. Like huntingtin, RRM2B is activated by the TP53 tumor suppressor [93], and is a known ATM interactor [94]. The ribonucleoside-diphosphate reductase activity could be critical downstream of PARG to salvage ADP back from hydrolyzed PAR chains during neuronal energy crisis. Thus, we can hypothesize a mechanism of RRM2B disease modification by catalyzing the conversion of poly ADP-ribose chains back to critical adenosine ribonucleosides. Given the significance of HD genetic modifier SNPs to some spinocerebellar ataxias, we may find an intersection of molecular mechanisms of disease between HD and other CAG repeat disorders with respect to DNA repair. One node is the ATM complex, but huntingtin has also been defined at the TCR complex [45] along with ataxin-3, the affected protein in SCA3, and PKNP, the protein mutated in MCSZ [27]. Ataxin-3 has also been implicated by others in the double-strand break response [95]. CAG expansion in the androgen receptor, a transcription factor involved in DNA damage repair signaling, leads to spinal and bulbar muscle atrophy, SBMA, or Kennedy’s disease [96, 97]. CAG expansion in the TATA box-binding protein (TBP) causes SCA17, and TBP localizes to damaged DNA [98]. DNA repair has also been implicated in SCA1, as overexpression of DNA repair factors replication protein A1 and high mobility group box 1 in mouse and Drosophila models corrects motor phenotypes [99, 100]. Thus, we may anticipate increased relevance of DNA repair in many late age–onset neurodegenerative diseases, as the natural increased ROS stress during human aging and effects on DNA/RNA oxidation are tempting mechanisms to explain why these diseases typically occur later in life. Increased DNA oxidation and hyper-PARylation appeared in neurodegenerative disease studies in the late 1990s [101, 102], but the early study of PAR chains and relevance of oxidation to disease mechanism were not further explored in favor of various amyloid hypotheses of late age–onset neurodegeneration. Although guanine oxidation products were the focus of these studies as a biomarker of DNA oxidation, it is not clear that all DNA bases are equally modified under oxidative stress nor processed in a similar manner. Adenosine bases are subject to nucleotide salvage in neurons and have unique utility after base excision repair to be salvaged back to adenosine nucleosides for energy production [85]. Neurons are highly metabolically active and thus generate high ROS loads, yet oxidative base damage to DNA cannot be repaired by DNA replication as in mitotic cell types. Brain subregions can also transiently flip to aerobic glycolysis or Warburg metabolism to generate ATP at times of energy stress [103], but this energy supply comes at a high cost of increased ROS stress. The burden of reactive oxygen levels on mitochondria, which have a diminished efficiency with human aging, might explain how mitochondrial dysfunction is a common aspect of all neurodegenerative diseases [104]. To fully understand the implications of defective DNA repair in neurodegeneration, it is important to understand how DNA repair imparts a severe energy stress on neurons. High rates of neuronal metabolism mean that even as energy is depleted, ROS by-products cause damage that further drains the energy supply. In this way, neurons must struggle to maintain energy levels and even a minor deficiency in a DNA repair protein would amount to undue neuronal death over time (Fig. 2). The involvement of DNA repair pathways in neurodegenerative diseases presents a number of opportunities for therapeutic intervention. Although drugs against key potential targets have been developed for cancer, preclinical data on their applicability for neurodegenerative diseases is