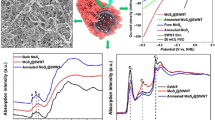
Abstract
Rational design and construction of low-cost and highly efficient electrocatalysts for hydrogen evolution reaction (HER) is meaningful but challenging. Herein, a robust three dimensional (3D) hollow CoSe2@ultrathin MoSe2 core@shell heterostructure (CoSe2@MoSe2) is proposed as an efficient HER electrocatalyst through interfacial engineering. Benefitting from the abundant heterogeneous interfaces on CoSe2@MoSe2, the exposed edge active sites are maximized and the charge transfer at the hetero-interfaces is accelerated, thus facilitating the HER kinetics. It exhibits remarkable performance in pH-universal conditions. Notably, it only needs an overpotential (η10) of 108 mV to reach a current density of 10 mA·cm−2 in 1.0 M KOH, outperforming most of the reported transition metal selenides electrocatalysts. Density functional theory (DFT) calculations unveil that the heterointerfaces synergistically optimize the Gibbs free energies of H2O and H* during alkaline HER, accelerating the reaction kinetics. The present work may provide new construction guidance for rational design of high-efficient electrocatalysts.

Similar content being viewed by others
References
Zhou, K. L.; Wang, Z.; Han, C. B.; Ke, X.; Wang, C.; **, Y.; Zhang, Q.; Liu, J.; Wang, H.; Yan, H. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nat. Commun. 2021, 12, 3783.
Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S. Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem., Int. Ed. 2015, 54, 52–65.
Shang, H. S.; Zhao, Z. H.; Pei, J. J.; Jiang, Z. L.; Zhou, D. N.; Li, A.; Dong, J. C.; An, P. F.; Zheng, L. R.; Chen, W. X. Dynamic evolution of isolated Ru-FeP atomic interface sites for promoting the electrochemical hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 22607–22612.
**u, L. Y.; Pei, W.; Zhou, S.; Wang, Z. Y.; Yang, P. J.; Zhao, J. J.; Qiu, J. S. Multilevel hollow MXene tailored low-Pt catalyst for efficient hydrogen evolution in full-pH range and seawater. Adv. Funct. Mater. 2020, 30, 1910028.
Yao, Y. C.; Hu, S. L.; Chen, W. X.; Huang, Z. Q.; Wei, W. C.; Yao, T.; Liu, R. R.; Zang, K. T.; Wang, X. Q.; Wu, G. et al. Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis. Nat. Catal. 2019, 2, 304–313.
Staszak-Jirkovsky, J.; Malliakas, C. D.; Lopes, P. P.; Danilovic, N.; Kota, S. S.; Chang, K. C.; Genorio, B.; Strmcnik, D.; Stamenkovic, V. R.; Kanatzidis, M. G. et al. Design of active and stable Co-Mo-Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 2016, 15, 197–203.
Wang, X. S.; Xu, C. C.; Jaroniec, M.; Zheng, Y.; Qiao, S. Z. Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions. Nat. Commun. 2019, 10, 4876.
Sheng, W. C.; Myint, M.; Chen, J. G.; Yan, Y. S. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 2013, 6, 1509–1512.
Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; van der Vliet, D.; Paulikas, A. P.; Stamenkovic, V. R.; Markovic, N. M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 2013, 5, 300–306.
Ma, F. X.; Wu, H. B.; **a, B. Y.; Xu, C. Y.; Lou, X. W. Hierarchical β-Mo2C nanotubes organized by ultrathin nanosheets as a highly efficient electrocatalyst for hydrogen production. Angew. Chem., Int. Ed. 2015, 54, 15395–15399.
Mao, J. J.; He, C. T.; Pei, J. J.; Chen, W. X.; He, D. S.; He, Y. Q.; Zhuang, Z. B.; Chen, C.; Peng, Q.; Wang, D. S. et al. Accelerating water dissociation kinetics by isolating cobalt atoms into ruthenium lattice. Nat. Commun. 2018, 9, 4958.
