Abstract
In recent years, different biomaterials have garnered more research attention due to their usefulness as adsorbents. The present study focuses on a chemical treatment process to improve the adsorption capacity of betel nut husk fibers for a textile effluent (methylene blue). The fibers of chemically modified material were assessed using Fourier transform infrared (FTIR) spectrometer and Brunauer–Emmett–Teller (BET) analyzer to determine the existing surface functional groups and surface area, respectively. Parameters including contact time, dye concentration, temperature, effects of pH and desorption efficiency were also evaluated to identify optimum adsorption performance. Adsorption followed the Freundlich isotherm model and pseudo-first-order kinetics, indicating physisorption was responsible for adsorption and its occurrence on multilayers. Adsorption capacity was 149.921 mg/g, 149.874 mg/g and 145.462 mg/g at 30, 40 and 50, respectively, and was best at 30 °C. ΔH° was found to be − 1.494 which suggests adsorption is exothermic in nature and thus satisfying the findings that the physical process of adsorption took place in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Bangladesh, around US$28 billion of export value originates from the textile industries which are nearly 82% of the country’s total export earnings (Hossain et al. 2018). More than 10,000 different textile dyes are currently available and 7.105 metric tons are produced annually (Carmen and Daniela 2012) and an unfixed ratio of dyes is always washed away along with water (Ghaly et al. 2014). Methylene blue (MB), one of the most widely used cationic dyes, can be detrimental to humans including vomiting, cyanosis, quadriplegia and tissue necrosis (Hasan et al. 2018). Outcomes like these have led to the adoption of many treatment techniques such as adsorption (Hasan et al. 2018), electrochemical oxidation (Singh et al. 2016), photocatalytic degradation (Rahman et al. 2017; Inamuddin. 2018) to separate dyes from solution. An alternative approach of treating contaminated water is biosorption where biomaterials separate pollutants from solutions (Ibisi and Asoluka 2018). In several studies it has been suggested that many inactive biomasses have the ability to accumulate pollutants (Romero-Cano et al. 2016). Biosorption relies on several mechanisms including ion exchange, Van Der Waals attraction, microprecipitation, complexation and covalent bindings where chelating, ion exchanging and complexion with functional groups and releasing [H3O]+ into aqueous solution tend to play most crucial role (Abdolali et al. 2014, Mashkoor et al. 2020a). Cost efficiency, easy storage and reusable compatibility have made this process desirable for wastewater treatment (Beni and Esmaeili 2019). Moreover, the method is widely preferred over other techniques as it does not produce any harmful and toxic by-products (Mashkoor and Nasar 2020b).
The residue lignocellulosic biomass materials have internal as well as external surfaces. Total accessible surface is the sum of these surfaces. The internal surface depends on the pore size and distribution whereas the external surface depends on the size as well as shape and a suitable pretreatment can increase these surfaces (Karimi and Taherzadeh 2016; Shafiei et al. 2015). Thus, properties of these residue materials can be enhanced by a physical, chemical or physical–chemical pre-modification process (Romero-Cano et al. 2016). Several chemical modifying agents such as acid, base and organic compounds have been reported in literatures (Salman et al. 2015). The chemical modification process alters the structural and textural characteristics providing the material with a larger surface area as new active sites are formed or functionalization of hydroxyl group takes place (Crini 2006; Gordobil et al. 2016). It is not as costly as the application of activated carbon (AC) at large scale (Manyangadze et al. 2020). Thus, the prospects of chemical modification of biomaterials are highly promising in terms of economic feasibility, performance efficiency and environmental sustainability. Of the many existing chemical activation processes, alkaline treatment has increased in popularity because of its capacity to increase the surface area, average pore volume and pore diameter significantly (Hokkanen et al. 2016). Sodium hydroxide (NaOH) is an ideal chemical modifier because it is cheaper, less corrosive, and environmentally friendly and requires only a low dosage (Cazetta et al. 2011).
Agro-waste biomaterials such as natural fibers are ecofriendly, inexpensive, low density and have comparable mechanical properties (Binoj et al. 2016). Betel nut is one of the most widely grown agro crops in tropical climate countries. Betel but husk (BNH) is the fibrous outer part of the crop and it contains cellulose, hemicellulose, lignin, pectin, and proto-pectin at varying compositions (Lazim and Sapuan 2018). This husk fiber when treated by NaOH, decreases in lignin content and more cellulose components are exposed which makes it possible for the surface to take up more metal ions (Lazim et al. 2014; Hokkanen et al. 2016). Moreover, in comparison to other alkaline reagents like KOH, sodium hydroxide is reported to provide a better activation for lignocellulosic materials that occurs through a non-intercalation mechanism (Lillo-Ródenas et al. 2007; Hina et al. 2010). Therefore, assumptions can be made that NaOH modified betel nut husk (BNH-CM) has the potential to separate cationic dyes from water. Approximately 91,681 tons in 2010 and 92,589 tons in 2011 BNH were produced in Bangladesh (Lazim et al. 2014). In general, the BNH is thrown away into the environment after the consumption of betel nut and this creates inconvenience for the environment. So, this large amount of BNH produced every year can be potentially used as a bio-adsorbent to remove dye from aqueous solutions. Evidence-based research has been done using homemade peach (Ribas et al. 2020), apricot stones (Albroomi et al. 2017), cucumber peels (Sayğılı and Güzel1 2016), banana peel, eucalyptus bark, maize cob and maize leaf fibers (Santos et al. 2019), bagasse fly ash (Leechart et al. 2016), walnut shell powder (Uddin and Nasar 2020a), Punica granatum peel (Shakoor and Nasar 2018), Punica granatum seeds (Uddin and Nasar 2020b), Quercus robur acorn peel (Kuppusamy et al. 2017), Schleichera oleosa bark (Khatoon et al. 2018), magnetized Tectona grandis sawdust (Mashkoor and Nasar 2020c), Cucumis sativus peel (Shakoor and Nasar 2017) and Artocarpus odoratissimus (Lim et al. 2016) using similar approach. Recently, pyrolyzed BNH was used in a study to investigate its ability to remove Fe2+ from water (Subramani et al. 2019). In addition, not long ago, hydrochar activated carbon and pyrolyzed betel nut husk has also studied in terms of their adsorption capability (Tabassum et al. 2020; Bardhan et al. 2020). However, the widespread use of activated carbon has always been considered to be impractical and is restricted because of its high cost and difficulties in operation (Shakoor and Nasar 2016; Al-Degs et al. 2009). For this reason, in this study, we have examined the adsorption capacity onto chemically modified betel nut husk for a cationic dye, which, to our best knowledge, was first of its kind.