needed as the goal of anticancer therapy is to kill affected cells, whereas the goal of antineurodegeneration therapy is to save affected cells. The immediate goals in researching DNA repair in neurodegeneration are to define the roles of DNA repair factors, in terms of their scaffolding versus enzymatic functions, to precisely define therapeutic mechanisms of either inhibiting enzymatic activity or modulating protein levels, or both. Testing in the most clinically relevant models and special attention to measures of genotoxicity will help pave the way to the clinic.Mismatch Repair and Somatic CAG Expansion
HD GWAS in the Bigger Picture of Polyglutamine Diseases
References
Macdonald M (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983
Andrade MA, Bork P (1995) HEAT repeats in the Huntington’s disease protein. Nat Genet 11:115–116
Takano H, Gusella JF (2002) The predominantly HEAT-like motif structure of huntingtin and its association and coincident nuclear entry with dorsal, an NF-kB/Rel/dorsal family transcription factor. BMC Neurosci 3:15
Brandi V, Di Lella V, Marino M, Ascenzi P, Polticelli F (2018) A comprehensive in silico analysis of huntingtin and its interactome. J Biomol Struct Dyn 36:3155–3171
Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90:537–548
Atwal RS, **a J, Pinchev D, Taylor J, Epand RM, Truant R (2007) Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Hum Mol Genet 16:2600–2615
Kim M, Velier J, Chase K, Laforet G, Kalchman MA, Hayden MR, Won L, Heller A, Aronin N, Difiglia M (1999) Forskolin and dopamine D1 receptor activation increase huntingtin’s association with endosomes in immortalized neuronal cells of striatal origin. Neuroscience 89:1159–1167
Marcora E, Gowan K, Lee JE (2003) Stimulation of NeuroD activity by huntingtin and huntingtin-associated proteins HAP1 and MLK2. Proc Natl Acad Sci U S A 100:9578–9583
Atwal RS, Desmond CR, Caron N, Maiuri T, **a J, Sipione S, Truant R (2011) Kinase inhibitors modulate huntingtin cell localization and toxicity. Nat Chem Biol 7:453–460
Godin JD, Colombo K, Molina-Calavita M, et al. (2010) Huntingtin is required for mitotic spindle orientation and mammalian neurogenesis. Neuron 67:392–406
Maiuri T, Woloshansky T, **a J, Truant R (2013) The huntingtin N17 domain is a multifunctional CRM1 and Ran-dependent nuclear and cilial export signal. Hum Mol Genet 22:1383–1394
Keryer G, Pineda JR, Liot G, et al. (2011) Ciliogenesis is regulated by a huntingtin-HAP1-PCM1 pathway and is altered in Huntington disease. J Clin Invest 121:4372–4382
Truant R, Atwal RS, Desmond C, Munsie L, Tran T (2008) Huntington’s disease: revisiting the aggregation hypothesis in polyglutamine neurodegenerative diseases. FEBS J 275:4252–4262
Castellani RJ, Smith MA (2011) Compounding artefacts with uncertainty, and an amyloid cascade hypothesis that is “too big to fail.”. J Pathol 224:147–152
Mangiarini L, Sathasivam K, Seller M, et al. (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493–506
Myers RH (2004) Huntington’s disease genetics. NeuroRx 1:255–262
Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium (2015) Identification of Genetic Factors that Modify Clinical Onset of Huntington’s Disease. Cell 162:516–526
Moss DJH, Pardiñas AF, Langbehn D, et al. (2017) Identification of genetic variants associated with Huntington’s disease progression: a genome-wide association study. Lancet Neurol 16:701–711
Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium, Lee J-M, Correia K, et al. (2019) Huntington’s disease onset is determined by length of uninterrupted CAG, not encoded polyglutamine, and is modified by DNA maintenance mechanisms. bioRxiv 529768
Bettencourt C, Hensman-Moss D, Flower M, et al. (2016) DNA repair pathways underlie a common genetic mechanism modulating onset in polyglutamine diseases. Ann Neurol 79:983–990
