Abstract
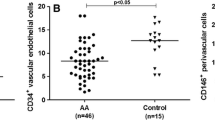
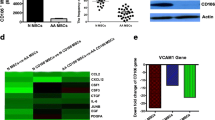
Bone marrow (BM) niches, including the osteoblastic, vascular, and perivascular niches, are numerically impaired in patients with aplastic anemia (AA). It remains unclear whether these niches are numerically restored in AA patients after allogenic hematopoietic stem cell transplantation (allo-HSCT). To investigate changes in BM niches, we monitored 52 patients with AA who had undergone allo-HSCT and performed immunohistochemical studies of BM niches using antibodies against CD34, CD146, and osteopontin. After allo-HSCT, patients with AA exhibited a remarkable increase in the number of cellular elements in the BM niches, including the vascular and perivascular cells. However, no significant differences in endosteal cells were detected. We explored the cause of this restoration by analyzing the origin of BM mesenchymal stem cells (BM-MSCs) and the expression of cytokines in BM plasma. STR-PCR revealed that the BM-MSCs were derived from the host, not the donor. In addition, significantly elevated levels of vascular endothelial growth factor (VEGF) were found after allo-HSCT. Our data indicates that vascular and perivascular niches are numerically restored, but the endosteal niche remains numerically impaired in patients with AA after allo-HSCT, and that levels of VEGF, but not donor-derived BM-MSCs, may correlate with the restoration of BM niches.


Similar content being viewed by others
References
Wu L, Mo W, Zhang Y, Deng H, Li Y, Zhou R, et al. Impairment of hematopoietic stem cell niches in patients with aplastic anemia. Int J Hematol. 2015;102:645–53.
Fureder W, Krauth MT, Sperr WR, Sonneck K, Simonitsch-Klupp I, Mullauer L, et al. Evaluation of angiogenesis and vascular endothelial growth factor expression in the bone marrow of patients with aplastic anemia. Am J Pathol. 2006;168:123–30.
Holmberg LA, Seidel K, Leisenring W, Torok-Storb B. Aplastic anemia: analysis of stromal cell function in long-term marrow cultures. Blood. 1994;84:3685–90.
Shipounova IN, Petrova TV, Svinareva DA, Momotuk KS, Mikhailova EA, Drize NI. Alterations in hematopoietic microenvironment in patients with aplastic anemia. Clin Transl Sci. 2009;2:67–74.
Wu L, Mo W, Zhang Y, Wang S. Vascular and perivascular niches but not osteoblastic niche restoration in patients with aplastic anemia after allogeneic hematopoietic stem cell transplantation by dynamic observation. Bone Marrow Transpl. 2016;51(Suppl 1):S473 (poster 672).
Park M, Park CJ, Jang S, Kim DY, Lee JH, Lee JH, et al. Reduced expression of osteonectin and increased natural killer cells may contribute to the pathophysiology of aplastic anemia. Appl Immunohistochem Mol Morphol. 2015;23:139–45.
Michelozzi IM, Pievani A, Pagni F, Antolini L, Verna M, Corti P, et al. Human aplastic anaemia-derived mesenchymal stromal cells form functional haematopoietic stem cell niche in vivo. Br J Haematol. 2016. doi:10.1111/bjh.14234.
Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiol (Bethesda). 2005;20:349–56.
Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–62.
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6.
Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125:2621–9.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36.
Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–19.
Chuncharunee S, Wong R, Rojnuckarin P, Chang CS, Chang KM, Lu MY, et al. Efficacy of rabbit antithymocyte globulin as first-line treatment of severe aplastic anemia: an Asian multicenter retrospective study. Int J Hematol. 2016;104:454–61.
Miano M, Dufour C. The diagnosis and treatment of aplastic anemia: a review. Int J Hematol. 2015;101:527–35.
Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–56.
Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997;150:815–21.