Thus, the objectives of the study were to: (1) generate as well as identify the characteristics of BNH activated by NaOH; (2) determine the influences of different process parameters on the adsorption procedure onto BNH-CM during methylene blue removal; (3) interpret the adsorption mechanism and thermodynamics of methylene blue onto BNH-CM.
Materials and methods
BNH collection and processing
In this research, raw betel nut husk was collected from local vendors in Gollamari, Khulna, Bangladesh. To remove moisture from the husk, it was dried in the sun for three days. After manual drying, it was kept in an oven at 105 °C for 24 h. After this step, grinding and sieving were carried out. The husk was sieved using a 1 mm mesh.
Chemical activation process
For the chemical modification process, BNH and NaOH pellets (LOBA Chemie, 97% purity) were mixed together in 1:1, 1:2 and 1:3 ratios (weight/weight). Deionized water was added to the mixtures and the samples were kept in a 100 mL beaker at 25 °C for 24 h. This chemically activated BNH (termed as BNH-CM) was washed with deionized water for achieving comparatively neutral pH using a pH meter the following day and then was retained in an oven until constant weight was obtained for further use.
Batch adsorption experiment
As after primary screening, the best result was obtained at 1:3 ratio and the experiments afterward were carried out at this ratio. The stock solution of 500 mg/L was made by mixing appropriate amounts of MB (C16H18CIN3S) (LOBA Chemie, 82%) in deionized water. Solutions with concentrations of 25, 50, 100, 150, 200, and 250 mg/L were prepared from the stock solution for isotherm and kinetic studies. The exact quantity of 200 mL MB solution was taken from the prepared solutions of different concentrations and 0.2 g of BNH-CM was added to each of them. The experiments were carried out at pH 7. Then, the entire dispersion was shaken at 150 rpm in a thermostatic water bath shaker (GEMMYCO, YCW-012S, Taiwan). The process parameters observed during this procedure were pH (2–12), contact time (0–24 h), initial dye concentration (25–250 mg/L) and temperature (30–50 °C). Desired pH adjustment was achieved using 0.1 M of NaOH and H2SO4. Instruments used to carry out these experiments were a double beam UV–vis spectrophotometer (SHIMADZU, Model UV 1800, Japan) at the wavelength of 668 nm and a pH meter (HANNA, pH 211 microprocessor pH Meter, USA).
The equations used to calculate the adsorption capacity of MB at the equilibrium (qe, mg/g), kinetics qt and removal percentage (R%) were given as follows:
where initial MB concentration (mg/L), concentration of MB at equilibrium (mg/L), MB solution volume (L) and mass of the material (g) are represented by Co, Ce, V and W, respectively.
In the pH point of zero charge experiments (pHpzc), initial pH of the 100 mL aqueous solutions (pH0) were calibrated to between 2 and 12 pH range by 0.1 M H2SO4 or NaOH. After that, 0.2 g of BNH-CM was added to each adjusted solution and shaken at 40 °C for 48 h. Finally, the required pH level was determined (pHf).
Characterization of modified material
Using a physisorption analyzer (Micromeritics, Model ASAP 2060, USA), the Brunauer–Emmett–Teller (BET) surface area and micropore volume were determined in the presence of N2 gas at − 196 °C. To identify the presence of surface functional groups of raw materials and/or before and after adsorption, Fourier Transform Infrared (FTIR) spectrometer (Model 2000, Perkin–Elmer, USA) was employed. Surface morphology was observed by a Scanning Electron Microscope (Jeol, JSM-7610F).
Determination of physical properties of the material
Bulk density and moisture content of BNH were calculated utilizing the following equations:
where Mf and Mc denote the weight of ground BNH-filled container and weight of container and Vc is the volume of container. M1 and M2 represent the initial and oven-dried weight of BNH, respectively.
Ash content (%), volatile matter (%) and fixed carbon (% yield) of BNH and BNH-AC were obtained using the muffle furnace (HYSC, MF-05, South Korea) and calculated by following the mathematical expressions written as follows:
where Wc is the weight of the crucible (g), Wrc is the weight of crucible and raw BNH (g), Wac is the total weight of crucible and ash (g) that was determined after experiment, and Ws is the sample weight (g). Temperature was fixed at 550 °C for 4 h for the purposes of determining ash content and particularly analyzing the volatile mater which was conducted at 900 °C for 7 min.
Desorption studies
Desorption experiments were conducted in order to evaluate the reusability of the modified husk. The adsorbents after being used in a preliminary equilibrium study containing MB were shaken at 30 °C in a water bath shaker for 6 h to extract access MB from its surface. This was done using the following solutions: methanol, sodium chloride (0.1 M/dm3) in water and sodium chloride (0.1 M/dm3) in methanol. Recovered materials were then again used for adsorption experiments for 100 ppm of MB solutions lasting 6 h. All the solutions were analyzed using a UV–Vis spectrophotometer.
