Abstract
Non-conventional torrefaction under partially oxidative conditions is an emerging cost-effective thermochemical pre-treatment method to improve the quality of biomass for energy applications. The literature lacks data on the combustion of biomass torrefied under oxygen-deficient atmosphere with actual reactor conditions (inevitable non-uniformities in the thermal environment). In this work, a dual mode fixed-bed biomass (torrefaction) reactor and combustor was operated on Australian biomass pellets, to torrefy the fuels at 275 °C for 30 min using partially oxidative atmosphere (O2: 5 vol%, balance N2) and then to combust them. Combustion behaviour with a particular focus on gaseous emissions of raw, blended (25% torrefied), and torrefied (100%) pellet fuels in a batch-type combustor was investigated. The decomposition behaviour was analysed in a thermogravimetric analyser to understand the impact of biomass constituents on the direct combustion of the tested samples. Results indicate that unlike the combustion of raw biomass, the fuels torrefied under partially oxidative conditions burned 45% faster, attained high packed-bed temperatures (1382 °C) and exhaust gas temperatures (657 °C) then latter (bed: 1128 °C, exhaust: 574 °C) at similar airflow. Additionally, 100% torrefied pellets emitted 38% less NOx compared to raw biomass pellets. However, low CO values for torrefied biomass were attained at higher primary airflows compared to raw. The combustion of 100% torrefied biomass in a fixed-bed was dominated by both flaming and smouldering phases with a modified combustion efficiency (MCE) value of 91%, whereas raw biomass combustion occurred in flaming phase with an MCE value of 98% at same airflow (0.35 kg·m−2·s−1). The outcomes of this work provide useful insights into the viability of using biomass fuels torrefied under partially oxidative conditions alongside other industrial processes generating (waste) heat and flue gases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Woody biomass is a carbon neutral (renewable) fuel but in its raw form has several limitations, i.e. high moisture, high oxygen, poor grindability, low calorific value and hygroscopic nature, all of which makes its use rather difficult for direct combustion [1]. Densification (through pelletisation) and thermal treatment (via torrefaction) helps overcome most of these drawbacks [2]. It is well reported in the literature that the torrefaction modifies the composition, i.e. hemicellulose, cellulose and lignin of the original biomass [3], all of which yields different ignition, burnout and peak temperatures during combustion [4]. Therefore, combustion of raw and torrefied biomass under similar reactor conditions (temperature controlled, airflow etc.) is not realistic.
The significance of torrefied biomass is that when the raw fuel is subjected to the typical 200–300 °C heating in an inert environment, this lowers the hemicellulose fraction binding the cellulose fibrils, thus improving grindability [5] and leading to other positive effects on the thermo-chemical and thermo-physical properties of biomass [6]. However, one of the challenges impeding the wider adoption of torrefied biomass in exiting coal-fired power plants [7] is the need to undertake such thermal treatment in an inert atmosphere. The lab-scale use of pure nitrogen makes the process costly if implemented at an industrial scale; therefore, adopting torrefied biomass remains challenging for large-scale industrial applications. To address this, recently torrefaction has been done in partially (or fully) oxidative environments by replacing nitrogen with air to make its adaptability easier in large-scale combustion plants [8, 9]. However, these attempts have only been reported to date at the small scale (few grammes) in relation to the combustion performance of woody pellets torrefied under oxidative environments. The quality of oxidative torrefaction depends on the concentration of oxygen in the carrier gas, i.e. high O2 concentration with high torrefaction temperature leads to faster reaction [10]. However, torrefaction under high O2 concentration negatively impacts the torrefaction performance indicators (solid yield, hydrophobicity) [8, 11]. Cheng at al. [8] studied the effect of various O2 concentrations and temperatures on torrefied agro-biomass pellets and suggested low O2 concentration <6 vol% for optimal torrefaction performance.
The merits of supplementing or replacing raw biomass (with torrefied fuels) arises from the fact that the combustion of (raw) biomass is linked with the high pollutant emissions particularly NOx, CO and greenhouse gas, i.e. CO2 [12]. One of the significant concerns related to biomass burning is the emissions of NOx that contributes to the formation of acid rain and photochemical smogs causing severe health and environmental pollution. Consequently, research has been conducted on the emissions behaviour of woody biomass [12, 13]. Yet, very few works have been done on the pollutant emissions during combustion of torrefied biomass [7, 14, 15]. Isemin et al. [16] found that torrefaction lowers the NOx emissions of straw pellets from 120 to 100 mg/m3, whereas Rokni et al. [17] reported higher NOx emissions during combustion of torrefied rice husk (230 ppm) as compared to raw biomass (185 ppm). The findings of these work highlights the dependence of pollutant emissions on the fuel type used.