Bartsch K, Al-Ali H, Reinhardt A, Franke C, Hudecek M, Kamprad M, et al. Mesenchymal stem cells remain host-derived independent of the source of the stem-cell graft and conditioning regimen used. Transplantation. 2009;87:217–21.
Villaron EM, Almeida J, Lopez-Holgado N, Alcoceba M, Sanchez-Abarca LI, Sanchez-Guijo FM, et al. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1421–7.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Bueno C, Roldan M, Anguita E, Romero-Moya D, Martin-Antonio B, Rosu-Myles M, et al. Bone marrow mesenchymal stem cells from patients with aplastic anemia maintain functional and immune properties and do not contribute to the pathogenesis of the disease. Haematologica. 2014;99:1168–75.
Zaim M, Karaman S, Cetin G, Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012;91:1175–86.
Purton LE, Mielcarek M, Torok-Storb B. Monocytes are the likely candidate ‘stromal’ cell in G-CSF-mobilized peripheral blood. Bone Marrow Transpl. 1998;21:1075–6.
Odriozola A, Riancho JA, Colorado M, Zarrabeitia MT. Evaluation of the sensitivity of two recently developed STR multiplexes for the analysis of chimerism after haematopoietic stem cell transplantation. Int J Immunogenet. 2013;40:88–92.
Schraml E, Daxberger H, Watzinger F, Lion T. Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Vienna experience. Leukemia. 2003;17:224–7.
Bacigalupo A, Socie G, Hamladji RM, Aljurf M, Maschan A, Kyrcz-Krzemien S, et al. Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696–702.
Maury S, Balere-Appert ML, Chir Z, Boiron JM, Galambrun C, Yakouben K, et al. Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica. 2007;92:589–96.
Xu LP, Wang SQ, Wu DP, Wang JM, Gao SJ, Jiang M, et al. Haplo-identical transplantation for acquired severe aplastic anaemia in a multicentre prospective study. Br J Haematol. 2016;175:265–74.
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7.
Wei X, Liu C, Wang H, Wang L, **ao F, Guo Z, et al. Surface phosphatidylserine is responsible for the internalization on microvesicles derived from hypoxia-induced human bone marrow mesenchymal stem cells into human endothelial cells. PLoS One. 2016;11:e0147360.
Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8.
Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16:239–53.
Stute N, Fehse B, Schroder J, Arps S, Adamietz P, Held KR, et al. Human mesenchymal stem cells are not of donor origin in patients with severe aplastic anemia who underwent sex-mismatched allogeneic bone marrow transplant. J Hematother Stem Cell Res. 2002;11:977–84.
Wang J, Liu K, Lu DP. Mesenchymal stem cells in stem cell transplant recipients are damaged and remain of host origin. Int J Hematol. 2005;82:152–8.
Chen JL, Hunt P, McElvain M, Black T, Kaufman S, Choi ES. Osteoblast precursor cells are found in CD34 + cells from human bone marrow. Stem Cells. 1997;15:368–77.
Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–57.
Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–6.
Gerber HP, Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. J Mol Med (Berl). 2003;81:20–31.
Gianelli U, Vener C, Raviele PR, Savi F, Somalvico F, Calori R, et al. VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol. 2007;128:966–73.
Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–8.
Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–11.
Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–9.
Acknowledgements
The authors would like to express gratitude for the excellent laboratory assistance of the pathologist Wei Xu. This work was supported by the Natural Science Foundation of Guangdong Province (2014A030313676), Guangzhou Municipal Science and Technology project (201508020254), Guangdong science and technology project (2014A020212521), the General guidance project of Guangzhou Health and Family Planning Commission (20161A011003) and the National Natural Science Foundation of China (81600147).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
L. Wu and W. Mo contributed equally to this work.
About this article
Cite this article
Wu, L., Mo, W., Zhang, Y. et al. Vascular and perivascular niches, but not the osteoblastic niche, are numerically restored following allogeneic hematopoietic stem cell transplantation in patients with aplastic anemia. Int J Hematol 106, 71–81 (2017). https://doi.org/10.1007/s12185-017-2217-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2217-1