Results and discussion
BNH-CM characterization
The results of proximate analysis of raw and chemically modified BNH are outlined in Table 1. As can be seen here, when the material undergoes a chemical treatment process, the percentage of fixed carbon increases significantly from 18.68 to 47.72% compared to the raw material; simultaneously the volatile matter and ash content decreases. Girish and George (2017) found a similar result while treating BNH with an acidic reagent. BNH when treated by an alkaline reagent like NaOH increases the moisture content. This elevation in the moisture content is supported by the data obtained from surface functional species analysis. The bulk density of betel nut husk was observed to be 0.245 (mg/L).
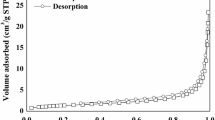
Textural characterization can be obtained by BET surface area analysis. The textural properties of BNH-CM are displayed in Table 1. The mean pore diameter of BNH-CM as is seen from the table is 6.92 nm, therefore, according to IUPAC classifications, chemically modified BNH prepared by NaOH can be identified as mesoporous (IUPAC 1972; Sing et al. 1985). Moreover, the updated IUPAC isotherm classification (Thommes et al. 2015) suggests the isotherm is a type IVa isotherm, one that indicates capillary condensation accompanied by hysteresis and type H4 hysteresis loop was observed (Fig. 1). This is very common in mesoporous carbon materials. The BET surface area of raw BNH was found to be 0.916 m2/g which rose slightly after being chemically treated (0.949 m2/g). The total pore volume and pore diameter were 0.0016 cm3/g and 6.92 nm, respectively, for BNH-CM.
The SEM of BNH (a), its state after modification (b), and after adsorption (c) is illustrated in Fig. 2. The characterizations were conducted at 3000 × magnification. As seen here, BNH-CM is visibly more porous than both the raw BNH and after being used as adsorbent (BNH-CMS). BNH-CM also demonstrates the roughest surface among the three micrographs. This is because when raw BNH is treated with an alkaline reagent like NaOH, the naturally occurring waxy layers on the surface such as pectin, wax and other components of lower molecule are removed creating hole-like pores on the surface (Lazim and Sapuan 2018). In Fig. 2c, the surface roughness and pores have again decreased after the adsorption of MB. Thus, alkali treatment can be regarded as an ideal option for increasing porosity and increasing the adsorption capacity of BNH.
Surface chemistry characterization
Characterization of the surface chemistry can be obtained by conducting FTIR analysis. FTIR spectra are useful in identifying surface functional groups of the adsorbent. Figure 3 represents the FTIR spectra of: (a) raw BNH, (b) chemically modified BNH (BNH-CM), and (c) BNH-CM after adsorption (BNH-CMS).
As can be seen from Fig. 3, the peaks found at around 1029.99 cm−1for BNH were due to C-O bond indicating the presence of alcohol, carboxylic acid, ether and ester groups which disappeared in BNH-CM and BNH-CMS. At 1550–1650 cm−1 the peaks existed due to the amine primary groups (N–H) in BNH which soften in BNH-CM and then again became intensified after adsorption. Therefore, most of the amine primary groups fell in number after chemical modification. In addition, the alkane groups (C–H) present at 2850–2925 cm−1 for the raw material disappeared after chemical modification and adsorption. –O–H stretching vibrations were responsible for peaks found after 3400 cm−1 which sharpened and broadened in the case of BNH-CMS. Therefore, it can be concluded that BNH when treated with NaOH increases in moisture content and this is demonstrated in another study (Chandra et al. 2016).
Effects of process parameters on adsorption
Effects of contact time and initial dye concentration at different temperatures.
To investigate the effect of contact time with respect to initial dye concentration in three different temperatures (30 °C, 40 °C, 50 °C), six different initial MB dye concentrations (25, 50, 100, 150, 200 and 250 mg/L) were taken at different time intervals (Fig. 4). In scenarios of lower dye concentrations, a rapid change was observed within 2 h for most cases. This happens because initially there are more active sites available for adsorption but these spots are only occupied gradually due to the competitive behavior of adsorbate molecules (Islam et al. 2017a). However, when the dye concentration is higher, the vacant adsorption sites become comparatively fewer which extends the time to reach equilibrium. Overall, for all studied concentrations, equilibrium tends to be attained at 6 h.
Higher qt values were obtained in higher initial dye concentrations. It was found that driving forces are higher when the initial dye concentration is high, which is why it is possible to overcome higher degrees of mass transfer resistance between adsorbent and the solutions (Feng et al. 2012). In addition to this, the equilibrium adsorption capacity of an adsorbent is subjected to the change in the experiment temperature for a particular adsorbate (Do˘gan et al. 2006). In general, temperature is likely to have a negative effect on the amount that is adsorbed (Ismadji and Bhatia. 2000). In this experiment, adsorption capacity has decreased when the experiment temperature increased. This is because with the increase in temperature, the physical bonding between organic compounds like dyes and the active sites of the adsorbent materials becomes weak. Moreover, the solubility of methylene blue increases with temperature like any other compounds (Chandra et al 2007). As a result, the prevailing interaction forces between MB and the solvent become much stronger than the force between MB and BNH-CM. Similar trends have also been found in case of adsorption capacity of activated carbon produced from durian shell and coconut husk (Chandra et al. 2007; Tan et al. 2008).
Effects of pH
Textile effluent collected from various industrial areas of Bangladesh (Savar, Gazipur, Narayangonj, Chittagong) were reported to be discharged at between 25 and 65 °C (Dey and Islam 2015). Therefore, pH experiments were conducted at 40° C. The pHpzc can be used to explain the mechanisms that may be involved in the electrostatic interaction between the material and the solution. It is the pH point where the net surface charge of the adsorbent is zero and therefore, the electrostatic force of ion species and adsorbent surface are balanced (Sayğılı1 and Güzel1 2016). The pHpzc which was found was recorded as being 7.05 (Fig. 5a).