Pan, Y.; Sun, K. A.; Lin, Y.; Cao, X.; Cheng, Y. S.; Liu, S. J.; Zeng, L. Y.; Cheong, W. C.; Zhao, D.; Wu, K. L. et al. Electronic structure and d-band center control engineering over M-doped CoP (M = Ni, Mn, Fe) hollow polyhedron frames for boosting hydrogen production. Nano Energy 2019, 56, 411–419.
Mao, J. J.; Yin, J. S.; Pei, J. J.; Wang, D. S.; Li, Y. D. Single atom alloy: An emerging atomic site material for catalytic applications. Nano Today 2020, 34, 100917.
Yang, Y.; Yao, H. Q.; Yu, Z. H.; Islam, S. M.; He, H. Y.; Yuan, M. W.; Yue, Y. H.; Xu, K.; Hao, W. C.; Sun, G. B. et al. Hierarchical nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a highly efficient electrocatalyst for overall water splitting in a wide pH range. J. Am. Chem. Soc. 2019, 141, 10417–10430.
Najafi, L.; Bellani, S.; Oropesa-Nuñez, R.; Ansaldo, A.; Prato, M.; Del Rio Castillo, A. E.; Bonaccorso, F. Engineered MoSe2-based heterostructures for efficient electrochemical hydrogen evolution reaction. Adv. Energy Mater. 2018, 8, 1703212.
Fu, Q.; Han, J. C.; Wang, X. J.; Xu, P.; Yao, T.; Zhong, J.; Zhong, W. W.; Liu, S. W.; Gao, T. L.; Zhang, Z. H. et al. 2D transition metal dichalcogenides: Design, modulation, and challenges in electrocatalysis. Adv. Mater. 2021, 33, 1907818.
Wang, Q. C.; Lei, Y. P.; Wang, Y. C.; Liu, Y.; Song, C. Y.; Zeng, J.; Song, Y. H.; Duan, X. D.; Wang, D. S.; Li, Y. D. Atomic-scale engineering of chemical-vapor-deposition-grown 2D transition metal dichalcogenides for electrocatalysis. Energy Environ. Sci. 2020, 13, 1593–1616.
Chia, X. Y.; Eng, A. Y. S.; Ambrosi, A.; Tan, S. M.; Pumera, M. Electrochemistry of nanostructured layered transition-metal dichalcogenides. Chem. Rev. 2015, 115, 11941–11966.
Li, H. Y.; Chen, S. M.; Zhang, Y.; Zhang, Q. H.; Jia, X. F.; Zhang, Q.; Gu, L.; Sun, X. M.; Song, L.; Wang, X. Systematic design of superaerophobic nanotube-array electrode comprised of transition-metal sulfides for overall water splitting. Nat. Commun. 2018, 9, 2452.
Li, H. Y.; Chen, S. M.; Jia, X. F.; Xu, B.; Lin, H. F.; Yang, H. Z.; Song, L.; Wang, X. Amorphous nickel-cobalt complexes hybridized with 1T-phase molybdenum disulfide via hydrazine-induced phase transformation for water splitting. Nat. Commun. 2017, 8, 15377.
Zhang, J. T.; Chen, Y. L.; Liu, M.; Du, K.; Zhou, Y.; Li, Y. P.; Wang, Z. J.; Zhang, J. 1T@2H-MoSe2 nanosheets directly arrayed on Ti plate: An efficient electrocatalytic electrode for hydrogen evolution reaction. Nano Res. 2018, 11, 4587–4598.
Zheng, X. R.; Han, X. P.; Cao, Y. H.; Zhang, Y.; Nordlund, D.; Wang, J. H.; Chou, S. L.; Liu, H.; Li, L. L.; Zhong, C. et al. Identifying dense NiSe2/CoSe2 heterointerfaces coupled with surface high-valence bimetallic sites for synergistically enhanced oxygen electrocatalysis. Adv. Mater. 2020, 32, 2000607.
