Abstract
We report the case of a 69-year-old man who presented acute leukemia with circulating blasts and numerous atypical mast cells in peripheral blood. Both blasts and mast cells presented identical cytochemistry (myeloperoxidase-negative, tartrate-resistant acid phosphatase–negative, esterase-negative; toluidine blue–positive; and acid phosphatase–positive). Flow cytometry showed a blast population MPO−, DR−, CD34−, CD117++, CD25+, and CD2−. Bone marrow histology revealed infiltration by CD25+, c-Kit+, and tryptase+ cells. Cytogenetics revealed 46,XY,t(8; 21)(q22; q22),der(12),t(12; 13)(p13; q12-14),del(13)(q14)[20]. The KIT D816V mutation was negative. Serum tryptase was very high. He received treatment with tyrosine kinase inhibitors and hydroxycarbamide without any response, and he died of respiratory distress with the finding at necropsy of pulmonary infiltration by mast cells. We highlight the rarity of the case that, in our opinion, could be classified as “acute” mast cell leukemia instead of systemic mastocytosis with an associated hematological neoplasia (WHO).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mast cell leukemia (MCL) is an aggressive form of systemic mastocytosis (SM). It accounts for less than 1% of all SM and may be de novo or secondary [1]. Its diagnosis is troublesome, and occasionally acute leukemia is observed accompanied by SM. The clinical manifestations are similar to those of acute leukemia, together with the characteristic activation symptoms: headache, diarrhea, skin lesions, gastrointestinal bleeding, fever, and pollakiuria, more frequent in patients with the KIT D816V mutation. Organomegaly are common in this type of leukemia.
For the diagnosis of MCL, the WHO requires SM criteria to be met and presence of more than 20% of atypical mast cells in the bone marrow or more than 10% in peripheral blood. However, aleukemic forms are frequent [2].
Clinical history
A 69-year-old male attended our center in May 2016 presenting atypical chest pain, asthenia, weight loss, and fever for 1 month. His personal history included type 2 diabetes mellitus, high blood pressure, dyslipidemia, hyperuricemia, sleep apnea, and ischemic heart disease; his medical history did not include any drug allergy or cutaneous symptoms. Physical examination revealed obesity and cutaneous ecchymosis.
The blood count showed leukocytosis with normocytic-normochromic anemia and thrombocytopenia. The results of other analyses highlighted prolonged prothrombin time and elevated d-dimer levels. The patient’s biochemistry showed high levels of LDH, vitamin B12, beta-2 microglobulin, and ferritin.
Materials and methods
Hemogram, biochemistry, and usual clinical tests
Study by peripheral blood (PB) smear. Other techniques used in this case study were modified Wright staining for bone marrow (BM) cytology, BM and PB staining with myeloperoxidase (MPO), dual esterase (chloroacetate esterase and butyrate esterase), toluidine blue, and tartrate-resistant acid phosphatase (TRAP). For the BM histology, hematoxylin-eosin (HE) staining was used, and immunohistochemistry was performed using peroxidase-antiperoxidase (PAP) and flow cytometry (FCM) (FACScalibur, Becton-Dickinson). The karyotype was performed by growing the metaphases in 10 ml in the MarrowPan medium for 24 h, with subsequent GTG banding. For the FISH technique, a vial 2 Vysis CLL FISH Probe kit 04no2-0022 (Abbot) was used; the assay was conducted in accordance with manufacturer’s guidelines.
Results
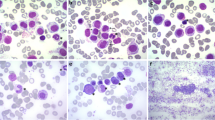
The presence of blasts in PB led to the suspicion of acute leukemia, and a cytology study, was performed (Wright). The PB smear revealed the following: 4% segmented neutrophils, 2% band neutrophils, 17% lymphocytes, 7% monocytes, 11% eosinophils, and 8% basophils. Furthermore, cells of fusiform appearance were observed, with eccentric nucleus and granulation located at one end, accounting for 13% of total peripheral blood cells, which were identified as type 1 atypical mast cells (Fig. 1b, d). Other cells accounting for 5% of cellularity were observed, with a very regular contour, bi- or multi-lobulated and cytoplasmic granulation similar to the typical granulation of basophiles that were identified morphologically as type 2 atypical mast cells (Fig. 1a). A cell population that was also observed constituted 31% of all cells; these cells were of blastic appearance and irregular in shape, with loss of the cytoplasm nucleus ratio. Their nucleus was highly basophilic and some had visible nucleoli (Fig. 1c, d). Other cells that were described were cells with hybrid granulation that accounted for 2% of the total (Fig. 1d).