Figure 5b illustrates the effects of solution pH of MB onto BNH-CM at 40 °C in the 2–12 range. MB adsorption is seen to be at its lowest at pH 2 and conspicuously rose with the increase of pH. This means the removal percentage increased as the pH also increased. This can be explained by the protonation and deprotonation of active sites subjected to initial pH. In acidic conditions, protonation causes a decline in negatively charged active sites on the surface of BNH which consequently hinders the adsorption of the positively charged cationic dye. Conversely, in basic conditions, deprotonation decreases the positively charged density of the surface of chemically modified BNH and subsequently enhances the attraction between MB and negatively charged surface. A decrease in the surface charge density with the increase of pH results in increasing the electrostatic force between the adsorbent and adsorbate (Islam et al. 2017a). Thus, the adsorption of MB dye on BNH-CM surface is driven by electrostatic force with the increase of initial pH. Similar results were found for cationic dyes adsorption on graphite oxides (Konicki et al. 2017).
Adsorption isotherm
The interaction between adsorbate and adsorbents, and the equilibrium distribution of adsorbate between the liquid and solid phase can be explained by adsorption isotherm (Njoku et al. 2014a). Simulations of isotherm data were conducted by applying the Langmuir and Freundlich isotherm models.
Figure 6 depicts the experimental amount of MB absorbed with respect to dye concentration. Adsorption isotherm was collected by plotting these data against each other. The Langmuir isotherm model (Langmuir 1918) describes the monolayer adsorption process on identical adsorption sites:
and one of its linear forms is given as follows:
where Ce is the equilibrium concentration, qm is the maximum adsorption capacity expressed in mg/g, and KL is Langmuir constant.
The Freundlich isotherm model (Freundlich 1906), on the other hand, describes a multilayer adsorption process assuming the adsorption surface is heterogeneous. The equation for this model is given as follows:
and the linear expression of this equation is given as follows:
where KF (mg/L (L/mg)1/n) and n are both Freundlich constant representing adsorption capacity and adsorption intensity, respectively.
By using the nonlinear form of these two models, the best isotherm model was recognized and validated by determining the coefficient (R2) and using the residual root-mean-square error (RMSE), respectively. Equations to determine R2 and RMSE are given as follows:
where experimental and model-predicted adsorption capacity is denoted by qe.means and qe.cal, respectively, and number of observation is denoted by n.
Table 2 compiles the determined parameters obtained from the abovementioned isotherm models. The Freundlich isotherm model overall demonstrates better R2 values with lower RMSE. Therefore, the adsorption process suits Freundlich isotherm model best where active sites present different values of energy. The results can be explained by suggesting that adsorption of MB on BNH-CM has occurred on the heterogeneous surface or multilayers and heat of the adsorption has occurred in a non-uniform manner. The 1/n values for all three temperatures were less than 1, indicating that the adsorption of methylene blue on the surface of BNH-CM occurred in favorable conditions. In many studies regarding methylene blue dye removal, the Freundlich isotherm model was best fitted the adsorption mechanism. Some examples of MB adsorption studies include Wodyetia bifurcata biochar (dos Santos et al. 2019), modified persimmon tannins (Wang et al. 2019) and Arundo donax activated carbon (Osman Üner 2019).
Furthermore, with the increase of temperature adsorption capacity decreases (149.921 mg/g, 149.874 mg/g, and 145.462 mg/g at 30, 40 and 50 °C, respectively). This may be because of the increase in the esca** tendency of MB as temperature increases. This also suggests that the adsorption mechanism of BNH-CM for MB adsorption involves a physical process. Therefore, chemically modified betel nut husk demonstrated multilayer physisorption. Moreover, BNH-CM exhibited higher adsorption capacity when compared to many other studies listed in Table 3.
Kinetics study
Adsorption kinetics study is important in terms of determining adsorption process efficiency, as it helps to design the process for an industrial scale treatment of wastewater. Two of the most widely used kinetic models—pseudo-first-order (PFO) and pseudo-second-order (PSO) models—were used in this study to understand the MB adsorption process onto BNH-CM. The Lagergren and Svenska (Langergren and Svenska 1998) pseudo-first-order model can be expressed as:
where k1 is the pseudo-first-order rate constant, t is the constant time in minute, and qe and qt are the absorbed amounts in mg/g, respectively, at equilibrium.
The pseudo-second-order model (Ho and McKay 1998) can be written as:
where k2 is the rate constant for pseudo-second-order adsorption.
The model that fitted best was calculated by the determination coefficient (R2), as well as two nonlinear error functions: average relative error function (ARE) and normalized standard deviation (NSD). The equations to find these three determinants were given as follows:
where qt.meas and qt.cal are the experimental and model-predicted MB adsorption capacity at t time and number of observations is expressed by n. Low ARE and NSD values refer to the better fitness to the model.
Figure 7 depicts nonlinear plots for the experimental kinetic data (qt) for PFO and PSO models with respect to time t for MB adsorption on BNH-CM at 40 °C. Based on comparatively higher R2 values and lower ARE and NSD values, pseudo-first-order model is much more reasonable when compared with experimental data than the pseudo-second-order system (Table 4). Adsorption capacity when investigated for wood apple shell, adsorption of malachite green with oil palm trunk fiber and removal of MB from aqueous solution by broad bean peels also complied with the PFO model as well (Sartape et al. 2017; Hameed and El-Khaiary 2008a, b).
Adsorption mechanism
The diffusion mechanism between particles and solutes cannot be explained by a kinetics study, so therefore the data obtained from kinetic studies were further analyzed using the Webber-Morris intraparticle diffusion model (Weber and Morris 1963). This can be expressed as:
where kid is rate constant (mg/g min1/2) is subjected to linear plot slopes of qt versus t1/2 (figure not shown) and C is boundary layer thickness.