Lee J-M, Chao MJ, Harold D, et al. (2017) A modifier of Huntington’s disease onset at the MLH1 locus. Hum Mol Genet 26:3859–3867
Morales F, Vásquez M, Santamaría C, Cuenca P, Corrales E, Monckton DG (2016) A polymorphism in the MSH3 mismatch repair gene is associated with the levels of somatic instability of the expanded CTG repeat in the blood DNA of myotonic dystrophy type 1 patients. DNA Repair 40:57–66
Wright GEB, Collins JA, Kay C, et al. (2019) Length of Uninterrupted CAG, Independent of Polyglutamine Size, Results in Increased Somatic Instability, Hastening Onset of Huntington Disease. Am J Hum Genet 104:1116–1126
Jiang B, Glover JNM, Weinfeld M (2017) Neurological disorders associated with DNA strand-break processing enzymes. Mech Ageing Dev 161:130–140
Takashima H, Boerkoel CF, John J, et al. (2002) Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat Genet 32:267–272
Moreira MC, Barbot C, Tachi N, et al. (2001) The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat Genet 29:189–193
Shen J, Gilmore EC, Marshall CA, et al. (2010) Mutations in PNKP cause microcephaly, seizures and defects in DNA repair. Nat Genet 42:245–249
Bras J, Alonso I, Barbot C, Costa MM, Darwent L, Orme T, Sequeiros J, Hardy J, Coutinho P, Guerreiro R (2015) Mutations in PNKP cause recessive ataxia with oculomotor apraxia type 4. Am J Hum Genet 96:474–479
Gao R, Liu Y, Silva-Fernandes A, et al. (2015) Inactivation of PNKP by mutant ATXN3 triggers apoptosis by activating the DNA damage-response pathway in SCA3. PLoS Genet 11:e1004834
Hoch NC, Hanzlikova H, Rulten SL, et al. (2017) XRCC1 mutation is associated with PARP1 hyperactivation and cerebellar ataxia. Nature 541:87–91
Amirifar P, Ranjouri MR, Yazdani R, Abolhassani H, Aghamohammadi A (2019) Ataxia-telangiectasia: A review of clinical features and molecular pathology. Pediatr Allergy Immunol. https://doi.org/10.1111/pai.13020
Stagni V, Cirotti C, Barilà D (2018) Ataxia-Telangiectasia Mutated Kinase in the Control of Oxidative Stress, Mitochondria, and Autophagy in Cancer: A Maestro With a Large Orchestra. Front Oncol 8:73
Paules RS, Levedakou EN, Wilson SJ, Innes CL, Rhodes N, Tlsty TD, Galloway DA, Donehower LA, Tainsky MA, Kaufmann WK (1995) Defective G2 checkpoint function in cells from individuals with familial cancer syndromes. Cancer Res 55:1763–1773
Cheng Q, Chen L, Li Z, Lane WS, Chen J (2009) ATM activates p53 by regulating MDM2 oligomerization and E3 processivity. EMBO J 28:3857–3867
Lu X-H, Mattis VB, Wang N, et al. (2014) Targeting ATM ameliorates mutant Huntingtin toxicity in cell and animal models of Huntington’s disease. Sci Transl Med 6:268ra178
Giuliano P, De Cristofaro T, Affaitati A, Pizzulo GM, Feliciello A, Criscuolo C, De Michele G, Filla A, Avvedimento EV, Varrone S (2003) DNA damage induced by polyglutamine-expanded proteins. Hum Mol Genet 12:2301–2309
Illuzzi J, Yerkes S, Parekh-Olmedo H, Kmiec EB (2009) DNA breakage and induction of DNA damage response proteins precede the appearance of visible mutant huntingtin aggregates. J Neurosci Res 87:733–747
Maiuri T, Mocle AJ, Hung CL, **a J, van Roon-Mom WMC, Truant R (2017) Huntingtin is a scaffolding protein in the ATM oxidative DNA damage response complex. Hum Mol Genet 26:395–406
Askeland G, Dosoudilova Z, Rodinova M, et al. (2018) Increased nuclear DNA damage precedes mitochondrial dysfunction in peripheral blood mononuclear cells from Huntington’s disease patients. Sci Rep 8:9817
Castaldo I, De Rosa M, Romano A, et al. (2019) DNA damage signatures in peripheral blood cells as biomarkers in prodromal huntington disease. Ann Neurol 85:296–301
Elfawy HA, Das B (2019) Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci 218:165–184
