Abstract
Background
There are limited numbers of studies focusing on renal effects of coronavirus disease 2019 (COVID-19) infection and proximal tubular dysfunction in children with COVID-19 infections. The purpose of this study was to evaluate the functions of the proximal tubule in hospitalized children with confirmed acute COVID-19.
Methods
The children who were hospitalized for confirmed COVID-19 were included in this prospective descriptive analysis. The presence of at least two of the following four abnormalities was used to diagnose proximal tubule injury: abnormal tubular reabsorption of phosphate, normoglycemic glycosuria, hyperuricosuria, and proteinuria.
Results
A total of 115 patients were included in the study. About a third of the individuals had elevated blood creatinine levels or proteinuria. In addition, abnormal renal tubular phosphate loss measured by renal tubular phosphate loss was found in 10 (8.7%) patients, as was hyperuricosuria in 28.6%. As a result, total proximal tubular dysfunction was found in 24 (20.9%) patients.
Conclusions
One in every five children with acute COVID-19 infections had proximal tubular dysfunction, according to our data. Although, the rate of proximal tubular dysfunction was lower than in adults, it should be noted. The recovery of proximal tubular function in children with COVID-19 should be followed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Except for multisystemic inflammatory syndrome associated in children (MIS-C) associated with coronavirus disease 2019 (COVID-19), the clinical course and outcome of COVID-19 are significantly better in pediatric patients than in adults [1, 2]. The studies conducted in China and Italy indicated that children are mildly affected, accounting for approximately 5% of cases and less than 1% of hospital admissions [1, 3]. Despite the fact that the majority of clinical presentations are due to pulmonary involvement, extra-pulmonary manifestations, such as gastrointestinal, cardiovascular, hematological, musculoskeletal, and endocrinological, as well as the renal system, may occur [4].
COVID-19-related renal impairments have been studied primarily in adult patients. Proteinuria and hematuria were the most frequently reported clinical findings in one study of 701 adults with COVID-19, occurring in 43.9% and 26.7% of patients, respectively and followed by elevated blood urea nitrogen, elevated serum creatinine, and low glomerular filtration rates [5]. The primary route of the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into cells is via angiotensin-converting enzyme 2 (ACE2) receptor. These receptors are predominantly found in the lung, but also in the heart, gastrointestinal organs, and kidneys [16]; (5) eGFR was determined using the modified Schwartz criteria [17]; (6) normoglycemic glucosuria is defined as increased urine glucose excretion in the presence of normal serum glucose levels. The presence of glucose positivity in the urine with a dipstick was intended to be significant; (7) hyperuricosuria: after the age of three, the result obtained using the formula "uric acid (mg/dL) × serum creatinine (mg/dL)/urine creatinine (mg/dL)", which also uses glomerular filtration rate, is greater than 0.56 mg/dL, indicating hyperuricosuria [18]; (8) urinary uric acid/creatinine ratio: it was evaluated and described as normal according to the previous study performed on Turkish children [19]; (9) hypouricemia: a blood uric acid level of less than 2 mg/kg is considered as hypouricemia [20]; (10) proteinuria: the upper limit of normal for the spot urine protein-to-creatinine ratio changes by age as following; < 0.5 mg protein/mg creatinine at age 6–24 months (infants and toddlers); < 0.2 mg protein/mg creatinine at age 24 months to 18 years (children and young adults). The spot urine protein-to-creatinine ratio that is indicative of nephrotic range proteinuria is > 3 mg protein/mg creatinine [21]; (11) diagnosis of proximal tubular dysfunction: the presence of at least two of the following four abnormalities [22]; including the presence of increased renal tubular phosphate loss, normoglycemic glycosuria, hyperuricosuria, and proteinuria, was used to diagnose proximal tubular dysfunction.
Statistical analysis
SPSS statistical software (version 22; SPSS, Chicago, IL, USA) was used for the statistical analysis. The Student’s t test was used to compare continuous parametric variables, the Mann–Whitney U test was used to compare continuous nonparametric variables, and χ2 or Fisher’s exact tests were used when appropriate. A two-tailed P value of < 0.05 was considered to be statistically significant.
Ethical consent
This study was approved by the local Ethical Committee of Dr. Behcet Uz Children's Training and Research Hospital with the registration number 452 (approval number: 166; date: 09.24.2020).
Results
The demographics of the study population
A total of 115 hospitalized patients who met the study’s criteria were included in the study. Seventy-three (63.5%) of the patients were male, and 42 (47.9%) were female. The median age of patients was 10 years (range: 1 months to 17 years). Regarding the age distribution, 18 (15.7%) of the patients were under 1-year-old and 68 (59.1%) were 12 years and older. The median body weight of the patients was 39.0 kg (range: 3.9–115 kg), and the median height was 145 cm (range: 54–186 cm). Fifteen (13.0%) patients had body mass index (BMI) ≥ 25. Eight (6.9%) patients were overweight (BMI = 25–29.9), and seven (6.0%) patients were obese (BMI ≥ 30).