Hu, X. M.; Zhang, S. L.; Sun, J. W.; Yu, L.; Qian, X. Y.; Hu, R. D.; Wang, Y. N.; Zhao, H. G.; Zhu, J. W. 2D Fe-containing cobalt phosphide/cobalt oxide lateral heterostructure with enhanced activity for oxygen evolution reaction. Nano Energy 2019, 56, 109–117.
Qian, Q. Z.; Zhang, J. H.; Li, J. M.; Li, Y. P.; **, X.; Zhu, Y.; Liu, Y.; Li, Z. Y.; El-Harairy, A.; **ao, C. et al. Artificial heterointerfaces achieve delicate reaction kinetics towards hydrogen evolution and hydrazine oxidation catalysis. Angew. Chem., Int. Ed. 2021, 60, 5984–5993.
Zhao, B.; Liu, J. W.; Xu, C. Y.; Feng, R. F.; Sui, P. F.; Luo, J. X.; Wang, L.; Zhang, J. J.; Luo, J. L.; Fu, X. Z. Interfacial engineering of Cu2Se/Co3Se4 multivalent hetero-nanocrystals for energy-efficient electrocatalytic co-generation of value-added chemicals and hydrogen. Appl. Catal. B:Environ. 2021, 285, 119800.
Liu, C. C.; Gong, T.; Zhang, J.; Zheng, X. R.; Mao, J.; Liu, H.; Li, Y.; Hao, Q. Y. Engineering Ni2P-NiSe2 heterostructure interface for highly efficient alkaline hydrogen evolution. Appl. Catal. B: Environ. 2020, 262, 118245.
Jiao, J. Q.; Yang, W. J.; Pan, Y.; Zhang, C.; Liu, S. J.; Chen, C.; Wang, D. S. Interface engineering of partially phosphidated Co@CoP@NPCNTs for highly enhanced electrochemical overall water splitting. Small 2020, 16, 2002124.
Lu, K.; Liu, Y. Z.; Lin, F.; Cordova, I. A.; Gao, S. Y.; Li, B. M.; Peng, B.; Xu, H. P.; Kaelin, J.; Coliz, D. et al. LixNiO/Ni heterostructure with strong basic lattice oxygen enables electrocatalytic hydrogen evolution with Pt-like activity. J. Am. Chem. Soc. 2020, 142, 12613–12619.
Li, Y. B.; Tan, X.; Tan, H.; Ren, H. J.; Chen, S.; Yang, W. F.; Smith, S. C.; Zhao, C. Phosphine vapor-assisted construction of heterostructured Ni2P/NiTe2 catalysts for efficient hydrogen evolution. Energy Environ. Sci. 2020, 13, 1799–1807.
Zhai, P. L.; Zhang, Y. X.; Wu, Y. Z.; Gao, J. F.; Zhang, B.; Cao, S. Y.; Zhang, Y. T.; Li, Z. W.; Sun, L. C.; Hou, J. G. Engineering active sites on hierarchical transition bimetal oxides/sulfides heterostructure array enabling robust overall water splitting. Nat. Commun. 2020, 11, 5462.
Yang, L.; Huang, L. T.; Yao, Y. H.; Jiao, L. F. In-situ construction of lattice-matching NiP2/NiSe2 heterointerfaces with electron redistribution for boosting overall water splitting. Appl. Catal. B:Environ. 2021, 282, 119584.
Chen, P. Z.; Xu, K.; Tao, S.; Zhou, T. P.; Tong, Y.; Ding, H.; Zhang, L. D.; Chu, W. S.; Wu, C. Z.; **e, Y. Phase-transformation engineering in cobalt diselenide realizing enhanced catalytic activity for hydrogen evolution in an alkaline medium. Adv. Mater. 2016, 28, 7527–7532.
Zhang, H.; Wang, T.; Sumboja, A.; Zang, W.; **e, J.; Gao, D.; Pennycook, S. J.; Liu, Z.; Guan, C.; Wang, J. Integrated hierarchical carbon flake arrays with hollow P-doped CoSe2 nanoclusters as an advanced bifunctional catalyst for Zn-air batteries. Adv. Funct. Mater. 2018, 28, 1804846.