Different cell types observed in peripheral blood smear (Wright stain magnification × 1000). a Atypical mast cell type II. It is symmetrical, morphologically similar to a basophil, but with greater granulation than this. b Atypical mast cell type I: elongated, fusiform cell, with polarized nucleus and lax chromatin, the granules are polarized at one end. c Blast cell: cell with loss of the cytoplasm nucleus ratio, large, with lax chromatin, and several visible nucleoli. d Atypical mast cell type I, blast cell, hybrid granulation cell
In the FCM study of PB, positivity was observed for CD38, CD22, CD13, CD33, CD117 (strong), CD123, CD4, CD25, CD64 (weak), and CD1a (weak). With other markers MPO, HLA-DR being negative, it was noteworthy that 53% of blasts were highly positive for CD117, positive for CD25, and negative for CD2. Besides, there was a small positive CD34 population representing 2.7% of total leukocytes (Fig. 2).
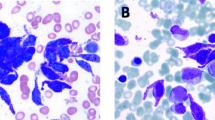
The bone marrow aspirate could not be assessed due to the inability to obtain marrow particles. In the bone marrow cylinder imprint, similar morphological characteristics with those described in peripheral blood were observed. Cytochemistry was performed on the imprint and in peripheral blood with the following findings: MPO, TRAP, chloroacetate, and butyrate esterase assays were negative (Fig. 3a, d), while toluidine blue and acid phosphatase tests were positive in the blastic population (Fig. 3b, c).
Cytochemistry stains. a Myeloperoxidase (magnification × 400): elongated fusiform cells, classified as atypical mast cells type I, are clearly negative for this staining, as well as blast cells. b Toluidine blue (magnification × 200). Staining was positive in atypical mast cells and blasts. c Acid phosphatase (magnification × 1000). Staining was very positive in fusiform cells identified as atypical mast cells. d Dual esterases (magnification × 200) performed in the bone marrow imprint, being negative for both stains
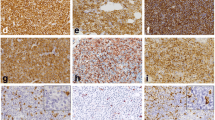
In the BM trephine biopsy, marrow was observed to be infiltrated by a proliferation of positive tryptase (Fig. 4d, e) and c-Kit cells (Fig. 4b) sometimes growing around lymph nodules, formed by lymphocytes with a predominance of the B CD20–positive population (Fig. 4a) located near the trabeculae. They were accompanied by reticulin fibrosis and intense eosinophilia CD15-positive. These cells were CD25-positive (Fig. 4f) and CD2-negative (Fig. 4c). A blastic population occupying the intertrabecular spaces was also observed, which was low-intensity MPO, and negative for CD34 and c-Kit.
Bone marrow immunohistochemistry. a Hematoxylin-eosin (magnification × 100): lymphoid nodule was observed. b c-Kit (magnification × 100): staining with anti-c-Kit was intensely positive. c CD2 (magnification × 100): staining with anti-CD2 was clearly negative. d Tryptase (magnification × 100): staining with anti-tryptase was clearly positive. e Tryptase (magnification × 200): detail of the fusiform cells identified as atypical mast cells type I, which are markedly striking for tryptase. f CD25 (magnification × 100): staining was clearly positive
Cytogenetics and molecular biology
A study of 20 metaphases was performed, demonstrating the presence of a cell clone with the following karyotype (complementary FISH was performed in order to conduct an adequate study): 46,XY,t(8;21)(q22;q22),der(12)t(12;13)(p13;q12-14),del(13)(q14)[20].ish der(12)t(12;13)(CEP12+,D13S319−,D13S1020+), which involved the translocations t(8;21)(q22;q22) with rearrangement of the RUNX1/RUNX1T1 genes, and t(12;13)(p13;q14) with rearrangement of ETV6. Quantitative PCR of t(8;21) was positive with an RUNX1/RUNX1T1 ratio of 2.005. FLT3 and NPM1 were not mutated, while the WT1 gene was positive with a ratio of 0.2617. The D816V mutation was also studied by PCR, with negative results, and the mutations of exons 10 and 17 of the KIT gene were studied by Sanger sequencing, also proving negative.