Whilst using raw biomass in direct thermal conversion (combustion, pyrolysis, gasification) has been widely researched [12, 18, 19], there are no studies into the supplementation of densified woody biomass torrefied under a partial oxidative atmosphere on its combustion. With the above in mind, it is noted that the literature on oxidative torrefied biomass does not address its eventual direct combustion characteristics. Rather, it is limited to qualitative aspects [9, 20, 21], its reaction kinetics [22] or performed on non-densified (non-pelletised) biomass [15] or in temperature programmed combustion reactors with a focus on particulate emissions in which actual environment of the industrial scale combustion is not considered, i.e. high temperature, reactor radiative heat loss and radial temperature gradients [8]. The present study makes the following contributions: (i) resolves the impact of oxidative torrefaction on the combustion behaviour compared to raw, blended (25% torrefied) and torrefied (100%) pellets in a direct combustion environment; (ii) ascertains the impact of blend ratio of biomass produced under partially oxidative conditions (25% torrefied, 75% raw biomass) on the gaseous emissions at varying (packed-bed) stoichiometry; and (iii) compares the outcomes of pelletised fuel thermal conversion in direct combustion to the micro-level combustion of the same fuels in a temperature-controlled environment using TGA. The outcomes of this work provide useful insights into the practical utilisation of torrefied biomass produced under partial oxidative conditions for large-scale industrial applications.
Materials and Methods
Raw Biomass Fuel
Commercially available Australian hardwood pellets were used as raw feedstock. The pellets had a diameter of 6.5 mm with varied lengths of <50 mm (as received basis). The raw biomass pellets, made from plantation timber waste, had a bulk density of 713 kg/m3. The ultimate analysis of raw and torrefied biomass was performed in CHNS/O elemental analyser (make: PerkinElmer, model: 2400 Series II), whereas the proximate analysis was determined using TGA (make: PerkinElmer, model: TGA 4000) with the method elaborated elsewhere [23]. The fuel properties are listed in Table 1.
Fixed-Bed Reactor and Combustor
The raw biomass pellets were torrefied in a dual-purpose, custom-built, fixed-bed reactor made of stainless steel with an inside diameter of 202 mm and a height of 1500 mm. The reactor is capable of being used for both torrefaction and a combustor. The details for production of torrefied pellets and their combustion are later discussed. The lab-scale reactor operated in torrefaction mode when it was (externally) heated and as a combustor when the fuel was ignited within. In both torrefaction and combustion, fuel was converted in batch mode [25, 26].
Torrefaction Experiments
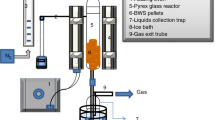
The reactor was divided into two zones: a torrefaction zone in which the (raw) biomass was assembled as a packed bed subjected to heating, and a free board zone. Figure 1 shows the fixed-bed reactor used for torrefaction of biomass. A detailed schematic and description of the torrefaction setup is present in the supplementary material accompanying the manuscript (Fig. S1). In this study, torrefaction in a partially oxidative atmosphere was conducted at 275 °C for 30 min (Fig. 2). The selection of temperatures is based on the findings of our earlier work [6, 27]. In each test run, about 1100 ±100 g of raw biomass pellets were charged into a (stainless steel mesh) basket inserted from the top of the reactor. A weakly oxidative mixture of 5 vol% O2 and 95 vol% N2 (industrial grade nitrogen) was used as carrier gas for torrefaction. The strongly inert nature of this carrier gas avoided initiating combustion of the raw biomass. The temperature within the fuel bed was continuously monitored with N-type thermocouple (TC2) positioned such that they extended from the shell of the reactor to its centreline. Once the reactor was cooled, the solid product called torrefied pellets were collected, weighed and placed in airtight container for further analysis and characterisation. The evolved gases during torrefaction treatment were analysed using a GC instrument and the details are presented in section S.2.3 of the supplementary material.
TG Analysis
The thermal degradation behaviour of raw and torrefied samples (produced in fixed-bed reactor) was investigated in a thermogravimetry (TGA) device (make: PerkinElmer, model: TGA 4000). Before TG analysis, randomly selected 10 torrefied pellets from each test run were mixed and ground to powder to ensure the TGA data represents the kinetic behaviour of multiple batches produced in the reactor.
In each TGA test, 10 ± 0.5 mg of powdered sample was heated in air atmosphere at 45 ml/min. The samples were heated from ambient temperature to 105 °C at 10 °C/min and held for 10 min to ensure removal of free moisture. Afterwards, the furnace temperature was ramped up to 850 °C. All tests were repeated at least twice to ensure reproducibility of the experimental results.
The obtained TGA data was used to determine the important combustion characteristics temperatures, i.e. ignition temperature (Ti), burnout temperature (Tb) and maximum temperature (Tmax). A high Ti value indicates the fuel is thermally stable and difficult to ignite and was calculated using intersection method as described by Lu et al. [28]. Tb refers to the temperature at which the rate of weight loss reaches < 1 wt% min−1 before the samples completely burned out [29]. The maximum temperature (Tmax) corresponds to the temperature of maximum weight loss in a DTG curve. Using characteristic temperatures (Ti, Tb), the comprehensive combustion index (S) was determined as follows [29];
where DTGmax (wt% min−1) is the maximum weight loss rate in DTG curve and DTGmean is the mean weight loss (wt% min−1) between Ti and Tb. The higher value of S indicates better combustion reactivity of the fuel [30].
Combustion Experiments
Combustion experiments, featuring raw, torrefied or blended biomass pellets, were carried out in batch mode. The experimental rig for combustion of biomass is presented in Fig. 3. For each test run, 1100 ±100 g of fuel was inserted into the combustor through the ignitor port. Air was introduced into the combustor through the plenum and passed through the perforated grate into the combustor column. Two N-type thermocouples (TC1–TC2) measured centreline temperatures within the fuel bed and in the freeboard region. Thermocouple data was acquired with a 16-channel National Instruments (NI) 9213 thermocouple module, interfaced with a NI data acquisition system (model: cRIO 9074). Temperature data was sampled at 10 second intervals through LabView data acquisition interface. The first thermocouple TC1 was positioned in the fuel bed whilst TC2 was placed at the top of the freeboard to monitor exhaust gas temperatures.