Zhang, L. L.; Zhang, T. T.; Dai, K. Q.; Zhao, L. Q.; Wei, Q. H.; Zhang, B.; **ang, X. Ultrafine Co3O4 nanolayer-shelled CoWP nanowire array: A bifunctional electrocatalyst for overall water splitting. RSC Adv. 2020, 10, 29326–29335.
Liu, H.; Liu, B. H.; Guo, H.; Liang, M. F.; Zhang, Y. H.; Borjigin, T.; Yang, X. F.; Wang, L.; Sun, X. L. N-doped C-encapsulated scalelike yolk-shell frame assembled by expanded planes few-layer MoSe2 for enhanced performance in sodium-ion batteries. Nano Energy 2018, 51, 639–648.
Lyu, F. L.; Bai, Y. C.; Li, Z. W.; Xu, W. J.; Wang, Q. F.; Mao, J.; Wang, L.; Zhang, X. W.; Yin, Y. D. Self-templated fabrication of CoO-MoO2 nanocages for enhanced oxygen evolution. Adv. Funct. Mater. 2017, 27, 1702324.
Zhang, J. T.; Hu, H.; Li, Z.; Lou, X. W. Double-shelled nanocages with cobalt hydroxide inner shell and layered double hydroxides outer shell as high-efficiency polysulfide mediator for lithium-sulfur batteries. Angew. Chem., Int. Ed. 2016, 55, 3982–3986.
Yu, L.; **a, B. Y.; Wang, X.; Lou, X. W. General formation of M-MoS3 (M = Co, Ni) hollow structures with enhanced electrocatalytic activity for hydrogen evolution. Adv. Mater. 2016, 28, 92–97.
Zhang, T. R.; Ge, J. P.; Hu, Y. X.; Zhang, Q.; Aloni, S.; Yin, Y. D. Formation of hollow silica colloids through a spontaneous dissolution-regrowth process. Angew. Chem., Int. Ed. 2008, 47, 5806–5811.
Li, S. F.; Yu, C.; Yang, J.; Zhao, C. T.; Zhang, M. D.; Huang, H. W.; Liu, Z. B.; Guo, W.; Qiu, J. S. A superhydrophilic “nanoglue” for stabilizing metal hydroxides onto carbon materials for high-energy and ultralong-life asymmetric supercapacitors. Energy Environ. Sci. 2017, 10, 1958–1965.
**a, C.; Jiang, Q.; Zhao, C.; Hedhili, M. N.; Alshareef, H. N. Selenide-based electrocatalysts and scaffolds for water oxidation applications. Adv. Mater. 2016, 28, 77–85.
Hou, P.; Li, D.; Yang, N. L.; Wan, J. W.; Zhang, C. H.; Zhang, X. Q.; Jiang, H. Y.; Zhang, Q. H.; Gu, L.; Wang, D. Delicate control on the shell structure of hollow spheres enables tunable mass transport in water splitting. Angew. Chem., Int. Ed. 2021, 60, 6926–6931.
Wang, J. Y.; Wan, J. W.; Wang, D. Hollow multishelled structures for promising applications: Understanding the structure-performance correlation. Acc. Chem. Res. 2019, 52, 2169–2178.
Yousaf, M.; Wang, Y. S.; Chen, Y. J.; Wang, Z. P.; Firdous, A.; Ali, Z.; Mahmood, N.; Zou, R. Q.; Guo, S. J.; Han, R. P. S. A 3D trilayered CNT/MoSe2/C heterostructure with an expanded MoSe2 interlayer spacing for an efficient sodium storage. Adv. Energy Mater. 2019, 9, 1900567.
Gao, M. R.; Xu, Y. F.; Jiang, J.; Zheng, Y. R.; Yu, S. H. Water oxidation electrocatalyzed by an efficient Mn3O4/CoSe2 nanocomposite. J. Am. Chem. Soc. 2012, 134, 2930–2933.