DiGiovanni LF, Mocle AJ, **a J, Truant R (2016) Huntingtin N17 domain is a reactive oxygen species sensor regulating huntingtin phosphorylation and localization. Hum Mol Genet 25:3937–3945
Bowie LE, Maiuri T, Alpaugh M, et al. (2018) N6-Furfuryladenine is protective in Huntington’s disease models by signaling huntingtin phosphorylation. Proc Natl Acad Sci U S A 115:E7081–E7090
Hung CL-K, Maiuri T, Bowie LE, et al. (2018) A Patient-Derived Cellular Model for Huntington’s Disease Reveals Phenotypes at Clinically Relevant CAG Lengths. Mol Biol Cell 29(23):2809-2820. mbc E18090590
Gao R, Chakraborty A, Geater C, et al. (2019) Mutant huntingtin impairs PNKP and ATXN3, disrupting DNA repair and transcription. Elife. https://doi.org/10.7554/eLife.42988
Telenius H, Kremer B, Goldberg YP, Theilmann J, Andrew SE, Zeisler J, Adam S, Greenberg C, Ives EJ, Clarke LA (1994) Somatic and gonadal mosaicism of the Huntington disease gene CAG repeat in brain and sperm. Nat Genet 6:409–414
Swami M, Hendricks AE, Gillis T, Massood T, Mysore J, Myers RH, Wheeler VC (2009) Somatic expansion of the Huntington’s disease CAG repeat in the brain is associated with an earlier age of disease onset. Hum Mol Genet 18:3039–3047
Shelbourne PF, Keller-McGandy C, Bi WL, et al. (2007) Triplet repeat mutation length gains correlate with cell-type specific vulnerability in Huntington disease brain. Hum Mol Genet 16:1133–1142
Pinto RM, Dragileva E, Kirby A, et al. (2013) Mismatch repair genes Mlh1 and Mlh3 modify CAG instability in Huntington’s disease mice: genome-wide and candidate approaches. PLoS Genet 9:e1003930
Manley K, Shirley TL, Flaherty L, Messer A (1999) Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat Genet 23:471–473
Gomes-Pereira M, Fortune MT, Ingram L, McAbney JP, Monckton DG (2004) Pms2 is a genetic enhancer of trinucleotide CAG.CTG repeat somatic mosaicism: implications for the mechanism of triplet repeat expansion. Hum Mol Genet 13:1815–1825
Tomé S, Manley K, Simard JP, et al. (2013) MSH3 polymorphisms and protein levels affect CAG repeat instability in Huntington’s disease mice. PLoS Genet 9:e1003280
Schmidt MHM, Pearson CE (2016) Disease-associated repeat instability and mismatch repair. DNA Repair 38:117–126
Hashida H, Goto J, Kurisaki H, Mizusawa H, Kanazawa I (1997) Brain regional differences in the expansion of a CAG repeat in the spinocerebellar ataxias: dentatorubral-pallidoluysian atrophy, Machado-Joseph disease, and spinocerebellar ataxia type 1. Ann Neurol 41:505–511
Peters AC, Young LC, Maeda T, Tron VA, Andrew SE (2003) Mammalian DNA mismatch repair protects cells from UVB-induced DNA damage by facilitating apoptosis and p53 activation. DNA Repair 2:427–435
Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J (2000) BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev 14:927–939
Luo Y, Lin F-T, Lin W-C (2004) ATM-mediated stabilization of hMutL DNA mismatch repair proteins augments p53 activation during DNA damage. Mol Cell Biol 24:6430–6444
Meyers M, Hwang A, Wagner MW, Bruening AJ, Veigl ML, Sedwick WD, Boothman DA (2003) A role for DNA mismatch repair in sensing and responding to fluoropyrimidine damage. Oncogene 22:7376–7388
Arora S, Huwe PJ, Sikder R, et al. (2017) Functional analysis of rare variants in mismatch repair proteins augments results from computation-based predictive methods. Cancer Biol Ther 18:519–533
Siehler SY, Schrauder M, Gerischer U, Cantor S, Marra G, Wiesmüller L (2009) Human MutL-complexes monitor homologous recombination independently of mismatch repair. DNA Repair 8:242–252
Kato N, Kawasoe Y, Williams H, et al. (2017) Sensing and Processing of DNA Interstrand Crosslinks by the Mismatch Repair Pathway. Cell Rep 21:1375–1385