At first, burning profile was established for raw wood pellets at five different airflows 280, 420, 560, 720 and 840 l/min which corresponds to 0.18, 0.26, 0.35, 0.46 and 0.53 kg·m−2·s−1. Once the burning profile was determined, the airflow at which the maximum burning rate was achieved for raw wood pellets was used for combustion of 100% torrefied pellets to determine the effect of 100% switch to thermally treated fuel on the burning characteristics compared to raw biomass pellets. Afterwards, the fuel blend (25% torrefied, 75% raw wood pellets) were also tested at all airflows to observe the wider effect on the combustion behaviour of raw biomass when co-fired with torrefied biomass pellets. Additional details related to the direct combustion experiments is present in section S.2.2 of the supplementary material.
Combustion Emissions
Gaseous emissions (CO2, CO, NOx) were acquired at the exit of the combustor using flue gas analyser (Testo-350). A gas sampling hose connected with an industrial-grade probe was placed at the centre of the exhaust pipe. To avoid overestimation of the results due to transient burning, all emission data were taken during steady-state burning and the average values are reported [12, 31]. To make results comparable, the emissions were benchmarked for 6 vol% O2 [7].
Furthermore, with each of the raw, torrefied and blended pellets, combustion emissions were also used to determine combustion performance using modified combustion efficiency (MCE) equation [32]:
Results and Discussion
TGA-Scale (Ground Fuel) Thermal Conversion
The combustion characteristics of ground samples derived from raw and torrefied woody biomass fuel pellets, as well as their blends (mix of 25% torrefied and 75% raw), are presented in Fig. 4. The thermal decomposition of woody biomass is complex and reflected in TGA testing through multiple peaks of varying decomposition rates [33, 34]. The behaviour in the DTG curves (Fig. 4) can be divided into two main stages. The lower temperature stage 1 corresponds to the oxidation of volatiles and appears to generally commence, and peak at higher temperatures as the ratio of torrefied fuel increases. The increase of torrefied fuel in the sample (from 25 to 100%) reduces the overall mass deficit during stage 1 but this is somewhat expected due to the earlier (thermal) treatment of these fuel samples unlike the raw fuel. At a higher temperature range, stage 2 is associated with the combustion of the remaining char [35, 36]. In contrast to stage 1, Fig. 4 shows that as the ratio of torrefied fuel increases, the peak weight loss in stage 2 tends to peak at a lower temperature. It is evident from Fig. 4 that the combustion behaviour of raw, blended and torrefied biomass varies reflecting hemicellulose, cellulose and lignin fractions in the fuel. In the first half of each stage 1, raw biomass (Fig. 4a) exhibited the most differential weight loss followed by blended biomass (Fig. 4b: 25% torrefied, 75% raw). This could be attributed to the higher portion of holocellulose fraction in the raw biomass. Contrary to both these trends, torrefied biomass underwent only 24.36% weight loss in stage 1 as compared to 47.69% for the blended (25% torrefied) and 57.53% in the (100%) torrefied fuel. The low weight loss in the fuel samples made of (100%) torrefied biomass over stage 1 is due to the excessive devolatisation of holocellulose fraction during torrefaction. This is reflected over the narrow temperature range 305–375 °C of torrefied biomass as compared to wider temperature range 260–395 °C of raw in stage 1. In comparison, a weight loss of 73.01% was observed for torrefied samples over stage 2. This higher weight loss values reflect char combustion which dominates the combustion of torrefied samples [36].
Similarly, the effect of torrefaction on the rate of weight loss (DTG) is also evident. For example, the peak weight loss shifted to stage 2 in torrefied samples; however, the intensity is reduced (6.23 wt% min−1) as compared to raw (9.36 wt% min−1) and the blend (8.79 wt% min−1) that achieved DTGmax in stage 1. This could be explained by the fact that cellulose contains higher volatile content (89.70%) as compared to hemicellulose (84.80%) and lignin (51.30%) [35]. Furthermore, the volatile release rates of holocellulose are faster than lignin [37]. Owing to the higher fraction of volatiles in holocellulose which undergoes major devolitisation during thermal treatment, the torrefied samples showed lower weight loss rates than raw samples and the blend. Lastly, the data in Fig. 4 shows that although when blending 25% torrefied biomass into a fuel batch, the effects are not significant during stage 1 with substantive variations only occurring with much larger proportions of torrefied fuel usage (100%). The more notable finding is that with (100%) torrefied fuels, the differential mass loss during char burnout (stage 2) appears to exceed those during the earlier combustion (stage 1).
Based on the TG-DTG data, several combustion performance indicators (Ti, Tp, Tb, S) were determined and listed in Table 2. The TGA thermal curve of raw, blended and torrefied biomass is presented in supplementary material (Fig. S3). It can be seen that torrefaction increased the Ti and Tb values as compared to raw biomass. The ignition temperature Ti increased from 298.16 °C (raw) to 346.2 °C (torrefied) whilst the burnout temperature Tb increased from 558 to 565 °C. The strong impact on the ignition temperature could be attributed with the removal of readily available light volatiles such as (CO2, CO, H2O) [3], during torrefaction which consequently increased the lignin fraction which has the higher ignition temperature compared to holocellulose [35]. However, at the 275 °C selected torrefaction temperature in this study, holocellulose fractions do not fully decompose as evident with the 24.36% weight loss (stage 1) during combustion of torrefied (100%) biomass. It is important to note that at a higher ignition temperature Ti, there is a lower risk of self-ignition, which is favourable for long-term safe storage of the torrefied biomass.