Further, to identify the actual slowest step in the adsorption process and distinguish between intraparticle diffusion and film diffusion, the Boyd model was employed. It can be formulated as follows:
where kfd is the liquid film diffusion constant.
The kid and C values are collected through the gradient and intercept of the linear plot qt and t1/2 (Fig. 8).
Table 5 outlines intraparticle and film diffusion parameters for adsorption of MB onto BNH-CM at different initial concentrations. The R2 value of the film diffusion model was higher of these two models. The finding means that film diffusion is the rate determining step controlling MB adsorption onto BNH-CM. In effect, the movement of solutes from the boundary layer to adsorbent surface occurred in a slow process. In many studies, i.e., activated carbon prepared from karanj fruit, the adsorption process followed the film diffusion model (Islam et al. 2017b). Moreover, based on the results from FTIR analysis, the surface of BNH-CM is contained different oxygen functional groups that might expect to play a pivotal role for the adsorption of MB dye (Jain et al. 2014) on the adsorbent. Although, this surface functional groups behavior on the adsorbent surface fluctuates with pH. Therefore, the adsorption efficiency of BNH-CM in the removal of MB dye was markedly increased from the pH 2–12 and the maximum adsorption occurred. The adsorption mechanisms were: at first, MB moves toward the BNH-CM through the solution by film diffusion then from the porous surface (as observed from the SEM) of the BNH-CM into the interior site by pore diffusion and finally, the MB is adsorbed into the active sites in the interior of the adsorbent (Foo and Hameed 2012b).
Adsorption thermodynamics
The process of adsorption causing the system to pass from its initial state to equilibrium and the process that takes place at equilibrium conditions can be explained by adsorption thermodynamics (Njoku et al. 2014b). Consequently, to evaluate other phenomena occurring simultaneously with adsorption, standard enthalpy (ΔH°), standard entropy (ΔS°), and standard free energy from the transfer of unit mole solute (ΔG°) from solution to the solid–liquid interface were calculated.
The values of ΔH° and ΔS° were calculated using the following equations:
where R (8.314 J/mol K) is the universal gas constant, T (k) is the absolute temperature of the solution and Kd is the distribution coefficient which is computed by:
where CAe(mg/L) and Ce are the amount of absorbed dyes by adsorbent at the equilibrium and dye concentrations, respectively.
ΔG° was obtained by:
Table 6 describes the thermodynamic parameters for the adsorption of methylene blue onto BNH-CM. Here, negative ΔG° value for all three experimental temperatures explains the adsorption process was spontaneous and feasible. The negative value of ΔH° meant that the interactions during the adsorption process were exothermic and may be subjected to either the improved desorption process of the adsorption system or a decrease in the attraction between methylene blue molecules or functioning surface sites of BNH-CM with an increase in temperature. This outcome also satisfies the process being subjected to physisorption and confirms that decrease in the adsorption capacity with the increase in temperature is likely to lead to an exothermic process. The positive ΔS° indicates randomness increased in the system so that during the adsorption process, MB molecules and BNH-CM surface had a good affinity with each other. This can be explained by electrostatic interactions which is a commonly occurring consequence of physical adsorption. Results obtained from palm date seed activated carbon (Islam et al. 2015a, b) and durian shell activated carbons (Foo and Hameed 2012a) were also found to be exothermic among many others.
Desorption studies
Results of desorption studies were expressed in percentage as desorption efficiency. From Fig. 9, it can be observed that desorption efficiency is 93.015% after being treated with sodium chloride in water (saline water) solution. The results obtained from treatment with sodium chloride in methanol solution also demonstrate good potential (71.554%). Methylene blue, a cationic dye, has the tendency to formulate molecular aggregates and can be adsorbed in a fibrous channel in diluted solutions (Giles and Smith 1974). Therefore, desorption efficiency may not be as highly promising as in 100 mg/L solution for higher dye concentrations. Like this study, desorption in the presence of sodium chloride has proven to be very effective in previous analyses, for example MB adsorption onto palygorskites and sepiolites (Moreira et al. 2016).
Conclusion
One of the major consequences of industrialization is textile dyes being discharged in the world’s river systems. Thus, the potential of BNH-CM as an adsorbent was investigated. BNH-CM can be regarded as a promising precursor in terms of its availability, cost efficiency and sustainability. Adsorption capacity was 149.921, 149.874 and 145.462 mg/g for 30, 40 and 50 °C according to Langmuir isotherm model. The kinetic mechanism fitted the pseudo-first-order model and decreased the adsorption capacity when an increase in temperature occurred. This suggested that adsorption is subject to physisorption rather than chemisorption. Adsorption followed the Freundlich isotherm model indicating the surface of the adsorbent is heterogeneous. Desorption efficiency was highly promising for sodium chloride added to the eluent. Such findings have led to establish that chemically modified betel nut husk can be used as a adsorbent for removing cationic dyes from water. However, further study is required to observe desorption capacity in higher MB concentrations.
Data availability
All data are available.