Limpose KL et al. BERing the burden of damage: Pathway crosstalk and posttranslational modification of base excision repair proteins regulate DNA damage management. - PubMed - NCBI. https://www.ncbi.nlm.nih.gov/pubmed/28629773. Accessed 24 Jun 2019
Fishel ML et al. DNA repair in neurons: so if they don’t divide what’s to repair? - PubMed - NCBI. https://www.ncbi.nlm.nih.gov/pubmed/16879837. Accessed 24 Jun 2019
Madabhushi R, Gao F, Pfenning AR, et al. (2015) Activity-Induced DNA Breaks Govern the Expression of Neuronal Early-Response Genes. Cell 161:1592–1605
** H, Cho Y (2017) Structural and functional relationships of FAN1. DNA Repair 56:135–143
Derheimer FA, Kevin Hicks J, Paulsen MT, Canman CE, Ljungman M (2009) Psoralen-Induced DNA Interstrand Cross-Links Block Transcription and Induce p53 in an Ataxia-Telangiectasia and Rad3-Related-Dependent Manner. Mol Pharmacol 75:599
Price NE, Johnson KM, Wang J, Fekry MI, Wang Y, Gates KS (2014) Interstrand DNA-DNA cross-link formation between adenine residues and abasic sites in duplex DNA. J Am Chem Soc 136:3483–3490
Zhao X-N, Usdin K (2018) FAN1 protects against repeat expansions in a Fragile X mouse model. DNA Repair 69:1–5
Goold R, Flower M, Moss DH, et al. (2019) FAN1 modifies Huntington’s disease progression by stabilizing the expanded HTT CAG repeat. Hum Mol Genet 28:650–661
Maiuri T, Bowie LE, Truant R (2019) DNA Repair Signaling of Huntingtin: The Next Link Between Late-Onset Neurodegenerative Disease and Oxidative DNA Damage. DNA Cell Biol 38:1–6
Aiken CT, Steffan JS, Guerrero CM, et al. (2009) Phosphorylation of threonine 3: implications for Huntingtin aggregation and neurotoxicity. J Biol Chem 284:29427–29436
Di Pardo A, Maglione V, Alpaugh M, et al. (2012) Ganglioside GM1 induces phosphorylation of mutant huntingtin and restores normal motor behavior in Huntington disease mice. Proc Natl Acad Sci U S A 109:3528–3533
Gu X, Greiner ER, Mishra R, Kodali R, Osmand A, Finkbeiner S, Steffan JS, Thompson LM, Wetzel R, Yang XW (2009) Serines 13 and 16 are critical determinants of full-length human mutant huntingtin induced disease pathogenesis in HD mice. Neuron 64:828–840
Barciszewski J, Mielcarek M, Stobiecki M, Siboska G, Clark BF (2000) Identification of 6-furfuryladenine (kinetin) in human urine. Biochem Biophys Res Commun 279:69–73
Barciszewski J, Siboska GE, Pedersen BO, Clark BF, Rattan SI (1997) A mechanism for the in vivo formation of N6-furfuryladenine, kinetin, as a secondary oxidative damage product of DNA. FEBS Lett 414:457–460
Wyszko E, Barciszewska MZ, Markiewicz M, Szymański M, Markiewicz WT, Clark BFC, Barciszewski J (2003) “Action-at-a distance” of a new DNA oxidative damage product 6-furfuryl-adenine (kinetin) on template properties of modified DNA. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression 1625:239–245
Carrette LLG, Madder A (2014) A synthetic oligonucleotide model for evaluating the oxidation and crosslinking propensities of natural furan-modified DNA. Chembiochem 15:103–107
Hertz NT, Berthet A, Sos ML, Thorn KS, Burlingame AL, Nakamura K, Shokat KM (2013) A Neo-Substrate that Amplifies Catalytic Activity of Parkinson’s-Disease-Related Kinase PINK1. Cell 154:737–747
Brace LE, Vose SC, Stanya K, et al. (2016) Increased oxidative phosphorylation in response to acute and chronic DNA damage. NPJ Aging Mech Dis 2:16022
Montenarh M (2016) Protein kinase CK2 in DNA damage and repair. Transl Cancer Res 5:49–63
Carrette LLG, Gyssels E, De Laet N, Madder A (2016) Furan oxidation based cross-linking: a new approach for the study and targeting of nucleic acid and protein interactions. Chem Commun 52:1539–1554
Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA (2010) NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci 30:2967–2978