The effect of torrefaction on the combustion performance was further evaluated by the Comprehensive Combustion Index donated by “S” [22]. Table 2 reveals that the torrefaction decreased the S value of 100% torrefied samples (4.29×10−7·min−2·°C−3) whilst the effect on blended samples was minimal. This could be due to the increase in fixed carbon owing to more lignin in the torrefied char leading to slow combustion with low DTGmax. However, in all cases, S exceeded 2×10−7·min−2·°C−3 demonstrating better combustion performance [38]. These results are consistent with the findings reported in the literature indicating that high torrefaction severity can lower the combustion reactivity [39].
The TGA derived combustion performance of 100% torrefied biomass and its blend based on 25% torrefied fuel further emphasises that torrefaction does not only increase the heating value of a fuel but its impact on other important combustion parameters also needs to be considered [6]. Data derived from the temperature-controlled (TGA) environment provided very useful information about the combustion performance of the torrefied biomass. However, it is evident that torrefaction alters the physicochemical characteristics of the biomass, i.e. loss of oxygen containing functional groups during torrefaction [6].
Reactor-Scale (Batch Fuel) Thermal Conversion
Biomass combustion occurs as a multistep kinetic phenomena, particularly for woody biomass where decomposition of hemicellulose, cellulose and lignin fractions occurs simultaneously over a wide range of temperatures [35]. Whilst the conversion of ground (and well mixed) crushed fuel samples in a controlled (uniform) thermal environment in TGA may provide valuable kinetics data, inevitable non-uniformities in the thermal environment at the reactor scale, through radial temperature gradients [40], reactor radiative heat loss near to walls [41] and various heat transfer processes within the packed fuel bed [40] mean that detailed studies into the effects of blending torrefied and raw fuels are also warranted at the reactor scale with a batch of pellets being used. The ensuing results are therefore a comparative analysis of raw, blended (25% torrefied) and (100%) torrefied pellet fuels in a batch type combustor.
The most important variable that influences the burning behaviour in a combustor is the supplied airflow rate [12, 31, 42,43,44]. Figure 5 presents the burning rate for raw, torrefied, and blended (25% torrefied) fuel batches at varying airflow rates. It can be seen that based on the primary airflow rate, stoichiometric conditions for the raw fuel are attained at around 0.35 kg·m−2·s−1 beyond which the burning rate tends to decrease. With the combustion of raw biomass divided into three different stages based on stoichiometry (fuel rich, stoichiometric and lastly quenching or convective cooling) [42], the initial increase in the burning over 0.18 and 0.26 kg·m−2·s−1 is considered fuel rich (oxygen limited) whereby volatiles and char oxidation are dominated by the amount of oxygen as the reaction front propagates within the fuel bed. The burning rate reaches a peak of 0.047 kg·m−2·s−1 for raw biomass pellets at an airflow of 0.35 kg·m−2·s−1. The same tests when conducted on batches of blended pellets (25% torrefied fuel) attained significantly higher burning rate at all flow rates. This magnitude of change is significantly different compared to that obtained earlier under the well-controlled TGA environment using samples that are ground and well homogenised. To further test the effect of using 100% torrefied fuel, at the conditions yielding optimal burning rate in raw and blended fuel, an additional test for these torrefied fuels is shown in Fig. 5. The 100% torrefied pellets were combusted with the airflow of 0.35 kg·m−2·s−1 at which the raw biomass attained maximum burning rate. As seen, the 100% torrefied biomass burned much faster (0.068 kg·m−2·s−1) than raw pellets (0.047 kg·m−2·s−1).
The interpretation of the above trends is that torrefaction decreases the particle size as well as moisture content and increases the porosity of the torrefied biomass relative to raw biomass [21, 45]. These changes in porosity and particle size provide more surface area and active sites which eventually expedite oxidation reactions within the bed promoting better heat transfer through radiation as the ignition front propagates downwards to the cold region of the bed. Another important observation made during the combustion of raw/torrefied blend is that unlike raw biomass, the reaction limited regime of the blend shifted towards the right (> 0.35 kg·m−2·s−1). In addition, compared to raw pellets, oxidative torrefied pellets contain more carbon and low oxygen content (low O/C) [8], thus leading to intense oxidation reaction in the char combustion zone.
Reactor-Scale Fuel bed and Exhaust Gas Temperature
The bed and exhaust gas temperatures of the combustion raw, torrefied and blended pellets are present in Fig. S4 (supplementary material). With the increase in airflow, the bed temperatures (859–1128 °C) of raw pellets increased in the oxygen-limited regime (0.18–0.35 kg·m−2·s−1), which however tends to decrease with further increase in the airflow, whereas the bed temperature of blended pellets was higher than that of raw pellets regardless of the airflow. The blended pellets attained the highest bed temperature (1208 °C) at the maximum airflow used in this study (0.53 kg·m−2·s−1). On the other hand, the bed temperature (1382 °C) of 100% torrefied was highest even at 0.35 kg·m−2·s−1. The higher combustion bed temperature with the 100% torrefied and blended (25% Torr.) pellets could be linked with the alteration in the fuel composition during torrefaction. The thermal degradation behaviour discussed above suggests incremental increase in the lignin fraction after torrefaction. The combustion temperature is generally dependent on the amount of lignin in the fuel which has the highest percent of fixed carbon [4]. These results suggest that direct combustion of 100% torrefied pellets could yield very high temperatures; therefore, lab-scale combustors need special design considerations for safe operation.