Chen, J.; Pan, A. Q.; Wang, Y. P.; Cao, X. X.; Zhang, W. C.; Kong, X. Z.; Su, Q.; Lin, J. D.; Cao, G. Z.; Liang, S. Q. Hierarchical mesoporous MoSe2@CoSe/N-doped carbon nanocomposite for sodium ion batteries and hydrogen evolution reaction applications. Energy Storage Mater. 2019, 21, 97–106.
Sun, Z. H.; Wu, X. L.; Xu, J. N.; Qu, D. Y.; Zhao, B. L.; Gu, Z. Y.; Li, W. H.; Liang, H. J.; Gao, L. F.; Fan, Y. Y. et al. Construction of bimetallic selenides encapsulated in nitrogen/sulfur Co-doped hollow carbon nanospheres for high-performance sodium/potassium-ion half/full batteries. Small 2020, 16, 1907670.
Najafi, L.; Bellani, S.; Oropesa-Nuñez, R.; Martín-García, B.; Prato, M.; Pasquale, L.; Panda, J. K.; Marvan, P.; Sofer, Z.; Bonaccorso, F. TaS2, TaSe2, and their heterogeneous films as catalysts for the hydrogen evolution reaction. ACS Catal. 2020, 10, 3313–3325.
Suo, G. Q.; Zhang, J. Q.; Li, D.; Yu, Q. Y.; Wang, W.; He, M.; Feng, L.; Hou, X. J.; Yang, Y. L.; Ye, X. H. et al. N-doped carbon/ultrathin 2D metallic cobalt selenide core/sheath flexible framework bridged by chemical bonds for high-performance potassium storage. Chem. Eng. J. 2020, 388, 124396.
Guo, Y. X.; Yao, Z. Y.; Shang, C. S.; Wang, E. K. P doped Co2Mo3Se nanosheets grown on carbon fiber cloth as an efficient hybrid catalyst for hydrogen evolution. J. Mater. Chem. A 2017, 5, 12043–12047.
Li, X. H.; Guo, S. H.; Li, W.; Ren, X. G.; Su, J.; Song, Q.; Sobrido, A. J.; Wei, B. Q. Edge-rich MoS2 grown on edge-oriented three-dimensional graphene glass for high-performance hydrogen evolution. Nano Energy 2019, 57, 388–397.
Lu, X.; Utama, M. I. B.; Lin, J. H.; Gong, X.; Zhang, J.; Zhao, Y. Y.; Pantelides, S. T.; Wang, J. X.; Dong, Z. L.; Liu, Z. et al. Large-area synthesis of monolayer and few-layer MoSe2 films on SiO2 substrates. Nano Lett. 2014, 14, 2419–2425.
Sun, D.; Feng, S. M.; Terrones, M.; Schaak, R. E. Formation and interlayer decoupling of colloidal MoSe2 nanoflowers. Chem. Mater. 2015, 27, 3167–3175.
Huang, Y. P.; Miao, Y. E.; Fu, J.; Mo, S. Y.; Wei, C.; Liu, T. X. Perpendicularly oriented few-layer MoSe2 on SnO2 nanotubes for efficient hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 16263–16271.
Chen, W. H.; Qiao, R.; Song, C. S.; Zhao, L. H.; Jiang, Z. J.; Maiyalagan, T.; Jiang, Z. Q. Tailoring the thickness of MoSe2 layer of the hierarchical double-shelled N-doped carbon@MoSe2 hollow nanoboxes for efficient and stable hydrogen evolution reaction. J. Catal. 2020, 381, 363–373.
Yoon, H.; Song, H. J.; Ju, B.; Kim, D. W. Cobalt phosphide nanoarrays with crystalline-amorphous hybrid phase for hydrogen production in universal-pH. Nano Res. 2020, 13, 2469–2477.
