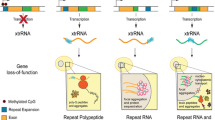
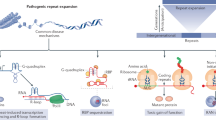
Abstract
Expansions of short tandem repeats (STRs) have been found to be present in more than 50 diseases and have a close connection with neurodegenerative diseases. Transcriptional silencing and R-LOOP formation, RNA-mediated sequestration of RNA-binding proteins (RBPs), gain-of-function (GOF) proteins containing expanded repeats, and repeat-associated non-AUG (RAN) translation of toxic repeat peptides are some potential molecular mechanisms underlying STR expansion disorders. R-LOOP, a byproduct of transcription, is a three-stranded nucleic acid structure with abnormal accumulation that participates in the pathogenesis of STR expansion disorders by inducing DNA damage and genome instability. R-LOOPs can engender a series of DNA damage, such as DNA double-strand breaks (DSBs), single-strand breaks (SSBs), DNA recombination, or mutations in the DNA replication, transcription, or repair processes. In this review, we provide an in-depth discussion of recent advancements in R-LOOP and systematically elaborate on its genetic destabilizing effects in several neurodegenerative diseases. These molecular mechanisms will provide novel targets for drug design and therapeutic upgrading of these devastating diseases.


Similar content being viewed by others
Data Availability
Not applicable.
References
Medina A, Mahjoub Y, Shaver L, Pringsheim T (2022) Prevalence and incidence of Huntington’s disease: an updated systematic review and meta-analysis. Mov Disord Off J Mov Disord Soc 37:2327–2335. https://doi.org/10.1002/mds.29228
Ruano L, Melo C, Silva MC, Coutinho P (2014) The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology 42:174–183. https://doi.org/10.1159/000358801
Stevanovski I, Chintalaphani SR, Gamaarachchi H, Ferguson JM, Pineda SS, Scriba CK, Tchan M, Fung V et al (2022) Comprehensive genetic diagnosis of tandem repeat expansion disorders with programmable targeted nanopore sequencing. Sci Adv 8:eabm5386. https://doi.org/10.1126/sciadv.abm5386
Talbott EO, Malek AM, Lacomis D (2016) The epidemiology of amyotrophic lateral sclerosis. Handb Clin Neurol 138:225–238. https://doi.org/10.1016/B978-0-12-802973-2.00013-6
Farg MA, Konopka A, Soo KY, Ito D, Atkin JD (2017) The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in amyotrophic lateral sclerosis. Hum Mol Genet 26:2882–2896. https://doi.org/10.1093/hmg/ddx170
Malik I, Kelley CP, Wang ET, Todd PK (2021) Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat Rev Mol Cell Biol 22:589–607. https://doi.org/10.1038/s41580-021-00382-6
Kim A, Wang GG (2021) R-loop and its functions at the regulatory interfaces between transcription and (epi)genome. Biochim Biophys Acta Gene Regul Mech 1864:194750. https://doi.org/10.1016/j.bbagrm.2021.194750
Niehrs C, Luke B (2020) Regulatory R-loops as facilitators of gene expression and genome stability. Nat Rev Mol Cell Biol 21:167–178. https://doi.org/10.1038/s41580-019-0206-3
Richard P, Manley JL (2017) R loops and links to human disease. J Mol Biol 429:3168–3180. https://doi.org/10.1016/j.jmb.2016.08.031
Sanz LA, Hartono SR, Lim YW, Steyaert S, Rajpurkar A, Ginno PA, Xu X, Chédin F (2016) Prevalent, dynamic, and conserved R-loop structures associate with specific epigenomic signatures in mammals. Mol Cell 63:167–178. https://doi.org/10.1016/j.molcel.2016.05.032
García-Muse T, Aguilera A (2019) R loops: from physiological to pathological roles. Cell 179:604–618. https://doi.org/10.1016/j.cell.2019.08.055
Groh M, Lufino MMP, Wade-Martins R, Gromak N (2014) R-loops associated with triplet repeat expansions promote gene silencing in Friedreich ataxia and fragile X syndrome. PLoS Genet 10:e1004318. https://doi.org/10.1371/journal.pgen.1004318
Kannan A, Cuartas J, Gangwani P, Branzei D, Gangwani L (2022) Mutation in senataxin alters the mechanism of R-loop resolution in amyotrophic lateral sclerosis 4. Brain J Neurol 145:3072–3094. https://doi.org/10.1093/brain/awab464