Apart from the lignin, other factors may also contribute to the increased bed temperature of the torrefied biomass and its blend. In the counter-current configurations, the fuel burns in the upper layer of the bed whilst the ignition front move downwards. The occurrence of devolatisation beneath the char combustion (top layer) preheats the lower layers of the bed through radiative and conductive heat [42]. Samples torrefied at severe torrefaction conditions (275 °C, slow heating) contain less volatiles [6], and almost insignificant moisture content as compared to raw wood pellets which limits the temperature gradient within the bed. Contrary to the observations made in this study, the results obtained in the temperature-controlled reactors may overlook the variation in heat dissipation as a result of modification in fuel composition after torrefaction. This further highlights the importance of combustion of torrefied samples in reactors more representative of direct industrial scale. On the other hand, the exhaust gas temperatures reflected similar behaviour to bed temperature except at low airflows (0.18–0.26 kg m−2 s−1) where minor difference was observed in between raw and torrefied wood pellets albeit with values within experimental error.
Gaseous Emissions
Figure 6 shows the gaseous emissions during combustion of raw, torrefied and blended pellets at different airflows. To make emission results comparable, the values of CO, CO2 and NOx were corrected at 6 vol% O2. It is evident that the highest CO concentration 13,768 ppm (raw) and 18,122 ppm (blend) was recorded at the low airflow (0.18 kg·m−2·s−1); however, CO tends to decrease with the increase in airflow with the exception of airflow value of 0.53 kg·m−2·s−1 (for raw wood pellets). Generally, CO emissions mainly depends on the temperature, air mixing and gas retention time inside the reactor [13]. Furthermore, it is considered the main indicator of combustion performance. Although the recorded CO values at 0.35 kg·m−2·s−1 and 0.46 kg·m−2·s−1 are comparable, however the former could be taken as optimum airflow for raw wood pellet combustion owing to its slightly higher burning rate and combustion temperatures compared to the latter. With further increase in the airflow (0.53 kg·m−2·s−1), the CO emissions tend to increase again due to the fact that the high airflow decreased the retention time of the gaseous emissions and may also have taken some heat whilst moving upwards.
Thus, short retention time combined with heat loss due to excess airflow contributed to the higher CO emissions for raw wood pellets at 0.53 kg·m−2·s−1
Contrary to the CO emission behaviour of raw wood pellets, the torrefied pellets shown an opposite trend. As seen (Fig. 6a), the CO emissions of the blended pellets continuously decreased from 18,122 to 1522 ppm with increasing airflow from 0.18 to 0.53 kg·m−2·s−1. This reduction could be attributed with the difference in oxygen requirement owing to low oxygen content in the torrefied wood pellets, i.e. that the reaction limited regime for the torrefied biomass blends shifted towards right (Fig. 5) as discussed in the previous section. In addition, the co-firing of raw and torrefied pellets could also affect the gaseous emissions. The difference in the fuel properties of pellets in a blended layer of fuel bed could potentially mismatch the demanded air supply and may overlap different combustion phases simultaneously thus resulting higher CO emissions at lower airflows (0.18–0.26 kg·m−2·s−1). Despite high burning rate and combustion temperature, the comparable CO (100% torrefied: 8599 ppm, blend: 7766 ppm) emitted from the combustion of 100% torrefied biomass at 0.35 kg·m−2·s−1 further strengthens this argument.
The NOx emissions from biomass are mainly dependent on the reactor temperature, configuration, retention time and the amount of volatiles which comprises of fuel bound nitrogen (fuel-N) [12, 14, 46]. As seen in Fig. 6c, with increasing airflow from 0.18 to 0.53 kg·m−2·s−1, the NOx emissions increased from 48 to 110 ppm for raw wood pellets, whereas low NOx values (min: 69 ppm, max: 88 ppm) were observed when the raw pellets were co-fired with torrefied woody pellets at varying airflows. In both cases, the higher values of NOx at higher airflow could be linked with multiple factors, i.e. high bed temperature (>1000 °C) availability of more oxygen leading to greater fuel-N conversion to NO and shorter retention time. The devolatisation during torrefaction reduces the amount of fuel-N as reported in the literature which contributed to the low NOx values of blended pellets [47]. This change is further evident during combustion of 100% torrefied woody pellets which yielded the highest bed temperature (1382 °C at 0.35 kg·m−2·s−1) emitting 38% less NOx compared to 100% raw woody pellet at a similar airflow.
The obtained emission data from this study at different airflows was used to evaluate the MCE, an index useful to determine the burning conditions, i.e. flaming or smouldering during combustion. In general, the MCE value near 100% indicates pure flame-dominated combustion whilst near to 80% indicates smouldering combustion [48]. The MCE value near to 90% represents a mix of flaming and smouldering combustion. As seen in Fig. S5, the MCE values for raw pellets attained minimum value of 90% at 0.18 kg·m−2·s−1, whereas maximum value 99% at 0.35 kg·m−2·s−1. Contrary to this, the blended pellets attained lowest MCE of 88% at 0.18 kg·m−2·s−1 and maximum value of 98% at 0.53 kg·m−2·s−1. These results suggest that with low airflow, flaming and smouldering combustion were happening simultaneously during burning of both raw and blended pellets.