References
Abdolali A, Guo WS, Ngo HH, Chen SS, Nguyen NC, Tung KL (2014) Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Biores Technol 160:57–66
Albroomi HI, Elsayed MA, Baraka A, Abdelmaged MA (2017) Batch and fixed-bed adsorption of tartrazine azo-dye onto activated carbon prepared from apricot stones. Appl Water Sci 7:2063–2074
Al-Degs YS, Khraisheh MAM, Allen SJ, Ahmad MN (2009) Adsorption characteristics of reactive dyes in columns of activated carbon. J Hazard Mater 165:944–949
Azzaz AA, Jellali S, Bousselmi L (2016) Optimization of a cationic dye adsorption onto a chemically modified agriculture by-product using response surface methodology. Int Renew Energy Congr. https://doi.org/10.1109/irec.2016.7478936
Bardhan M, Novera TM, Tabassum M, Islam MA, Jawad AH, Islam MA (2020) Adsorption of methylene blue onto betel nut husk based- activated carbon prepared by sodium hydroxide activation process. Water Sci Technol. https://doi.org/10.2166/wst.2020.451
Beni AA, Esmaeili A (2019) Biosorption, an efficient method for removing heavy metals from industrial effluents: a review. Environ Technol Innov 17:100503
Binoj JS, Raj RE, Sreenivasan VS, Thusnavis GR (2016) Morphological, physical, mechanical, chemical and thermal characterization of sustainable Indian areca fruit husk fibers (Areca catechu L.) as ars. J Bionic Eng 13(1):156–165
Boyd GE, Adamson AW, Myers LS (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites. Kinet J Am Chem Soc 69(11):2836–2848
Carmen Z, Daniela S (2012) Organic pollutants ten years after the Stockholm convention: environmental and analytical update. Intech Publisher Inc., Rijeka
Cazetta AL, Vargas AMM, Nogami EM, Kunita MH, Guilherme MR, Martins AC, Silva TL, Moraes JCG, Almeida VC (2011) NaOH-activated carbon of high surface area produced from coconut shell: kinetics and equilibrium studies from the methylene blue adsorption. Chem Eng J 174(1):117–125
Chandra TC, Mirna MM, Sudaryanto Y, Ismadji S (2007) Adsorption of basic dye onto activated carbon prepared from durian shell: Studies of adsorption equilibrium and kinetics. Chem Eng J 127(1–3):121–129
Chandra CSJ, George N, Narayanankutty SK (2016) Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydr Polym 142:158–166
Chen XG, Lv SS, Liu ST, Zhang PP, Zhang AB, Sun J, Ye Y (2012) Adsorption of methylene blue by rice hull ash. Sep Sci Technol 47(1):147–156
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97(9):1061–1085
Dey S, Islam A (2015) A review on textile wastewater characterization in Bangladesh. Resour Environ 5(1):15–44
Djilani C, Zaghdoudi R, Djazi F, Bouchekima B, Lallam A, Modarressi A, Rogalski M (2015) Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. J Taiwan Inst Chem Eng 53:112–121
Doğan M, Alkan M, Demirbaş Ö, Özdemir Y, Özmetin C (2006) Adsorption kinetics of maxilon blue GRL onto sepiolite from aqueous solutions. Chem Eng J 124(1–3):89–101
Dos Santos KJL, de Souza dos Santos G, de Sá ÍMG L, Ide AH, da Silva Duarte JL, Vieira de Carvalho SH, Meili L (2019) Wodyetia bifurcata Biochar for methylene blue removal from aqueous matrix. Biores Technol 122093
Feng Y, Zhou H, Liu G, Qiao J, Wang J, Lu H, Yang L, Wu Y (2012) Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: equilibrium, kinetic and adsorption mechanisms. Bioresour Technol 125:138–144
Foo KY, Hameed BH (2012a) Textural porosity, surface chemistry and adsorptive properties of durian shell derived activated carbon prepared by microwave assisted NaOH activation. Chem Eng J 187:53–62
Foo KY, Hameed BH (2012b) Microwave-assisted preparation and adsorption performance of activated carbon from biodiesel industry solid reside: influence of operational parameters. Bioresour Technol 103:398–404
Freundlich H (1906) Über die adsorption in lösungen (adsorption in solution). Phys Chem 57:384–470
Fu J, Chen Z, Wang M, Liu S, Zhang J, Zhang J, Han R, Xu Q (2015) Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): kinetics, isotherm, thermodynamics and mechanism analysis. Chem Eng J 259(1):53–61
Ghaly AE, Ananthashankar R, Alhattab M, Ramakrishnan VV (2014) Production, characterization and treatment of textile effluents: a critical review. J Chem Eng Process Technol 5:182
Giles CH, Smith D (1974) A general treatment and classification of the solute adsorption isotherm. J Colloid Interface Sci 47:755–765
Girish CR, George GM (2017) Phenol removal from wastewater using arecanut husk (Areca Catechu) as adsorbent. IJMET 8(12):1020–1028
Gordobil O, Moriana R, Zhang L, Labidi J, Sevastyanova O (2016) Assessment of technical lignins for uses in biofuels and biomaterials: structure-related properties, proximate analysis and chemical modification. Ind Crop Prod 83:155–165
Hameed BH, El-Khaiary MI (2008a) Batch removal of malachite green from aqueous solutions by adsorption on oil palm trunk fibre: Equilibrium isotherms and kinetic studies. J Hazard Mater 154(1–3):237–244
Hameed BH, El-Khaiary MI (2008b) Sorption kinetics and isotherm studies of a cationic dye using agricultural waste: broad bean peels. J Hazard Mater 154(1–3):639–648
Hasan R, Chong CC, Setiabudi HD, Jusoh R, Jalil AA (2018) Process optimization of methylene blue adsorption onto eggshell-treated palm oil fuel ash. Environ Technol Innov 13:62–73
Hina K, Bishop P, Arbestain MC, Calvelo-Pereira R, Maciá-Agulló JA, Hindmarsh J, Hedley MJ (2010) Producing biochars with enhanced surface activity through alkaline pretreatment of feedstocks. Aust J Soil Res 48(7):606
