Abstract
Purpose
Older adults with COVID-19 have a high prevalence of complications and mortality during hospitalization. Given the large proportion of older adults requiring admission to an intensive care unit (ICU), we aimed to describe the management and outcomes of older adults with COVID-19 requiring ICU care and identify predictors of hospital mortality.
Methods
We included consecutive patients ≥ 65 yr of age who were admitted between 11 March 2020 and 30 June 2021 to one of five Toronto (ON, Canada) ICUs with a primary diagnosis of SARS-CoV-2 infection in a retrospective cohort study. Patient characteristics, ICU treatment, and outcomes were recorded. We used multivariable logistic regression to identify predictors of in-hospital mortality.
Results
Of the 273 patients, the median [interquartile range] age was 74 [69–80] yr, 104 (38.1%) were female, and 164 (60.1%) required invasive mechanical ventilation. One hundred and forty-two patients (52.0%) survived their hospital stay. Compared with survivors, nonsurvivors were older (74 [70–82] yr vs 73 [68–78] yr; P = 0.03), and a smaller proportion was female (39/131, 29.8% vs 65/142, 45.8%; P = 0.01). Patients had long hospital (19 [11–35] days) and ICU (9 [5–22] days) stays, with no significant differences in ICU length of stay or duration of invasive mechanical ventilation between the two groups. Higher APACHE II score, increasing age, and the need for organ support were independently associated with higher in-hospital mortality while female sex was associated with lower mortality.
Conclusions
Older critically ill COVID-19 patients had long ICU and hospital stays, and approximately half died in hospital. Further research is needed to identify individuals who will benefit most from an ICU admission and to evaluate posthospitalization outcomes.
Résumé
Objectif
Les personnes âgées atteintes de la COVID-19 ont une prévalence élevée de complications et de mortalité pendant l’hospitalisation. Compte tenu de la forte proportion de personnes âgées nécessitant une admission dans une unité de soins intensifs (USI), nous avons cherché à décrire la prise en charge et les devenirs des personnes âgées atteintes de COVID-19 nécessitant des soins intensifs et à identifier les prédicteurs de mortalité hospitalière.
Méthode
Nous avons inclus des patient·es consécutif·ves âgé·es de ≥ 65 ans admis·es entre le 11 mars 2020 et le 30 juin 2021 dans l’une des cinq unités de soins intensifs de Toronto (ON, Canada) avec un diagnostic primaire d’infection par le SRAS-CoV-2 dans une étude de cohorte rétrospective. Les caractéristiques des patient·es, le traitement en USI et les devenirs ont été enregistrés. Nous avons utilisé une régression logistique multivariable pour identifier les prédicteurs de mortalité hospitalière.
Résultats
Parmi les 273 patient·es, l’âge médian [écart interquartile] était de 74 [69-80] ans, 104 (38,1 %) étaient des femmes et 164 (60,1 %) ont nécessité une ventilation mécanique invasive. Cent quarante-deux personnes (52,0 %) ont survécu à leur séjour à l’hôpital. Comparativement aux personnes survivantes, les personnes qui n’ont pas survécu étaient plus âgées (74 [70-82] ans vs 73 [68–78] ans; P = 0,03), et une plus faible proportion était de sexe féminin (39/131, 29,8 % vs 65/142, 45,8 %; P = 0,01). Les séjours des patient·es à l’hôpital (19 [11-35] jours) et à l’USI (9 [5-22] jours) étaient longs, sans différence significative dans la durée du séjour en USI ou la durée de la ventilation mécanique invasive entre les deux groupes. Un score APACHE II plus élevé, un âge plus avancé et le besoin de mesures de soutien d’organes étaient indépendamment associés à une mortalité plus élevée à l’hôpital, tandis que le sexe féminin était associé à une mortalité plus faible.