Yu, B.; Qi, F.; Zheng, B. J.; Hou, W. Q.; Zhang, W. L.; Li, Y. R.; Chen, Y. F. Self-assembled pearl-bracelet-like CoSe2-SnSe2/CNT hollow architecture as highly efficient electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 1655–1662.
Kong, D. S.; Wang, H. T.; Lu, Z. Y.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900.
Xu, C. Y.; Li, Q. H.; Shen, J. L.; Yuan, Z.; Ning, J. Q.; Zhong, Y. J.; Zhang, Z. Y.; Hu, Y. A facile sequential ion exchange strategy to synthesize CoSe2/FeSe2 double-shelled hollow nanocuboids for the highly active and stable oxygen evolution reaction. Nanoscale 2019, 11, 10738–10745.
Li, K. D.; Zhang, J. F.; Wu, R.; Yu, Y. F.; Zhang, B. Anchoring CoO domains on CoSe2 nanobelts as bifunctional electrocatalysts for overall water splitting in neutral media. Adv. Sci. 2016, 3, 1500426.
Qiu, B. C.; Wang, C.; Zhang, N.; Cai, L. J.; **ong, Y. J.; Chai, Y. CeO2-induced interfacial Co2+ octahedral sites and oxygen vacancies for water oxidation. ACS Catal. 2019, 9, 6484–6490.
Gao, J. Y.; Li, Y. P.; Shi, L.; Li, J. J.; Zhang, G. Q. Rational design of hierarchical nanotubes through encapsulating CoSe2 nanoparticles into MoSe2/C composite shells with enhanced lithium and sodium storage performance. ACS Appl. Mater. Interfaces 2018, 10, 20635–20642.
Ma, M. Z.; Zhang, S. P.; Yao, Y.; Wang, H. Y.; Huang, H. J.; Xu, R.; Wang, J. W.; Zhou, X. F.; Yang, W. J.; Peng, Z. Q. et al. Heterostructures of 2D molybdenum dichalcogenide on 2D nitrogen-doped carbon: Superior potassium-ion storage and insight into potassium storage mechanism. Adv. Mater. 2020, 32, 2000958.
Zhu, H.; Zhang, J. F.; Yanzhang, R. P.; Du, M. L.; Wang, Q. F.; Gao, G. H.; Wu, J. D.; Wu, G. M.; Zhang, M.; Liu, B. et al. When cubic cobalt sulfide meets layered molybdenum disulfide: A core-shell system toward synergetic electrocatalytic water splitting. Adv. Mater. 2015, 27, 4752–4759.
Tang, B. S.; Yu, Z. G.; Zhang, Y. X.; Tang, C. H.; Seng, H. L.; Seh, Z. W.; Zhang, Y. W.; Pennycook, S. J.; Gong, H.; Yang, W. F. Metal-organic framework-derived hierarchical MoS2/CoS2 nanotube arrays as pH-universal electrocatalysts for efficient hydrogen evolution. J. Mater. Chem. A 2019, 7, 13339–13346.
Zhang, J. J.; Wu, M. H.; Liu, T.; Kang, W. P.; Xu, J. Hierarchical nanotubes constructed from interlayer-expanded MoSe2 nanosheets as a highly durable electrode for sodium storage. J. Mater. Chem. A 2017, 5, 24859–24866.
Ma, Y. F.; Chen, M.; Geng, H. B.; Dong, H. F.; Wu, P.; Li, X. M.; Guan, G. Q.; Wang, T. J. Synergistically tuning electronic structure of porous β-Mo2C spheres by Co do** and Mo-vacancies defect engineering for optimizing hydrogen evolution reaction activity. Adv. Funct. Mater. 2020, 30, 2000561.
Chen, Y. J.; Ren, Z. Y.; Fu, H. Y.; Zhang, X.; Tian, G. H.; Fu, H. G. NiSe-Ni0.85Se heterostructure nanoflake arrays on carbon paper as efficient electrocatalysts for overall water splitting. Small 2018, 14, 1800763.
Li, Y. G.; Wang, H. L.; **e, L. M.; Liang, Y. Y.; Hong, G. S.; Dai, H. J. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299.