However, MCE of 94% for blended pellets with the airflow of 0.35 kg·m−2·s−1 reflects that the air demand for raw and torrefied pellets differs. This difference is evident with low MCE value (91%) of 100% torrefied pellets compared to raw with an MCE value of 98% at similar airflow (0.35 kg·m−2·s−1). There is lack of information available in the literature on the combustion behaviour of torrefied pellets at different airflow. However, the MCE trends for raw pellets are consistent with the earlier findings reported in the literature [49].
Conclusions
In this work, direct combustion behaviour of torrefied biomass pellets produced in a fixed-bed reactor under oxygen-deficient atmosphere was investigated and compared with raw pellets. The results suggest that torrefied pellets burned faster, attained higher bed and exhaust gas temperatures, whereas it reduced the NOx emissions during direct combustion. Co-firing of blended (25% torrefied, 75% raw) biomass showed an increase in air demand for lower gaseous emissions. The outcomes of this work suggest the potential of reducing the cost associated with thermally processing biomass fuels if (waste) process heat and flue gases are used.
Change history
20 February 2023
Missing Open Access funding information has been added in the Funding Note.
References
Chen W-H, Lin B-J, Lin Y-Y, Chu Y-S, Ubando AT, Show PL, Ong HC, Chang J-S, Ho S-H, Culaba AB (2021) Progress in biomass torrefaction: principles, applications and challenges. Prog Energy and Combust Sci 82:100887. https://doi.org/10.1016/j.pecs.2020.100887
Ghiasi B, Kumar L, Furubayashi T, Lim CJ, Bi X, Kim CS, Sokhansanj S (2014) Densifed biocoal from woodchips: is it better to do torrefaction before or after densifcation? Appl Energy 134:133–142. https://doi.org/10.1016/j.apenergy.2014.07.076
Chen D, Gao A, Cen K, Zhang J, Cao X, Ma Z (2018) Investigation of biomass torrefaction based on three major components: hemicellulose, cellulose, and lignin. Energy Conversat Manag 169:228–237. https://doi.org/10.1016/j.enconman.2018.05.063
Wang S, Zou C, Yang H, Lou C, Cheng S, Peng C, Wang C, Zou H (2021) Effects of cellulose, hemicellulose, and lignin on the combustion behaviours of biomass under various oxygen concentrations. Bioresour Technol 320:124375. https://doi.org/10.1016/j.biortech.2020.124375
Adeleke AA, Odusote JK, Ikubanni PP, Lasode OA, Malathi M, Paswan D (2021) Essential basics on biomass torrefaction, densification and utilization. Int J Energy Res 45(2):1375–1395. https://doi.org/10.1002/er.5884
Riaz S, Al-Abdeli YM, Oluwoye I, Altarawneh M (2021) Torrefaction of densified woody biomass: the effect of pellet size on thermochemical and thermophysical characteristics. Bioenergy Res 15:544–558. https://doi.org/10.1007/s12155-021-10319-8
Ndibe C, Grathwohl S, Paneru M, Maier J, Scheffknecht G (2015) Emissions reduction and deposits characteristics during cofiring of high shares of torrefied biomass in a 500 kW pulverized coal furnace. Fuel 156:177–189. https://doi.org/10.1016/j.fuel.2015.04.017
Cheng W, Zhu Y, Zhang W, Jiang H, Hu J, Zhang X, Yang H, Chen H (2022) Effect of oxidative torrefaction on particulate matter emission from agricultural biomass pellet combustion in comparison with non-oxidative torrefaction. Renew Energy 189:39–51. https://doi.org/10.1016/j.renene.2022.03.032
Wang Q, Sun S, Zhang X, Liu H, Sun B, Guo S (2021) Influence of air oxidative and non-oxidative torrefaction on the chemical properties of corn stalk. Bioresour Technol 332:125120. https://doi.org/10.1016/j.biortech.2021.125120
Wang C, Peng J, Li H, Bi XT, Legros R, Lim C, Sokhansanj S (2013) Oxidative torrefaction of biomass residues and densification of torrefied sawdust to pellets. Bioresour Technol 127:318–325. https://doi.org/10.1016/j.biortech.2012.09.092
Brachi P, Chirone R, Miccio M, Ruoppolo G (2019) Fluidized bed torrefaction of biomass pellets: a comparison between oxidative and inert atmosphere. Powder Technol 357:97–107. https://doi.org/10.1016/j.powtec.2019.08.058
Rashidian B, Al-Abdeli YM, Patiño D, Guzzomi FG, Yeoh GH (2016) Effect of freeboard deflectors in the fixed bed combustion of biomass. Appl Therm Eng 103:543–552. https://doi.org/10.1016/j.applthermaleng.2016.04.140
Roy MM, Dutta A, Corscadden K (2013) An experimental study of combustion and emissions of biomass pellets in a prototype pellet furnace. Appl Energy 108:298–307. https://doi.org/10.1016/j.apenergy.2013.03.044
Maxwell D, Gudka B, Jones J, Williams A (2020) Emissions from the combustion of torrefied and raw biomass fuels in a domestic heating stove. Fuel Process Technol 199:106266. https://doi.org/10.1016/j.fuproc.2019.106266