Fever (51, 44.3%) and cough (48, 41.7%) were the most common symptoms, followed by headache (29, 25.2%), myalgia (25, 21.7%), sore throat (19, 16.5%), diarrhea (18, 15.6%), chest pain (16, 14.0%), anosmia (15, 13.2%), vomiting (9, 7.8%), nasal discharge (8, 6.9%), rash (2, 1.7%).
The baseline characteristics of the laboratory tests
The average serum creatinine level was 0.6 ± 0.1 mg/dL (range: 0.4–1.00 mg/dL) with 38 (33.0%) patients having high creatinine levels (Table 1). The mean serum phosphate level was 4.5 ± 0.8 mg/dL. Hypophosphatemia was found in 10 (8.7%) patients of the whole group. The mean eGFR was 85.98 ± 18.38 (range: 44.6–131.4).
Evaluation of diagnostic criteria for proximal tubular injury
The median urinary protein/urinary creatinine ratio was 0.17 (range: 0.03–2.62) indicating 39 (33.9%) patients had proteinuria. The mean age in the patients with proteinuria was 87.54 ± 68.98 months (range: 1 month to 17 years) and the mean age of those without proteinuria was 118.57 ± 68.71 months (range: 2 months to 17 years). Mean age was significantly lower in the proteinuria group (P = 0.024). In the patients without proteinuria, 15.8% (n = 12) of the patients had microalbuminuria (Table 2).
Tubular phosphate leakage measured by TRP was present in 27 (23.5%) patients. TMP/eGFR values also revealed abnormal renal tubular phosphate loss (< 2.8 mg/dL) in 10 (8.7%) patients and normal range in 105 (91.3%) patients. Hypophosphatemia was found in 10 (8.7%) patients of the whole group. Two (1.73%) patients had normoglycemic glycosuria. Hyperuricosuria was present at 28.6% (n = 33) of the population. The median age in the patients with hyperuricosuria was 4 years (range: 1 month to 16 years), while that of patients without hyperuricosuria was 12.5 years (range: 2 months to 17 years). The median age was significantly lower in the patients with hyperuricosuria (P < 0.001). The percentage of patients with hypouricemia was 4.3% (n = 5).
Evaluation of patients for proximal tubule dysfunction
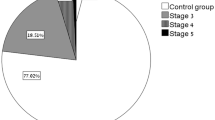
As shown in Table 2, 7 (6.0%) patients had both proteinuria and increased renal tubular phosphate loss measured by TmP/eGFR, 18 (15.6%) patients had both proteinuria and hyperuricosuria, and 3 (2.6%) patients had both increased renal tubular phosphate loss and hyperuricosuria. There were 24 (20.9%) patients with proximal tubular pathology based on the presence of two diagnostic criteria positivity (Table 2). Four (3.5%) patients had at least three positive diagnostic criteria (Table 2). The probability of occurrence of PTD was 0.209 (95% confidence interval 0.134–0.283).
The percentage of male patients was 58.3% (n = 14) in the PTD group and was 64.8% (n = 59) in the patients with normal proximal tubule function. No significant difference was present between these two groups (P > 0.05). The median age was 6 years (range: 1 month to 17 years) in the patients with PTD and was 10 years (range: 2 months to 17 years) in the patients without PTD. The median age was significantly lower in the patients with PTD (P = 0.028). A binary logistic regression model that specified presence/absence of PTD as a function of age (months) was not statistically significant, [χ2 = F(1, 115) = 79.1, P > 0.05], indicating that age could not be used to distinguish between presence or absence of PTD.
Discussion
In this prospective study, PTD was evaluated in children with COVID-19. About a third of the patients had higher serum creatinine levels or proteinuria. In addition, abnormal renal tubular phosphorus loss measured by TMP/eGFR was found in 10 (8.7%) patients, and hyperuricosuria was found in 28.6%. As a result, overall PTD was found in one of five patients in our study.
The kidney has been identified as a specific target for SARS-CoV-2 infection in several investigations [23,24,25,26]. The postmortem examination of the kidney revealed the accumulation of SARS-CoV-2 antigens in the renal epithelial cells [23]. Despite the published articles, limited studies have focused on COVID-19’s effect on proximal tubule functions [7, 22, 27]. Detection of viral particles of SARS-CoV-2 in the proximal tubular epithelium and podocytes associated with foot process effacement, vacuolation, and detachment of podocytes from the glomerular basement membrane indicated the involvement of the proximal tubules by SARS-CoV-2 [26]. Another renal impairment was SARS-CoV-2-related acute kidney injury and was generally reported in the critically ill patients [28, 29]. In our patient's group, no acute kidney injury related to SARS-CoV-2 was observed. This result is primarily related to the study group’s characteristics. We focused on children who did not require intensive care unit admission and were thus less likely to be affected by COVID-19 infections.