Conclusion
Les personnes plus âgées gravement malades atteintes de la COVID-19 ont eu de longs séjours en soins intensifs et à l’hôpital, et environ la moitié sont décédées à l’hôpital. D’autres recherches sont nécessaires pour identifier les personnes qui bénéficieraient le plus d’une admission à l’USI et pour évaluer les devenirs post-hospitalisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Older adults have been disproportionately impacted by SARS-CoV-2, frequently requiring more intensive medical care and experiencing worse outcomes.1,2 Atypical syndromes such as functional decline and weakness, and absence of classic symptoms of fever or cough may result in delayed diagnoses.1 The higher prevalence of comorbidities, combined with the physiologic effects of aging and frailty, may contribute to different clinical courses, treatment effects, and outcomes.1,2
Outcomes of older patients with COVID-19 critical illness have been reported from the USA and other countries, but the Canadian experience has not been well characterized.3 Prior studies have shown that older age, need for invasive mechanical ventilation (IMV), acute kidney injury (AKI), and acute respiratory distress syndrome (ARDS) are independently associated with fewer days alive.3,4 A meta-analysis of all COVID-19 patients also revealed significant regional differences in patient outcomes, highlighting the need to formulate region-specific management strategies reflecting variable resources, population health status, and/or viral strains.4
Across Canada, between January 2020 and December 2021, more than 25% of COVID-19-related hospital stays included admission to the intensive care unit (ICU), and 60% of these patients required mechanical ventilation.2 Patients who did not survive their hospital stay were older than those who were discharged alive (median age, 73 vs 57 yr).2 In a Canadian retrospective study of 1,671 adults older than 65 yr of age who were hospitalized for COVID-19, 24.1% required ICU admission and 23.5% died; advanced age, need for ICU admission, frailty and delirium were independently associated with hospital mortality.1
Given the large proportion of older adults requiring ICU care and the implications for health systems, we aimed to describe the management and outcomes of COVID-19 patients ≥ 65 yr of age across five Toronto ICUs and to identify predictors of mortality. Such data on outcomes and risk factors can guide management and inform patient and clinician decision-making regarding ICU admission and organ support.
Methods
Study design
This was an ICU substudy of a multicentre retrospective cohort study, which investigated the atypical presentation of COVID-19 among older adults.1 The protocol was prospectively published in Open Science Framework (https://osf.io/k4g7a/) and the study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Electronic Supplementary Material [ESM] eTable 1).5 Research Ethics approval was obtained through Clinical Trials Ontario (3186-OPIA-Apr/2020-38044) and individual patient consent was not required.
Setting and participants
The study was conducted in five academic medical centres in Toronto, ON, Canada (Mount Sinai Hospital, University Health Network – Toronto General Hospital and Toronto Western Hospital, St. Michael’s Hospital, and Sunnybrook Health Sciences Centre). We included consecutive adults ≥ 65 yr who were admitted between 11 March 2020 and 30 June 2021 to one of these ICUs with a primary ICU diagnosis of polymerase chain reaction-confirmed acute SARS-CoV-2 infection. We excluded patients who were 1) readmitted more than 24 hr after an index ICU admission for COVID-19 and 2) required ICU management for non-COVID-19-related conditions, as defined by the site’s ICU and infection control teams (e.g., admitted for an elective procedure).
Data collection
Patients meeting the eligibility criteria were identified by the data analytics service at each site, using the same case detection protocol for public health reporting. Deidentified data were extracted from electronic patient records and entered into a REDCap database by a trained chart assessor (K. H.) using a standardized data abstraction form.6 The chart assessor (K. H.) was trained by physician investigators at each hospital site (S. M., E. W., R. N.). The first five charts were extracted in duplicate with the physician investigator, and additional charts were reviewed as needed.
From the electronic patient records, we recorded the following: 1) patient characteristics including age, sex, place of residence, code status, clinical frailty score,7 and comorbidities; 2) daily respiratory parameters (e.g., lowest ratio of arterial partial pressure of oxygen and fraction of inspiratory oxygen [PaO2/FIO2], median tidal volume, lowest arterial pH); 3) ICU complications as defined by the ICU team (e.g., ARDS, AKI, bacterial pneumonia, myocardial infarction [MI] delirium, prolonged fever); 4) analgosedation (sedatives, opioids, antipsychotics) and neuromuscular blockers; and 5) laboratory variables such as hemoglobin, creatinine, and bilirubin at ICU admission. Prolonged fever was defined as a body temperature > 38.3 °C for more than seven days. We calculated illness severity scores (Sequential Organ Failure Assessment [SOFA] and Acute Physiological and Chronic Health Evaluation [APACHE] II) using data from the first 24 hr of ICU admission if arterial blood gas results were available.8,9,10 Hospital and ICU outcomes included delirium prevalence as defined by validated tools used clinically at each site11,12 and a validated chart review method,13 duration of mechanical ventilation, length of stay, and mortality. Delirium was assessed during the entire hospitalization (ICU and wards), with at least one positive delirium score indicating delirium.