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70(2):115–124
Hokkanen S, Bhatnagar A, Sillanpää M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173
Hong JM, Lin B, Jiang JS, Chen BY, Chang CT (2014) Synthesis of pore expanded mesoporous materials using waste quartz sand and the adsorption effects of methylene blue. J Ind Eng Chem 20(5):3667–3671
Hossain L, Sarker SK, Khan MS (2018) Evaluation of present and future wastewater impacts of textile dyeing industries in Bangladesh. Environ Dev 26:23–33
Huang XY, Mao XY, Bu HT, Yu XY, Jiang GB, Zeng MH (2011) Chemical modification of chitosan by tetraethylenepentamine and adsorption study for anionic dye removal. Carbohyd Res 346(10):1232–1240
Ibisi NE, Asoluka CA (2018) Use of agro-waste (Musa paradisiaca peels) as a sustainable biosorbent for toxic metal ions removal from contaminated water. Chem Int 4(1):52–59
Inamuddin (2018) Xanthan gum/titanium dioxide nanocomposite for photocatalytic degradation of methyl orange dye. Int J Biol Macromol 121:1046–1053
Islam MA, Benhouria A, Asif M, Hameed BH (2015a) Methylene blue adsorption on factory-rejected tea activated carbon prepared by conjunction of hydrothermal carbonization and sodium hydroxide activation processes. J Taiwan Inst Chem Eng 52:57–64
Islam MA, Tan IAW, Benhouria A, Asif M, Hameed BH (2015b) Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chem Eng J 270:187–195
Islam MA, Ahmed MJ, Khanday WA, Asif M, Hameed BH (2017a) Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol Environ Saf 138:279–285
Islam MA, Sabar S, Benhouria A, Khanday WA, Asif M, Hameed BH (2017b) Nanoporous activated carbon prepared from karanj (Pongamia pinnata) fruit hulls for methylene blue adsorption. J Taiwan Inst Chem Eng 74:96–104
Ismadji S, Bhatia SK (2000) Adsorption of flavour esters on granular activated carbon. Can J of Chem Eng 78(5):892–901
IUPAC (1972) Manual of symbols and terminology. Pure Appl Chem 31:587
Jain A, Jayaraman S, Balasubramanian R, Srinivasan M (2014) Hydrothermal pretreatment for mesoporous carbon synthesis: enhancement of chemical activation. J Mater Chem A 2:520–528
Karimi K, Taherzadeh MJ (2016) A critical review on analysis in pretreatment of lignocelluloses: degree of polymerization, adsorption/desorption, and accessibility. Biores Technol 203:348–356
Khatoon A, Uddin MK, Rao RAK (2018) Adsorptive remediation of Pb(II) from aqueous media using Schleichera oleosa bark. Environ Technol Innov 11:1–14
Konicki W, Aleksandrzak M, Mijowska E (2017) Equilibrium, kinetic and thermodynamic studies on adsorption of cationic dyes from aqueous solutions using graphene oxide. Chem Eng Res Des 123:35–49
Kuppusamy S, Venkateswarlu K, Thavamani P, Lee YB, Naidu R, Megharaj M (2017) Quercus robur acorn peel as a novel coagulating adsorbent for cationic dye removal from aquatic ecosystems. Ecol Eng 101:3–8
Langergren S, Svenska BK (1998) Zurtheorie der sogenannten adsorption geloesterstoffe. Veternskapsakad Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Lazim Y, Sapuan SM (2018) Properties of betel nut husk reinforced vinyl ester composites. Nat Fibre Reinf Vinyl Ester Vinyl Polym Compos. https://doi.org/10.1016/B978-0-08-102160-6.00006-8
Lazim Y, Salit MS, Zainudin ES, Mustapha M, Jawaid M (2014) Nut husk alkaline treatment. BioResources 9(4):7721–7736
Leechart P, Inthorn D, Thiravetyan P (2016) Adsorption of antimony by bagasse fly ash: chemical modification and adsorption mechanism. Water Environ Res 88(9):907–912
Lillo-Ródenas MA, Marco-Lozar JP, Cazorla-Amoros D, Linares-Solano A (2007) Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J Anal Appl Pyrolysis 80:166–174
Lim LBL, Priyantha N, Mohamad NAHZ, Jamil UAN, Chieng HI, Zehra T, Liyandeniya A (2016) Chemical modification of artocarpus odoratissimus skin for enhancement of their adsorption capacities toward toxic malachite green dye. J Mater Environ Sci 7(9):3211–3224
Manyangadze M, Chikuruwo NHM, Narsaiah TB, Chakra CS, Radhakumari M, Danha G (2020) Enhancing adsorption capacity of nano-adsorbents via surface modification: a review. South African J Chem Eng 31:25–32
Mashkoor F, Nasar A (2018) Preparation, characterization and adsorption studies of the chemically modified Luffa aegyptica peel as a potential adsorbent for the removal of malachite green from aqueous solution. J Mol Liq 274:315–327
Mashkoor F, Nasar A (2020a) Magsorbents: potential candidates in wastewater treatment technology—a review on the removal of methylene blue dye. J Magn Mag Mater 500:166408
Mashkoor F, Nasar A (2020b) Magnetized Tectona grandis sawdust as a novel adsorbent: preparation, characterization, and utilization for the removal of methylene blue from aqueous solution. Cellulose 27:2613–2635
Mashkoor F, Nasar A, Inamuddin (2020) Carbon nanotube-based adsorbents for the removal of dyes from waters: a review. Environ Chem Lett 2020:1–25
Moreira MA, Ciuffi KJ, Rives V, Vicente MA, Trujillano R, Gil A, Korili SA, Faria EH (2016) Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl Clay Sci 135:394–404
Nasuha N, Zurainan HZ, Maarof HI, Zubir NA, Amri N (2011) Effect of cationic and anionic dye adsorption from aqueous solution by using chemically modified papaya seed. 2011 Int. Conf. Environ. Sci. Eng. IPCBEE vol.8 (2011), IACSIT Press, Singapore
Njoku VO, Islam MA, Asif M, Hameed BH (2014a) Preparation of mesoporous activated carbon from coconut frond for the adsorption of carbofuran insecticide. J Anal Appl Pyrolysis 110:172–180