Andrabi SA, Umanah GKE, Chang C, Stevens DA, Karuppagounder SS, Gagné J-P, Poirier GG, Dawson VL, Dawson TM (2014) Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc Natl Acad Sci U S A 111:10209–10214
Sasaki Y, Hozumi M, Fujimori H, Murakami Y, Koizumi F, Inoue K, Masutani M (2016) PARG Inhibitors and Functional PARG Inhibition Models. Curr Protein Pept Sci 17:641–653
Fasullo M, Endres L (2015) Nucleotide salvage deficiencies, DNA damage and neurodegeneration. Int J Mol Sci 16:9431–9449
Andrabi SA, Dawson TM, Dawson VL (2008) Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci 1147:233–241
Kam T-I, Mao X, Park H, et al. (2018) Poly(ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson’s disease. Science. https://doi.org/10.1126/science.aat8407
Dahl J-U, Gray MJ, Jakob U (2015) Protein quality control under oxidative stress conditions. J Mol Biol 427:1549–1563
Weids AJ, Ibstedt S, Tamás MJ, Grant CM (2016) Distinct stress conditions result in aggregation of proteins with similar properties. Sci Rep 6:24554
Berger NA, Besson VC, Boulares AH, et al. (2018) Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br J Pharmacol 175:192–222
Levin AA (2019) Treating Disease at the RNA Level with Oligonucleotides. N Engl J Med 380:57–70
Gorman GS, Pitceathly RDS, Turnbull DM, Taylor RW (2013) RRM2B-Related Mitochondrial Disease. Mitochondrial Disorders Caused by Nuclear Genes 171–182
Cho EC, Yen Y (2016) Novel regulators and molecular mechanisms of p53R2 and its disease relevance. Biochimie 123:81–84
Chang L, Zhou B, Hu S, Guo R, Liu X, Jones SN, Yen Y (2008) ATM-mediated serine 72 phosphorylation stabilizes ribonucleotide reductase small subunit p53R2 protein against MDM2 to DNA damage. Proc Natl Acad Sci U S A 105:18519–18524
Pfeiffer A, Luijsterburg MS, Acs K, et al. (2017) Ataxin-3 consolidates the MDC1-dependent DNA double-strand break response by counteracting the SUMO-targeted ubiquitin ligase RNF4. EMBO J 36:1066–1083
Thompson TC, Li L (2017) Connecting androgen receptor signaling and the DNA damage response: Development of new therapies for advanced prostate cancer. Mol Cell Oncol 4:e1321167
Cortes CJ, La Spada AR (2018) X-Linked Spinal and Bulbar Muscular Atrophy: From Clinical Genetic Features and Molecular Pathology to Mechanisms Underlying Disease Toxicity. Adv Exp Med Biol 1049:103–133
Vichi P, Coin F, Renaud JP, Vermeulen W, Hoeijmakers JH, Moras D, Egly JM (1997) Cisplatin- and UV-damaged DNA lure the basal transcription factor TFIID/TBP. EMBO J 16:7444–7456
Taniguchi JB, Kondo K, Fujita K, et al. (2016) RpA1 ameliorates symptoms of mutant ataxin-1 knock-in mice and enhances DNA damage repair. Hum Mol Genet 25:4432–4447
Ito H, Fujita K, Tagawa K, et al. (2015) HMGB1 facilitates repair of mitochondrial DNA damage and extends the lifespan of mutant ataxin-1 knock-in mice. EMBO Mol Med 7:78–101
Gabbita SP, Lovell MA, Markesbery WR (1998) Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J Neurochem 71:2034–2040
Love S, Barber R, Wilcock GK (1999) Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer’s disease. Brain 122 ( Pt 2):247–253
Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME (2010) Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A 107:17757–17762
Murphy MP, Hartley RC (2018) Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. https://doi.org/10.1038/nrd.2018.174
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 499 kb)
Rights and permissions
About this article
Cite this article
Maiuri, T., Suart, C.E., Hung, C.L.K. et al. DNA Damage Repair in Huntington’s Disease and Other Neurodegenerative Diseases. Neurotherapeutics 16, 948–956 (2019). https://doi.org/10.1007/s13311-019-00768-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-019-00768-7