The determination of serum tryptase showed very high values, 998 ng/ml (normal values 0.0–11.0 ng/ml).
With all these results in mind, it was concluded that the patient was suffering from “acute” mast cell leukemia, which could be classified as Mast Cell Leukemia, WHO code: 9742/3 [1, 2].
Patient evolution
Treatment was started with tyrosine kinase inhibitors (TKI) and hydroxycarbamide [3]. In the first 15 days, an improvement in cytopenias was observed, but 5 days later, the patient experienced sudden dyspnea, tachypnea, and severe hypoxemia. A CT scan of the chest was performed, and multi-lobular bilateral consolidations with minimal pleural effusion and pulmonary edema were detected (Fig. 5); bronchoscopy revealed pigmented lesions similar to petechial lesions that were diffusely distributed throughout the trachea, carina, and both bronchial trees (Fig. 5). Four days later, the patient suffered rectal bleeding and a colonoscopy was performed without any significant findings. The patient began to present hemodynamic instability requiring admission to the ICU and died in June of the same year.
Thoracic CT scan, bronchoscopy images: Striking bilateral infiltrates are observed. Bronchoscopy petechial lesions in the area of the bronchial carina. Lung histology postmortem. a Hematoxylin-eosin (× 40). b CD25 (× 40). c CD2 (× 40). d CD117 (× 100). e Tryptase (× 100). Infiltration of cells with the phenotype observed in the peripheral blood (CD25-positive, CD2-negative, CD117-positive, tryptase-positive)
Ribcage necropsy was performed, and the same petechial lesions found during bronchoscopy were observed. The immunohistochemical study of these lesions showed positivity for CD117, and so the cells were interpreted as being mast cells (Fig. 5e), being their infiltration a very rare finding according to the current literature.
Discussion
This clinical picture meets the required criteria for classification as MCL with a high percentage of circulating mast cell precursors as well as undifferentiated blasts, together with a cytogenetic alteration (t(8;21)) typical of acute myeloid leukemia [4]. However, it does present some peculiarities that could lead to the classification of this case as “acute” mast cell leukemia rather than two concomitant hematological processes [5].
The patient had typical symptoms of any acute leukemia, with no previous history or typical symptoms of systemic mastocytosis activation, without organomegaly or KIT D816V mutation. It should be noted that lung infiltration by mast cells is a very rare finding.
In peripheral blood smears, typical precursor cells of mast cells were observed along with other undifferentiated blasts which, in the immunophenotypic study, were CD117-positive (strong)/CD25-positive/CD2-negative, a typical phenotype of mast cells together with other myeloid markers, in a uniform MPO-negative population [6, 7]. The cytochemistry results found in blasts were superimposable to those of mast cells [8].
There is no typical cytogenetic abnormality of mast cell leukemia; although cases with normal karyotypes del(5q), del(7), t(10;16) (q22; q13q22) and t(8; 21) (q22; q22) have been described in the literature [1], we have not found any case with the karyotype of our patient. There are only cases of t(8;21) when SM is associated with acute leukemia concomitantly [5]. While it is true that the current WHO classification categorizes the t(8;21) RUNX1/RUNX1T1 mutation as an independent category from acute myeloid leukemia, it does not, however, contemplate the fact that it has another associated translocation as with our patient [9].
Recently, a new entity has been described: myelo-mast cell leukemia, although it does not have such high tryptase concentrations nor atypical circulating mast cells and neither is the phenotype consistent with our case [10, 11].
In our case, the KIT D816V mutation was not detected. In the literature, cases are reported without mutations associated with this gene, as well as with mutations in exon 9, exon 11, and exon 13 (in these cases, the sequencing of other genes was not performed during diagnosis) [1, 6, 7]. The fact of not having the KIT D816V mutation could mean a worse prognosis, which could justify our patient’s poor evolution.