Statistical analysis
Patient demographics and clinical characteristics are described using proportions (frequencies) and means (standard deviation) or medians [interquartile range (IQR)] as appropriate. We used the Chi square test to determine statistical significance for proportions. We identified statistical significance using ANOVA and the Kruskal–Wallis test for normally distributed and skewed continuous data, respectively.14
To identify the association between patient risk factors and in-hospital mortality, we used a multivariable logistic regression model. The following variables were chosen a priori for clinical relevance guided by previously reported associations with death in ICU patients: age,15 sex,16 number of comorbidities,17 APACHE II score,10 clinical frailty score,18 and the need for organ support (i.e., one or more of mechanical ventilation, continuous renal replacement therapy [CRRT], and vasopressors).19 We used multiple imputation to address missing data in the models (“mice” R package; R Foundation for Statistical Computing, Vienna, Austria), with the assumption that variables were missing at random.20 The data are presented using unadjusted and adjusted odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and P values. All data were analyzed using R version 4.2.1.21
Results
Baseline characteristics
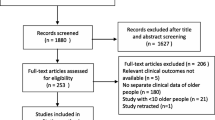
During the study period, 273 patients ≥ 65 yr who were admitted to the ICU for COVID-19 were included. The median [IQR] age was 74 [69–80] yr and 104 (38.1%) patients were female. Across the cohort, 199 (81.6%) were full-code at admission, 29 (11.9%) had a do-not-resuscitate (DNR) designation, and 16 (6.6%) were for intubation only (Table 1). The median [IQR] number of comorbidities was 2 [1–3], with hypertension (194, 71.1%) and diabetes (129, 47.4%) being among the most prevalent. A large proportion of patients developed complications (228, 83.5%) including ARDS, AKI, prolonged fever, and bacterial pneumonia (Table 2). Patients required prolonged median [IQR] hospital (19 [11–35] days) and ICU (9 [5–22] days) stays, with 142 (52.0%) surviving their hospital stay (Table 3).
Compared with hospital survivors, patients who died in hospital were older (median [IQR], 74 [70–82] vs 73 [68–78] yr; P = 0.03), and a smaller proportion of nonsurvivors were female (39/131, 29.8% vs 65/142, 45.8%; P < 0.01). There were no significant differences in the prevalence of comorbidities, except for chronic kidney disease, which was more frequent among nonsurvivors than survivors (44/131, 33.6% vs 16/142, 11.3%; P < 0.001). On ICU admission, nonsurvivors had significantly lower hemoglobin, neutrophils, and platelets, and higher creatinine compared with survivors (Table 1).
At ICU admission, nonsurvivors had lower mean (SD) Glasgow Coma Scores (8.7 [5.3] vs 10.1 [5.3]; P = 0.03), and higher SOFA (10.3 [5.0] vs 8.8 [4.9]; P = 0.03) and APACHE II (25.0 [8.0] vs 22.0 [7.8]; P < 0.01) scores (Table 1). In addition, more nonsurvivors had a DNR order (20/131, 15.3% vs 9/142, 6.3%; P = 0.02).
Intensive care unit interventions and organ dysfunction
There were no differences in the proportion of patients receiving different COVID-19 treatments across the two cohorts (Table 2). There was no difference between survivors and nonsurvivors in the proportion of patients requiring IMV (79/142, 55.6% vs 85/131, 64.9%; P = 0.15). Patients who died in-hospital had significantly higher rates of ARDS (119/131, 90.8% vs 105/142, 73.9%; P < 0.01), AKI (74/131, 56.5% vs 36/142, 25.4%; P < 0.001), and MI (13/131, 9.9% vs 3/142, 2.1%; P = 0.01), and a larger proportion required CRRT (35/131, 26.7% vs 9/142, 6.3%; P < 0.001), compared with those who survived. There was no difference in the prevalence of delirium between survivors and nonsurvivors (93/142, 65.5% vs 77/131, 58.8%; P = 0.24).