Wan, J. W.; Zhao, Z. H.; Shang, H. S.; Peng, B.; Chen, W. X.; Pei, J. J.; Zheng, L. R.; Dong, J. C.; Cao, R.; Sarangi, R. et al. In situ phosphatizing of triphenylphosphine encapsulated within metal-organic frameworks to design atomic Co1-P1N3 interfacial structure for promoting catalytic performance. J. Am. Chem. Soc. 2020, 142, 8431–8439.
Deng, S. J.; Zhong, Y.; Zeng, Y. X.; Wang, Y. D.; Yao, Z. J.; Yang, F.; Lin, S. W.; Wang, X. L.; Lu, X. H.; **a, X. H. et al. Directional construction of vertical nitrogen-doped 1T-2H MoSe2/graphene shell/core nanoflake arrays for efficient hydrogen evolution reaction. Adv. Mater. 2017, 29, 1700748.
Zhao, G. Q.; Li, P.; Rui, K.; Chen, Y. P.; Dou, S. X.; Sun, W. P. CoSe2/MoSe2 heterostructures with enriched water adsorption/dissociation sites towards enhanced alkaline hydrogen evolution reaction. Chem. Eur. J. 2018, 24, 11158–11165.
Duan, J. J.; Chen, S.; Ortíz-Ledón, C. A.; Jaroniec, M.; Qiao, S. Z. Phosphorus vacancies that boost electrocatalytic hydrogen evolution by two orders of magnitude. Angew. Chem., Int. Ed. 2020, 59, 8181–8186.
Chen, X. S.; Liu, G. B.; Zheng, W.; Feng, W.; Cao, W. W.; Hu, W. P.; Hu, P. A. Vertical 2D MoO2/MoSe2 core-shell nanosheet arrays as high-performance electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2016, 26, 8537–8544.
Chen, W. S.; Gu, J. J.; Du, Y. P.; Song, F.; Bu, F. X.; Li, J. H.; Yuan, Y.; Luo, R. C.; Liu, Q. L.; Zhang, D. Achieving rich and active alkaline hydrogen evolution heterostructures via interface engineering on 2D 1T-MoS2 quantum sheets. Adv. Funct. Mater. 2020, 30, 2000551.
Zhou, P.; Lv, X. S.; **ng, D. N.; Ma, F. H.; Liu, Y. Y.; Wang, Z. Y.; Wang, P.; Zheng, Z. K.; Dai, Y.; Huang, B. B. High-efficient electrocatalytic overall water splitting over vanadium doped hexagonal Ni0.2Mo0.8N. Appl. Catal. B:Environ. 2020, 263, 118330.
Wang, Y. K.; Zhang, R. F.; Chen, J.; Wu, H.; Lu, S. Y.; Wang, K.; Li, H. L.; Harris, C. J.; **, K.; Kumar, R. V. et al. Enhancing catalytic activity of titanium oxide in lithium-sulfur batteries by band engineering. Adv. Energy Mater. 2019, 9, 1900953.
Acknowledgements
The authors thank the National Natural Science Foundation of China (Nos. U1804140, U20041100 and 21801015) for support. This work is also supported by Bei**g Institute of Technology Research Fund Program for Young Scholars (No. 3090012221909). The authors thank BL10B and BL12B in the National Synchrotron Radiation Laboratory (NSRL) for help with characterizations.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2021_3887_MOESM1_ESM.pdf
Interfacial engineering of 3D hollow CoSe2@ultrathin MoSe2 core@shell heterostructure for efficient pH-universal hydrogen evolution reaction
Rights and permissions
About this article
Cite this article
Zhang, L., Lei, Y., Zhou, D. et al. Interfacial engineering of 3D hollow CoSe2@ultrathin MoSe2 core@shell heterostructure for efficient pH-universal hydrogen evolution reaction. Nano Res. 15, 2895–2904 (2022). https://doi.org/10.1007/s12274-021-3887-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3887-9