Njoku VO, Foo KY, Asif M, Hameed BH (2014b) Preparation of activated carbons from rambutan (Nephelium lappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chem Eng J 250:198–204
Rahman AFA, Jalil AA, Triwahyono S, Ripin A, Aziz FFA, Fatah NAA (2017) Strategies for introducing titania onto mesostructured silica nanoparticles targeting enhanced photocatalytic activity of visible-light-responsive Ti-MSN catalysts. Clean Prod 143:948–959
Ribas MC, de Franco MAE, Adebayo MA, Lima EC, Parkes GMB, Feres LA (2020) Adsorption of procion red MX-5B dye from aqueous solution using homemade peach and commercial activated carbons. Appl Water Sci 10:154
Romero-Cano LA, Gonzalez-Gutierrez LV, Baldenegro-Perez LA, Medina-Montes MI (2016) Preparation of orange peels by instant controlled pressure drop and chemical modification for its use as biosorbent of organic pollutants. Rev Mex Ing Quím 15(2):481–491
Salman M, Athar M, Farooq U (2015) Biosorption of heavy metals from aqueous solutions using indigenous and modified lignocellulosic materials. Rev Environ Sci Bio Technol 14(2):211–228
Santos PF, Neris JB, MartínezLuzardo FH, Velasco FG, Tokumoto MS, da Cruz RS (2019) Chemical modification of four lignocellulosic materials to improve the Pb2+ and Ni2+ ions adsorption in aqueous solutions. J Environ Chem Eng 7:103363
Sartape AS, Mandhare AM, Jadhav VV, Raut PD, Anuse MA, Kolekar SS (2017) Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab J Chem 10:S3229–S3238
SayğılıGüzel GAF (2016) Chemical modification of a cellulose-based material to improve its adsorption capacity for anionic dyes. J Dispers Sci Technol 38(3):381–392
Sebeia N, Jabli M, Ghith A, Elghoul Y, Alminderej FM (2019) Production of cellulose from Aegagropila Linnaei macro-algae: Chemical modification, characterization and application for the bio-sorption of cationic and anionic dyes from water. Int J Bio Macromol 135:152–162
Shafiei M, Kumar R, Karimi K (2015) Pretreatment of lignocellulosic Biomass. Biofuel and Bioref Technol. https://doi.org/10.1007/978-3-319-14033-9_3
Shakoor S, Nasar A (2016) Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J Taiwan Inst Chem Eng 66:154–163
Shakoor S, Nasar A (2017) Adsorptive treatment of hazardous methylene blue dye from artificially contaminated water using cucumis sativus peel waste as a low-cost adsorbent. Groundw Sustain Dev 5:152–159
Shakoor S, Nasar A (2018) Utilization of Punica granatum peel as an eco-friendly biosorbent for the removal of methylene blue dye from aqueous solution. J Appl Biotechnol Bioeng 5(4):242–249
Sing KSW, Everett DH, Haul RAW, Moscou L, Pieroti RA, Rouquerol J, Siemieniewska T (1985) Physical and biophysical chemistry division commission on colloid and surface chemistry including catalysis. Pure Appl Chem 57:603–619
Singh S, Lo SL, Srivastava VC, Hiwarkar AD (2016) Comparative study of electrochemical oxidation for dye degradation: parametric optimization and mechanism identification. J Env Chem Eng 4(3):2911–2921
Subramani BS, Shrihari S, Manu B, Babunarayan KS (2019) Evaluation of pyrolyzed areca husk as a potential adsorbent for the removal of Fe2+ ions from aqueous solutions. J Environ Manage 246:345–354
Tabassum M, Bardhan M, Novera TM et al (2020) NaOH-Activated betel nut husk hydrochar for efficient adsorption of methylene blue dye. Water Air Soil Pollut 231:398
Tan IAW, Ahmad AL, Hameed BH (2008) Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater 154(1–3):337–346
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KS (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069
Uddin MK, Nasar A (2020a) Walnut shell powder as a low-cost adsorbent for methylene blue dye: isotherm, kinetics, thermodynamic, desorption and response surface methodology examinations. Sci Rep. https://doi.org/10.1038/s41598-020-64745-3
Uddin MK, Nasar A (2020b) Decolorization of basic dyes solution by utilizing fruit seed powder. KSCE J Civ Eng 24(2):345–355
Üner O (2019) Hydrogen storage capacity and methylene blue adsorption performance of activated carbon produced from Arundo donax. Mater Chem Phys 237:121858
Wang Z, Gao M, Li X, Ning J, Zhou Z, Li G (2019) Efficient adsorption of methylene blue from aqueous solution by graphene oxide modified persimmon tannins. Mater Sci Eng C 108:110196
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon solution. J Sanit Eng Div 89(2):31–59
Yao Y, Gao B, Fang J, Zhang M, Chen H, Zhou Y, Creamer AE, Sun W, Yang L (2014) Characterization and environmental applications of clay–biochar composites. Chem Eng J 242:136–143
Acknowledgements
This research was funded by Ministry of Education, Government of Bangladesh, under advanced research in education scheme (Grant Nos.: 37. 20. 0000. 004. 003. 020. 2016). Bangladesh Council of Scientific and Industrial Research (BCSIR) and Universiti Teknologi Mara, Malaysia cooperated in SEM-EDX, CHNS and BET analysis, respectively.
Funding
This research was funded by Ministry of Education, Government of Bangladesh, under advanced research in education scheme (Grant Nos.: 37. 20. 0000. 004. 003. 020. 2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Novera, T.M., Tabassum, M., Bardhan, M. et al. Chemical modification of betel nut husk prepared by sodium hydroxide for methylene blue adsorption. Appl Water Sci 11, 66 (2021). https://doi.org/10.1007/s13201-021-01394-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01394-5