There were no between-group differences in the proportion of patients requiring high-flow nasal oxygen (HFNO) or prone positioning (ESM eTable 2). Nevertheless, across patients receiving HFNO, survivors had a longer duration of use than those who died (median [IQR], 5 [3–7] vs 3 [2–4] days; P < 0.001). During the first seven ICU days, nonsurvivors had lower arterial pH and mean arterial blood pressure (ESM eTable 2; ESM eFigs 1–6). Nonsurvivors also had higher plateau pressures; however, this was no longer significant after Bonferroni correction. There were no significant differences in the PaO2/FIO2 ratio, tidal volume, or positive end-expiratory pressure between the two groups (ESM eTable 2; ESM eFigs 1–6).
A significantly greater proportion of nonsurvivors received midazolam (61/131, 46.6% vs 39/142, 27.5%; P < 0.01) and hydromorphone (59/131, 45.0% vs 38/142, 26.8%; P < 0.01) infusions. Among those receiving midazolam and/or hydromorphone, survivors received longer durations of infusion than nonsurvivors did (midazolam mean [SD] 12.1 [9.3] days vs 7.6 [6.3] days; P < 0.01, and hydromorphone 15.0 [11.4] vs 10.7 [8.0] days; P = 0.04). There was also a greater need for vasopressor support in the nonsurvivor cohort than the survivors cohort (95/131, 72.5% vs 80/142, 56.3%; P < 0.01). While restraint use was similar, survivors had a longer duration of restraint use (9.1 [8.6] days vs 4.6 [4.0] days; P < 0.01). Survivors were more likely to have a tracheostomy performed (29 [20.4%] vs 9 [6.9%]; P < 0.01) than nonsurvivors were (Table 2). Across the cohort, 170 (62.3%) patients developed delirium during their hospital stay; with comparable rates in survivors and nonsurvivors (93/142, 65.5% vs 77/131, 58.8%; P = 0.24).
Patient outcomes
There were no differences in the durations of IMV or ICU stay between the two groups (Table 3). Hospital stay was significantly longer among survivors than nonsurvivors (median [IQR], 27 [15–55] vs 14 [9–24] days; P < 0.001; Table 3). Of the 131 nonsurvivors, 103 (78.6%) died in the ICU and 28 (21.4%) died in hospital following ICU discharge.
Using a multivariable logistic regression model, older age (adjusted OR [aOR], 1.42; 95% CI, 1.16 to 1.72; P < 0.001), higher APACHE II score (aOR, 1.05; 95% CI, 1.00 to 1.09; P = 0.04), and the need for organ support (aOR, 2.10; 95% CI, 1.04 to 4.27; P = 0.04) were independently associated with increased odds of in-hospital mortality after adjusting for total number of comorbidities, sex, and clinical frailty scale (Table 4). Need for organ support was defined as IMV, CRRT, and/or vasopressors. Female sex (aOR, 0.42; 95% CI, 0.24 to 0.73; P < 0.01) was independently associated with decreased odds of in-hospital mortality.
Discussion
In this multicentre Toronto cohort of 273 patients ≥ 65 yr admitted to the ICU with COVID-19, 60% required IMV for an average of over two weeks, 38% died in the ICU, and 48% died in the hospital. Nonsurvivors were older, had higher severity of illness, and were more likely to have ARDS, AKI, and MI than survivors were. A higher APACHE II score was independently associated with higher hospital mortality while female sex was associated with lower mortality. Furthermore, increasing age and the need for organ support were also associated with greater odds of mortality.