Finally, we would like to emphasize the low frequency of this disease and its difficult diagnosis and cataloging which require the collaboration of different specialists. Treatment is not fully defined, and the prognosis is poor. The recent approval of midostaurin could be a useful tool in the treatment of these diseases.
Abbreviations
- MCL:
-
Mast cell leukemia
- SM:
-
Systemic mastocytosis
- PB:
-
Peripheral blood
- BM:
-
Bone marrow
- MPO:
-
Myeloperoxidase
- TRAP:
-
Tartrate-resistant acid phosphatase
- H-E:
-
Hematoxylin-eosin
- PAP:
-
Peroxidase-antiperoxidase
- FCM:
-
Flow cytometry
- TKI:
-
Tyrosine kinase inhibitors
References
Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G (2013) Mast cell leukemia. Blood 121(8):1285–1295
Horny HP, Akin C, Arber DA et al (2017) Mastocytosis. In: Swerdlow SH, Campo E, Harris NL et al (eds) WHO classification of tumors of haematopoietic and lymphoid tissues, 4th edn. IARC, Lyon, pp 62–69
Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, Castells M, Sperr WR, Kluin-Nelemans HC, Hamdy NA, Lortholary O, Robyn J, van Doormaal J, Sotlar K, Hauswirth AW, Arock M, Hermine O, Hellmann A, Triggiani M, Niedoszytko M, Schwartz LB, Orfao A, Horny HP, Metcalfe DD (2007) Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Investig 37(6):435–453
Bernd HW, Sotlar K, Lorenzen J, Osieka R, Fabry U, Valent P, Horny HP (2004) Acute myeloid leukaemia with t(8;21) associated with “occult” mastocytosis. Report of an unusual case and review of the literature. J Clin Pathol 57:324–328
Arredondo AR, Gotlib J, Shier L, Medeiros B, Wong K, Cherry A, Corless C, Arber DA, Valent P, George TI (2010) Myelomastocytic leukemia versus mast cell leukemia versus systemic mastocytosis associated with acute myeloid leukemia: a diagnostic challenge. Am J Hematol 85(8):600–606
Teodosio C, Garcia-Montero AC, Jara-Acevedo M et al (2012) An immature immunophenotype of bone marrow mast cells predicts for multilineage D816V KIT mutation in systemic mastocytosis. Leukemia 26(5):951–958
Daniel MT, Flandrin G, Bernard J (1975) Acute mast cell leukemia. Cytochemical and ultrastructural study, about a particular case. Nouv Rev Fr Hematol 15(3):319–332
Noack F, Sotlar K, Notter M, Thiel E, Valent P, Horny HP (2004) Aleukemic mast cell leukemia with abnormal immunophenotype and c-kit mutation D816V. Leuk Lymphoma 45(11):2295–2302
Pullarkat ST, Pullarkat V, Kroft SH, Wilson CS, Ahsanuddin AN, Mann KP, Thein M, Grody WW, Brynes RK (2009) Systemic mastocytosis associated with t(8;21)(q22;q22)acute myeloid leukemia. J Hematop 2:27–33
Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A, George TI, Kluin-Nelemans HC, Hermine O, Butterfield JH, Hägglund H, Ustun C, Hornick JL, Triggiani M, Radia D, Akin C, Hartmann K, Gotlib J, Schwartz LB, Verstovsek S, Orfao A, Metcalfe DD, Arock M, Horny HP (2014) Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol 25:1691–1700
Baghestanian M, Bankl H, Sillaber C et al (1996) A case of malignant mastocytosis with circulating mast cell precursors: biologic and phenotypic characterization of the malignant clone. Leukemia 10(1):159–166
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The patient before his death gave his consent in the publication of these images.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lerma-Verdejo, A., Forés, R., Espinosa-Hevia, L. et al. Mast cell leukemia with t(8;21) and t(12;13): can we classify it as acute mast cell leukemia?. J Hematopathol 13, 57–62 (2020). https://doi.org/10.1007/s12308-020-00385-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-020-00385-w