Lasek JA, Kopczyński M, Janusz M, Iluk A, Zuwała J (2017) Combustion properties of torrefied biomass obtained from flue gas-enhanced reactor. Energy 119:362–368. https://doi.org/10.1016/j.energy.2016.12.079
Isemin R, Mikhalev A, Klimov D, Grammelis P, Margaritis N, Kourkoumpas D-S, Zaichenko V (2017) Torrefaction and combustion of pellets made of a mixture of coal sludge and straw. Fuel 210:859–865. https://doi.org/10.1016/j.fuel.2017.09.032
Rokni E, Ren X, Panahi A, Levendis YA (2018) Emissions of SO2, NOx, CO2, and HCl from Co-firing of coals with raw and torrefied biomass fuels. Fuel 211:363–374. https://doi.org/10.1016/j.fuel.2017.09.049
Bandara JC, Jaiswal R, Nielsen HK, Moldestad BM, Eikeland MS (2021) Air gasification of wood chips, wood pellets and grass pellets in a bubbling fluidized bed reactor. Energy 233:121149. https://doi.org/10.1016/j.energy.2021.121149
Santamaria L, Beirow M, Mangold F, Lopez G, Olazar M, Schmid M, Li Z, Scheffknecht G (2021) Influence of temperature on products from fluidized bed pyrolysis of wood and solid recovered fuel. Fuel 283:118922. https://doi.org/10.1016/j.fuel.2020.118922
Chen D, Chen F, Cen K, Cao X, Zhang J, Zhou J (2020) Upgrading rice husk via oxidative torrefaction: characterization of solid, liquid, gaseous products and a comparison with non-oxidative torrefaction. Fuel 275:117936. https://doi.org/10.1016/j.fuel.2020.117936
Zhao Z, Feng S, Zhao Y, Wang Z, Ma J, Xu L, Yang J, Shen B (2022) Investigation on the fuel quality and hydrophobicity of upgraded rice husk derived from various inert and oxidative torrefaction conditions. Renew Energy 189:1234–1248. https://doi.org/10.1016/j.renene.2022.03.087
Dong K, Hu Z, Xue Y, Zhou Y, Lei T, Cheng Z (2022) Effect of torrefaction on thermal oxidative degradation kinetics of lignite using multi-distributed activation energy model. Fuel 324:124488. https://doi.org/10.1016/j.fuel.2022.124488
Huang C-W, Li Y-H, **ao K-L, Lasek J (2019) Cofiring characteristics of coal blended with torrefied Miscanthus biochar optimized with three Taguchi indexes. Energy 172:566–579. https://doi.org/10.1016/j.energy.2019.01.168
Parikh J, Channiwala S, Ghosal G (2005) A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 84(5):487–494. https://doi.org/10.1016/j.fuel.2004.10.010
Junejo A, Al-Abdeli YM, Porteiro J (2022) Role of primary freeboard on staged combustion of hardwood pellets in a fixed bed combustor. Bioenergy Res Document type: Early Access
Onsree T, Tippayawong N (2020) Torrefaction of maize residue pellets with dry flue gas. Bioenergy Res 13(1):358–368. https://doi.org/10.1007/s12155-019-10058-x
Riaz S, Oluwoye I, Al-Abdeli YM (2022) Oxidative torrefaction of densified woody biomass: performance, combustion kinetics and thermodynamics. Renew Energy 199:908–918. https://doi.org/10.1016/j.renene.2022.09.023
Lu J-J, Chen W-H (2015) Investigation on the ignition and burnout temperatures of bamboo and sugarcane bagasse by thermogravimetric analysis. Appl Energy 160:49–57. https://doi.org/10.1016/j.apenergy.2015.09.026
Bermejo SP, Prado-Guerra A, Pérez AIG, Prieto LFC (2020) Study of quinoa plant residues as a way to produce energy through thermogravimetric analysis and indexes estimation. Renew Energy 146:2224–2233. https://doi.org/10.1016/j.renene.2019.08.056
Ma Q, Han L, Huang G (2017) Evaluation of different water-washing treatments effects on wheat straw combustion properties. Bioresour Technol 245:1075–1083. https://doi.org/10.1016/j.biortech.2017.09.052
Rashidian B, Al-Abdeli YM, Yeoh GH, Patiño D, Guzzomi F (2017) Methodologies for processing fixed bed combustor data. Combust Sci Technol 189(1):79–102. https://doi.org/10.1080/00102202.2016.1193497
Bertrand A, Stefenelli G, Bruns EA, Pieber SM, Temime-Roussel B, Slowik JG, Prévôt AS, Wortham H, El Haddad I, Marchand N (2017) Primary emissions and secondary aerosol production potential from woodstoves for residential heating: influence of the stove technology and combustion efficiency. Atmos Environ 169:65–79. https://doi.org/10.1016/j.atmosenv.2017.09.005
Barzegar R, Yozgatligil A, Olgun H, Atimtay AT (2020) TGA and kinetic study of different torrefaction conditions of wood biomass under air and oxy-fuel combustion atmospheres. J Energy Inst 93(3):889–898. https://doi.org/10.1016/j.joei.2019.08.001
Riaz S, Al-Abdeli Y (2021) Effects of constant and staged torrefaction temperatures on biomass combustion and pyrolysis. Proceedings of the Australian Combustion Symposium 2021:171–185. Australian and New Zealand Section of the Combustion Institute. Available online https://anz-combustioninstitute.org/root/ACS2021/ACS2021_proceedings.pdf. Accessed on 16 Jun 2022