Comparison with existing evidence
A recent narrative review identified 19 studies reporting data on critically ill older adults (≥ 60 yr) with COVID-19; however, only one study exclusively focused on this population.22 Consistent with our findings, across seven studies, the proportion of older patients requiring IMV ranged from 52% to 91%.22 The high mortality rate in our cohort is comparable to that in other studies. A multicentre retrospective study of 239 patients reported an overall 60-day mortality rate of 61.5%, and noted higher mortality in patients older than 65 yr compared with those younger than 65 yr (73% vs 51%).23 The high prevalence of comorbidities is also consistent with a large cohort study which found that all patients over 80 yr and 76% of those over 60 yr had at least one comorbidity.24 The greater prevalence of ARDS among nonsurvivors in our cohort is also consistent with some prior studies,25,26 while other studies have not reported this finding.27,28 Our modelling revealed that older age was independently associated with a higher odds of hospital mortality, similar to a retrospective study of 1,591 critically ill adults with COVID-19, 436 of whom were older than 60 yr.24 Another study also found significantly higher 90-day mortality rates among those over 80 yr compared with those aged 75–79 yr and 70–74 yr (67% vs 47% vs 39%; P < 0.001, respectively).29 In contrast to our findings, they reported higher 90-day mortality among those requiring intubation, and patients with higher clinical frailty scores. We also found that female sex was associated with decreased in-hospital mortality. This is consistent with a meta-analysis, which reported that male sex was an important risk factor for COVID-19-related adverse events.30 Another study noted that this difference may be attributable to multiple factors, including differential hormonal regulation of immune responses including a greater antiviral response in females, greater dysregulated inflammation in males, and the higher prevalence of underlying cardiovascular comorbidities in males.31
Across the cohort, more than 60% of patients experienced delirium during their hospital stay; however, we did not find a significant difference in the prevalence of delirium between survivors and nonsurvivors. A cohort study of 213 critically ill patients ≥ 18 yr with COVID-19 reported that the mortality rate among those who experienced delirium was not significantly different from those without delirium (25% vs 34%; P = 0.17).32 In contrast, a retrospective study of 235 noncritically ill adults ≥ 65 yr hospitalized with COVID-19 reported that delirium was associated with increased mortality (hazard ratio, 2.1; 95% CI, 1.2 to 3.7; P = 0.01).33 Similarly, in a large cohort of noncritically ill older adults admitted to the same five Toronto hospitals, delirium increased the odds of mortality overall.1 This raises the concern that delirium assessment in critically ill patients may not be accurate because of confounding medications and sepsis.34 Nevertheless, this finding must be interpreted with caution given the limited sample size, and the potential for the presence of hypoactive delirium and detection bias.
We found a significantly higher use of midazolam infusions among hospital nonsurvivors than survivors. This may be attributable to the higher illness severity, higher ARDS incidence, or palliation, which necessitated the use of additional sedative agents. The high use of midazolam in the entire cohort is inconsistent with the Choosing Wisely Canada guidelines, which recommend avoidance of benzodiazepines in older adults, particularly given the suggested association with increased mortality.35 A multicentred, propensity matched study of mechanically ventilated patients ≥ 18 yr reported lower hospital mortality in patients sedated with propofol vs midazolam (RR, 0.76; 95% CI, 0.69 to 0.82).36 A prospective, multicentre study also found that a higher cumulative dose of benzodiazepines was associated with increased odds of 90-day mortality.37
More nonsurvivors had a DNR designation at ICU admission than survivors did. This could be attributable to care teams and families recognizing the risk of poor outcomes among these patients. Nevertheless, nonsurvivors had long stays in the ICU before they died, and approximately half of them died in the ICU following withdrawal of life support. Our data reinforces the importance of early goals-of-care conversations in providing patient-centred care, as well as time-limited ICU trials.
Strengths and limitations
This study is among the first to characterize the management and outcomes of critically ill, older adults with COVID-19. The multicentre retrospective design allowed us to include a larger cohort of consecutive patients across different Toronto ICUs, improving the precision and representativeness of the findings. A study investigator supervised the chart review at each site to ensure consistent data abstraction. Our study has limitations. The data were collected retrospectively, and some data were missing. While techniques such as multiple imputation were used to address missing data, the accuracy and availability of our data were also limited to electronic medical records. We were unable to evaluate the impact of different COVID-19 variants on patient management and outcomes. Furthermore, because only a small proportion of patients received a COVID-19 vaccine, we were unable to investigate the role of vaccination status on outcomes. The impact of other sociodemographic variables such as race and ethnicity were not assessed. We also lack data on morbidity and mortality beyond the hospital stay, and on quality-of-life following recovery from serious COVID-19 illness.
Conclusions
In this retrospective cohort of older adults admitted to the ICU with COVID-19, approximately half died in hospital. Across all patients, over half required IMV and vasopressors. A higher APACHE II score, increasing age, and the need for organ support were associated with increased odds of hospital mortality while female sex was associated with decreased odds. Further prospective research is required to better understand the risk factors for adverse events and provide more long-term data on physical and cognitive outcomes.
References
Wong EK, Watt J, Zou H, et al. Mortality in hospitalized older adults with COVID-19 during three waves: a multicenter retrospective cohort study. Health Sci Rep 2022; 5: e603. https://doi.org/10.1002/hsr2.603
Canadian Institute for Health Information. COVID-19 hospitalization and emergency department statistics, 2023. Available from URL: https://www.cihi.ca/en/covid-19-hospitalization-and-emergency-department-statistics (accessed February 2023).