Werion et al. reported that 70–80% of the patients with COVID-19 had low-molecular-weight proteinuria and that 46% and 19% of the patients had an inappropriate urinary loss of uric acid and phosphate, respectively [7]. In another study 75% of the COVID-19 patients had at least two of the four criteria for PTD [22]. Patients requiring pediatric critical care units were more likely to show signs of renal involvement, such as proximal tubule injury, according to recent studies [22]. Despite the fact that the present study used similar diagnostic criteria, the rate of PTD was 20.8%, which was lower than the previous study. The higher rates of PTD in the previous study could have been attributed to a smaller sample size and to more patients being followed up in the critical care unit, which may be different from our study [22]. The administration of considerably more nephrotoxic medications to patients in the intensive care unit may be associated with a higher rate of PTD as a result of having concomitant diseases. For this reason, in our study patients receiving any nephrotoxic drugs and patients with comorbid diseases were excluded to provide homogeneity of the study group and to prevent any possible confounding with patients in the intensive care units. As a result, it is possible that PTD will be found in a significant number of children with COVID-19 and that enrolling patients in intensive care units will almost certainly raise the proportion with PTD.
According to our findings, proximal tubule dysfunction is found not only in adults but also in children with COVID-19. Children were reported to have fewer symptoms than adults, and the attributable mortality in the children was considerably lower than adults [1]. Although the pathogenesis of age-related differences of severity was not clearly understood, the variety of ACE2 and transmembrane protease, serine-subtype-2 (TMPRSS2) was one of the important candidates for pathogenesis [30]. One of the most popular hypotheses is that ACE and TMPRSS2 expression and affinity increase with age, while the opposing hypothesis is that ACE2 has anti-inflammatory properties [31,32,33,34]. Proximal tubular cells have been found to express high levels of ACE2 and TMPRSS2, making them a promising target for SARS-CoV-2 at the early stage [35]. Apart from renal cells, ACE2 is found in the parietal epithelium of Bowman’s capsule, collecting ducts, a thick ascending limb of Helen, podocytes, proximal cell brush border, and mesangial cells, implying that SARS-CoV-2 targets various sites [36]. As previously stated, the greater affinity of SARS-spike CoV-2’s protein for ACE was linked to disease severity [37]. This notion was supported by the fact that the proportion of children with PTD was lower than that of adults in investigations. On the other hand, the significantly younger age in the patients with PTD supported the ACE-related hypothesis and revealed unknown complicated mechanisms and interactions in addition to SARS-CoV-2 and ACE receptors on the proximal tubule cells.
Normoglycemic glycosuria was one of the PTD was defining criteria. Werion et al. reported that although other elements of PTD were present in the patient cohort with different rates, no normoglycemic glucosuria was recorded [7]. While, Korman et al. reported that normoglycemic glucosuria was present in 11 (28%) of the patients and that the majority of these patients came from intensive care units [22]. Only two (1.73%) patients in our study exhibited normoglycemic glucosuria, indicating that the children had a low rate compared to most adult studies. There were numerous causes for the difference in PTD and COVID-19 infections between adults and children in the literature. Besides the better prognosis of the COVID-19 infections, the comparatively low burden of chronic diseases and concomitant medications, the low rate of intensive care, and the mechanical ventilation requirement in children are key explanations for the difference.
Our study had several limitations, including a small number of measurements for some markers, lack of renal biopsy, and failure to detect SARS-CoV-2 at the proximal tubule cells, as well as the study’s single-center methodology. The current study’s findings, on the other hand, may be more credible because the study included more unvarying groups by the excluding patients in intensive care units, who may have more possible confounding factors. A better marker, such as low molecular weight proteins including beta-2 microglobulin and retinol-binding protein, might be more helpful; however, we were unable to conduct these tests. To our knowledge, this is one of the first comprehensive studies focusing on the effect of SARS-CoV-2 infections on the proximal tubule functions in children with COVID-19 infections. Our findings could help to design a screening test. In addition, the longer follow-up of the patients for recovery of PTD was missing, which would be planned in the future.
In conclusion, our findings suggested that 20.8% of the children with COVID-19 infections had proximal tubular dysfunction. Proximal tubular dysfunction has been documented not only in adults but also in children. Children with COVID-19 infections should be followed up for recovery of proximal tubule dysfunction. More research for the evaluation of renal effects of SARS-CoV-2 on pediatric patients is needed.
Data availability
The data and materials are available from the authors on reasonable request.
References
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–95.
Tagarro A, Epalza C, Santos M, Sanz-Santaeufemia FJ, Otheo E, Moraleda C, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid Spain. JAMA Pediatr. 2020;8:e201346.