Cao W, Li J, Martí-Rosselló T, Zhang X (2019) Experimental study on the ignition characteristics of cellulose, hemicellulose, lignin and their mixtures. J Energy Inst 92(5):1303–1312. https://doi.org/10.1016/j.joei.2018.10.004
**ao Z, Wang S, Luo M, Cai J (2022) Combustion characteristics and synergistic effects during co-combustion of lignite and lignocellulosic components under oxy-fuel condition. Fuel 310:122399. https://doi.org/10.1016/j.fuel.2021.122399
Wang S, Zou C, Lou C, Yang H, Mei M, **g H, Cheng S (2020) Effects of hemicellulose, cellulose and lignin on the ignition behaviors of biomass in a drop tube furnace. Bioresour Technol 310:123456. https://doi.org/10.1016/j.biortech.2020.123456
Parshetti GK, Quek A, Betha R, Balasubramanian R (2014) TGA–FTIR investigation of co-combustion characteristics of blends of hydrothermally carbonized oil palm biomass (EFB) and coal. Fuel Process Technol 118:228–234. https://doi.org/10.1016/j.fuproc.2013.09.010
He Q, Ding L, Gong Y, Li W, Wei J, Yu G (2019) Effect of torrefaction on pinewood pyrolysis kinetics and thermal behavior using thermogravimetric analysis. Bioresour Technol 280:104–111. https://doi.org/10.1016/j.biortech.2019.01.138
Rashidian B, Al-Abdeli YM, Yeoh GH, Guzzomi FG (2015) Effect of freeboard deflectors on the temperature distribution in packed beds. Appl Therm Eng 89:134–143. https://doi.org/10.1016/j.applthermaleng.2015.05.045
Sazali S, Al-attab K, Zainal Z (2019) Gasification enhancement and tar reduction using air fogging system in a double walled downdraft biomass gasifier. Energy 186:115901. https://doi.org/10.1016/j.energy.2019.115901
Porteiro J, Patino D, Collazo J, Granada E, Moran J, Miguez J (2010) Experimental analysis of the ignition front propagation of several biomass fuels in a fixed-bed combustor. Fuel 89(1):26–35. https://doi.org/10.1016/j.fuel.2009.01.024
Khodaei H, Al-Abdeli YM, Guzzomi F, Yeoh GH (2015) An overview of processes and considerations in the modelling of fixed-bed biomass combustion. Energy 88:946–972. https://doi.org/10.1016/j.energy.2015.05.099
Rashidian B, Al-Abdeli YM (2017) Effect of freeboard deflectors on the exergy in a fixed bed combustor. Appl Therm Eng 118:62–72. https://doi.org/10.1016/j.applthermaleng.2017.02.082
Mei Y, Liu R, Yang Q, Yang H, Shao J, Draper C, Zhang S, Chen H (2015) Torrefaction of cedarwood in a pilot scale rotary kiln and the influence of industrial flue gas. Bioresour Technol 177:355–360. https://doi.org/10.1016/j.biortech.2014.10.113
Oluwoye I, Zeng Z, Mosallanejad S, Altarawneh M, Gore J, Dlugogorski BZ (2021) Controlling NOx emission from boilers using waste polyethylene as reburning fuel. Chem Eng J 411:128427. https://doi.org/10.1016/j.cej.2021.128427
McNamee P, Darvell L, Jones J, Williams A (2015) The combustion characteristics of high-heating-rate chars from untreated and torrefied biomass fuels. Biomass and Bioenerg 82:63–72. https://doi.org/10.1016/j.biombioe.2015.05.016
Stockwell C, Yokelson R, Kreidenweis S, Robinson A, DeMott P, Sullivan R, Reardon J, Ryan K, Griffith D, Stevens L (2014) Trace gas emissions from combustion of peat, crop residue, domestic biofuels, grasses, and other fuels: configuration and Fourier transform infrared (FTIR) component of the fourth Fire Lab at Missoula Experiment (FLAME-4). Atmos Chem Phys 14(18):9727–9754. https://doi.org/10.5194/acp-14-9727-2014
Pokhrel RP, Gordon J, Fiddler MN, Bililign S (2021) Impact of combustion conditions on physical and morphological properties of biomass burning aerosol. Aerosol Sci Technol 55(1):80–91. https://doi.org/10.1080/02786826.2020.1822512
Acknowledgements
The first author would like to acknowledge the School of Engineering, ECU, Joondalup, Australia, for providing the laboratory facilities used in this research work. The authors are grateful to Dr. Humair Ahmed Baloch from the School of Engineering, RMIT University, for his assistance in elemental analyser and Karine Barclay from the School of Science, ECU for her support in GC analysis.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The first author is grateful for the financial support provided for the PhD grant via ECU-HEC Joint Scholarship 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riaz, S., Al-Abdeli, Y.M. & Oluwoye, I. Partially Oxidative Torrefaction of Woody Biomass Pellets: Burning Behaviour and Emission Analysis. Bioenerg. Res. 16, 2331–2341 (2023). https://doi.org/10.1007/s12155-023-10572-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10572-z