Pun BT, Badenes R, La Calle GH, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med 2021; 9: 239–50. https://doi.org/10.1016/s2213-2600(20)30552-x
Chang R, Elhusseiny KM, Yeh YC, Sun WZ. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes—a systematic review and meta-analysis. PLoS One 2021; 16: e0246318. https://doi.org/10.1371/journal.pone.0246318
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–7. https://doi.org/10.7326/0003-4819-147-8-200710160-00010
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. https://doi.org/10.1016/j.jbi.2008.08.010
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–95. https://doi.org/10.1503/cmaj.050051
Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26: 1793–800. https://doi.org/10.1097/00003246-199811000-00016
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–10. https://doi.org/10.1001/jama.2016.0287
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–29.
Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001; 29: 1370–9. https://doi.org/10.1097/00003246-200107000-00012
Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med 2001; 27: 859–64. https://doi.org/10.1007/s001340100909
Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc 2005; 53: 312–8. https://doi.org/10.1111/j.1532-5415.2005.53120.x
Mishra P, Pandey CM, Singh U, Gupta A, Sahu C, Keshri A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth 2019; 22: 67–72. https://doi.org/10.4103/aca.aca_157_18
Vallet H, Schwarz GL, Flaatten H, de Lange DW, Guidet B, Dechartres A. Mortality of older patients admitted to an ICU: a systematic review. Crit Care Med 2021; 49: 324–34. https://doi.org/10.1097/ccm.0000000000004772
Modra L, Higgins A, Vithanage R, Abeygunawardana V, Bailey M, Bellomo R. Sex differences in illness severity and mortality among adult intensive care patients: a systematic review and meta-analysis. J Crit Care 2021; 65: 116–23. https://doi.org/10.1016/j.jcrc.2021.05.019
Simpson A, Puxty K, McLoone P, Quasim T, Sloan B, Morrison DS. Comorbidity and survival after admission to the intensive care unit: a population-based study of 41,230 patients. J Intensive Care Soc 2021; 22: 143–51. https://doi.org/10.1177/1751143720914229
Flaatten H, De Lange DW, Morandi A, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years). Intensive Care Med 2017; 43: 1820–8. https://doi.org/10.1007/s00134-017-4940-8
Bingold TM, Lefering R, Zacharowski K, et al. Individual organ failure and concomitant risk of mortality differs according to the type of admission to ICU—a retrospective study of SOFA score of 23,795 patients. PLoS One 2015; 10: e0134329. https://doi.org/10.1371/journal.pone.0134329
GitHub. mice: Multivariate imputation by chained equations. Available from URL: https://github.com/amices/mice (accessed February 2023).
The R Foundation. The R project in statistical computing. Available from URL: https://www.R-project.org/ (accessed February 2023).
Gkoufa A, Maneta E, Ntoumas GN, et al. Elderly adults with COVID-19 admitted to intensive care unit: a narrative review. World J Crit Care Med 2021; 10: 278–89. https://doi.org/10.5492/wjccm.v10.i5.278
Xu J, Yang X, Yang L, et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care 2020; 24: 394. https://doi.org/10.1186/s13054-020-03098-9
Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323: 1574–81. https://doi.org/10.1001/jama.2020.5394
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–81. https://doi.org/10.1016/s2213-2600(20)30079-5
Alshukry A, Ali H, Ali Y, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients in Kuwait. PLoS One 2020; 15: e0242768. https://doi.org/10.1371/journal.pone.0242768
Aleva FE, van Mourik L, Broeders ME, Paling AJ, de Jager CP. COVID-19 in critically ill patients in North Brabant, the Netherlands: patient characteristics and outcomes. J Crit Care 2020; 60: 111–5. https://doi.org/10.1016/j.jcrc.2020.08.001
Larsson E, Brattström O, Agvald-Öhman C, et al. Characteristics and outcomes of patients with COVID-19 admitted to ICU in a tertiary hospital in Stockholm, Sweden. Acta Anaesthesiol Scand 2021; 65: 76–81. https://doi.org/10.1111/aas.13694