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702.
Adukia SA, Ruhatiya RS, Maheshwarappa HM, Manjunath RB, Jain GN. Extrapulmonary features of COVID-19: a concise review. Indian J Crit Care Med. 2020;24:575–80.
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–38.
Ni W, Yang X, Yang D, Bao J, Li R, **ao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422.
Werion A, Belkhir L, Perrot M, Schmit G, Aydin S, Chen Z, et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98:1296–307.
Finer G, Landau D. Clinical approach to proximal renal tubular acidosis in children. Adv Chronic Kidney Dis. 2018;25:351–7.
Stewart DJ, Hartley JC, Johnson M, Marks SD, du Pré P, Stojanovic J. Renal dysfunction in hospitalised children with COVID-19. Lancet Child Adolesc Health. 2020;4:e28–9.
Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–8.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9.
World Health Organization. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. Accessed 19 Feb 2021.
Fang F, Chen Y, Zhao D, Liu T, Huang Y, Qiu L, et al. Recommendations for the diagnosis, prevention and control of the 2019 novel coronavirus infection in children-the Chinese perspectives. Front Pediatr. 2020;8:553394.
Alon U, Hellerstein S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol. 1994;8:250–1.
Payne RB. Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35:201–6.
Ashoor IF, Somers MJG. Physiology of the develo** kidney: fluid and electrolyte homeostasis and therapy of basic disorders. In: Avner E, editor. Pediatric nephrology. Berlin Heidelberg: Springer-Verlag; 2016. p. 1355–61.
Schwartz GJ, Furth SL. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol. 2007;22:1839–48.
Hoppe B, Leumann E, Milliner DS. Urolithiasis and nephrocalcinosis in childhood. In: Geary DF, Schaefer F, editors. Comprehensive pediatric nephrology. 1st ed. Philadelphia: Mosby; 2008. p. 499–526.
Poyrazoğlu HM, Düşünsel R, Yazici C, Durmaz H, Dursun I, Sahin H, et al. Urinary uric acid: creatinine ratios in healthy Turkish children. Pediatr Int. 2009;51:526–9.
Ogino K, Hisatome I, Saitoh M, Miyamoto J, Ishiko R, Hasegawa J, et al. Clinical significance of hypouricemia in hospitalized patients. J Med. 1991;22:76–82.
Houser M. Assessment of proteinuria using random urine samples. J Pediatr. 1984;104:845–8.
Kormann R, Jacquot A, Alla A, Corbel A, Koszutski M, Voirin P, et al. Coronavirus disease 2019: acute Fanconi syndrome precedes acute kidney injury. Clin Kidney J. 2020;13:362–70.
Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021;12:2506.
Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. 2020;31:1683–7.
Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46:1114–6.
Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–27.
Braun F, Huber TB, Puelles VG. Proximal tubular dysfunction in patients with COVID-19: what have we learnt so far? Kidney Int. 2020;98:1092–4.
Wang L, Li X, Chen H, Yan S, Li D, Li Y, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51:343–8.
Yang X, Yu Y, Xu J, Shu H, **a J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81.
Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2020. https://doi.org/10.1136/archdischild-2020-320338.
Muus C, Luecken MD, Eraslan G, Waghray A, Heimberg G, Sikkema L, et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv. 2020. https://doi.org/10.1101/2020.04.19.049254.
Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157:104833.
Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–9.
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–6.
Chen QL, Li JQ, **ang ZD, Lang Y, Guo GJ, Liu ZH. Localization of cell receptor-related genes of SARS-CoV-2 in the kidney through single-cell transcriptome analysis. Kidney Dis (Basel). 2020;6:258–70.
Martinez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol. 2020;318:1454–62.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271-80.e8.
Funding
None.
Author information
Authors and Affiliations
Contributions
DF involved in protocol development and manuscript writing. BE, KE, ŞS, CE, DM involved in data collection. KAA, AKÖ and KA involved in data collection, and microbiological investigations. YE and DN involved in protocol development and data collection. BN involved in protocol development, data collection. DI involved in data analysis and manuscript writing and will act as guarantor for the paper. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the local Ethical Committee of Dr. Behcet Uz Children's Training and Research Hospital with the registration number 452 (approval number: 166; date: 09.24.2020). Written consent for publication of the case details together with imaging or videos were obtained from participants (or their parent or legal guardian in the case of children under 16).
Conflict of interest
Ilker Devrim had educational grant from BD and Ilker Devrim has educational webinars for BD and Pfizer. However, all other authors including İlker Devrim have no conflicts of interest about this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devrim, F., Böncüoğlu, E., Kıymet, E. et al. Evaluation of proximal tubule functions in children with COVID-19: a prospective analytical study. World J Pediatr 18, 607–612 (2022). https://doi.org/10.1007/s12519-022-00552-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-022-00552-2