Dres M, Hajage D, Lebbah S, et al. Characteristics, management, and prognosis of elderly patients with COVID-19 admitted in the ICU during the first wave: insights from the COVID-ICU study: prognosis of COVID-19 elderly critically ill patients in the ICU. Ann Intensive Care 2021; 11: 77. https://doi.org/10.1186/s13613-021-00861-1
Galbadage T, Peterson BM, Awada J, et al. Systematic review and meta-analysis of sex-specific COVID-19 clinical outcomes. Front Med 2020; 7: 348. https://doi.org/10.3389/fmed.2020.00348
Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res 2020; 116: 2197–206. https://doi.org/10.1093/cvr/cvaa284
Williamson CA, Faiver L, Nguyen AM, Ottenhoff L, Rajajee V. Incidence, predictors and outcomes of delirium in critically ill patients with COVID-19. Neurohospitalist 2022; 12: 31–7. https://doi.org/10.1177/19418744211034815
Mendes A, Herrmann FR, Périvier S, Gold G, Graf CE, Zekry D. Delirium in older patients with COVID-19: prevalence, risk factors, and clinical relevance. J Gerontol A Biol Sci Med Sci 2021; 76: e142–6. https://doi.org/10.1093/gerona/glab039
Oxenbøll-Collet M, Egerod I, Christensen V, Jensen J, Thomsen T. Nurses’ and physicians’ perceptions of confusion assessment method for the intensive care unit for delirium detection: focus group study. Nurs Crit Care 2018; 23: 16–22. https://doi.org/10.1111/nicc.12254
Klein S, Mayer D. Choosing Wisely Canada recommendations. Can Fam Physician 2017; 63: e473.
Lonardo NW, Mone MC, Nirula R, et al. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am J Respir Crit Care Med 2014; 189: 1383–94. https://doi.org/10.1164/rccm.201312-2291oc
Aragón RE, Proaño A, Mongilardi N, et al. Sedation practices and clinical outcomes in mechanically ventilated patients in a prospective multicenter cohort. Crit Care 2019; 23: 130. https://doi.org/10.1186/s13054-019-2394-9
Author contributions
Eric K. C. Wong, Jennifer Watt, Richard Norman, Katrina Piggott, Sharon E. Straus, Barbara Liu, and Sangeeta Mehta contributed to the study conception and design. Data collection was performed by Kiyan Heybati, Eric K. C. Wong, Hanyan Zou, Arthana Chandraraj, and Alissa W. Zhang. Data analysis was performed by Eric Kai-Chung Wong. The first draft of the manuscript was written by Kiyan Heybati, Eric K. C. Wong, and Sangeeta Mehta and all authors commented on previous versions of the manuscript.
Disclosures
None.
Funding statement
Academic Health Science Centre Alternate Funding Plans Innovative Funds from Unity Health Toronto; Sinai Health/University Health Network Healthy Ageing and Geriatrics Program and its Geriatrics Summer Scholars Program; Division of Geriatric Medicine and General Internal Medicine, Sunnybrook Health Sciences Centre. The sponsor has no role in this study’s design, method, patient recruitment, data collection, analysis, or the manuscript. SES is funded by a Tier 1 Canada Research Chair. EKW is funded by the Clinician Scientist Training Program at the University of Toronto and the Vanier Scholarship from the Canadian Institutes of Health Research.
Prior conference presentations
Dr. Heybati presented this research at the Critical Care Canada Forum (28 November–1 December 2022, Toronto, ON, Canada).
Editorial responsibility
This submission was handled by Dr. Alexis F. Turgeon, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12630_2023_2518_MOESM1_ESM.pdf
Supplementary file1 (PDF 705 KB)—eTable 1 STROBE checklist. eTable 2 Additional ventilatory interventions and parameters, stratified by hospital outcome. eFig. 1 Lowest PaO2/FIO2 ratio on ICU days 1 to 7. eFig. 2 Median tidal volume on ICU days 1 to 7. eFig. 3 Lowest arterial pH on ICU days 1 to 7. eFig. 4 Lowest arterial pH on ICU days 1 to 7. eFig. 5 Highest plateau pressure on ICU days 1 to 7. eFig. 6 Lowest mean arterial pressure on ICU days 1 to 7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heybati, K., Wong, E.K.C., Watt, J. et al. Outcomes of critically ill older adults with COVID-19: a multicentre retrospective cohort study. Can J Anesth/J Can Anesth 70, 1371–1380 (2023). https://doi.org/10.1007/s12630-023-02518-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02518-y