Reddy K, Tam M, Bowater RP, Barber M, Tomlinson M, Nichol Edamura K, Wang Y-H, Pearson CE (2011) Determinants of R-loop formation at convergent bidirectionally transcribed trinucleotide repeats. Nucleic Acids Res 39:1749–1762. https://doi.org/10.1093/nar/gkq935
Bustos BI, Billingsley K, Blauwendraat C, Gibbs JR, Gan-Or Z, Krainc D, Singleton AB, Lubbe SJ (2023) Genome-wide contribution of common short-tandem repeats to Parkinson’s disease genetic risk. Brain J Neurol 146:65–74. https://doi.org/10.1093/brain/awac301
Chédin F (2016) Nascent connections: R-loops and chromatin patterning. Trends Genet TIG 32:828–838. https://doi.org/10.1016/j.tig.2016.10.002
Aguilera A, García-Muse T (2012) R loops: from transcription byproducts to threats to genome stability. Mol Cell 46:115–124. https://doi.org/10.1016/j.molcel.2012.04.009
Pan X, Jiang N, Chen X, Zhou X, Ding L, Duan F (2014) R-loop structure: the formation and the effects on genomic stability. Yi Chuan Hered 36:1185–1194. https://doi.org/10.3724/SP.J.1005.2014.1185
Rinaldi C, Pizzul P, Longhese MP, Bonetti D (2020) Sensing R-loop-associated DNA damage to safeguard genome stability. Front Cell Dev Biol 8:618157. https://doi.org/10.3389/fcell.2020.618157
Petermann E, Lan L, Zou L (2022) Sources, resolution and physiological relevance of R-loops and RNA-DNA hybrids. Nat Rev Mol Cell Biol 23:521–540. https://doi.org/10.1038/s41580-022-00474-x
Lockhart A, Pires VB, Bento F, Kellner V, Luke-Glaser S, Yakoub G, Ulrich HD, Luke B (2019) RNase H1 and H2 are differentially regulated to process RNA-DNA hybrids. Cell Rep 29:2890-2900.e5. https://doi.org/10.1016/j.celrep.2019.10.108
Manzo SG, Hartono SR, Sanz LA, Marinello J, De Biasi S, Cossarizza A, Capranico G, Chedin F (2018) DNA topoisomerase I differentially modulates R-loops across the human genome. Genome Biol 19:100. https://doi.org/10.1186/s13059-018-1478-1
Ginno PA, Lott PL, Christensen HC, Korf I, Chédin F (2012) R-loop formation is a distinctive characteristic of unmethylated human CpG island promoters. Mol Cell 45:814–825. https://doi.org/10.1016/j.molcel.2012.01.017
Chen L, Chen J-Y, Zhang X, Gu Y, **ao R, Shao C, Tang P, Qian H et al (2017) R-ChIP using inactive RNase H reveals dynamic coupling of R-loops with transcriptional pausing at gene promoters. Mol Cell 68:745-757.e5. https://doi.org/10.1016/j.molcel.2017.10.008
García-Muse T, Aguilera A (2016) Transcription-replication conflicts: how they occur and how they are resolved. Nat Rev Mol Cell Biol 17:553–563. https://doi.org/10.1038/nrm.2016.88
Crossley MP, Bocek M, Cimprich KA (2019) R-Loops as cellular regulators and genomic threats. Mol Cell 73:398–411. https://doi.org/10.1016/j.molcel.2019.01.024
Hamperl S, Bocek MJ, Saldivar JC, Swigut T, Cimprich KA (2017) Transcription-replication conflict orientation modulates R-loop levels and activates distinct DNA damage responses. Cell 170:774-786.e19. https://doi.org/10.1016/j.cell.2017.07.043
Jones L, Houlden H, Tabrizi SJ (2017) DNA repair in the trinucleotide repeat disorders. Lancet Neurol 16:88–96. https://doi.org/10.1016/S1474-4422(16)30350-7
Srivatsan A, Tehranchi A, MacAlpine DM, Wang JD (2010) Co-orientation of replication and transcription preserves genome integrity. PLoS Genet 6:e1000810. https://doi.org/10.1371/journal.pgen.1000810
Zeman MK, Cimprich KA (2014) Causes and consequences of replication stress. Nat Cell Biol 16:2–9. https://doi.org/10.1038/ncb2897
Magdalou I, Lopez BS, Pasero P, Lambert SAE (2014) The causes of replication stress and their consequences on genome stability and cell fate. Semin Cell Dev Biol 30:154–164. https://doi.org/10.1016/j.semcdb.2014.04.035
Saponaro M, Kantidakis T, Mitter R, Kelly GP, Heron M, Williams H, Söding J, Stewart A et al (2014) RECQL5 controls transcript elongation and suppresses genome instability associated with transcription stress. Cell 157:1037–1049. https://doi.org/10.1016/j.cell.2014.03.048
Groh M, Silva LM, Gromak N (2014) Mechanisms of transcriptional dysregulation in repeat expansion disorders. Biochem Soc Trans 42:1123–1128. https://doi.org/10.1042/BST20140049
Lans H, Hoeijmakers JHJ, Vermeulen W, Marteijn JA (2019) The DNA damage response to transcription stress. Nat Rev Mol Cell Biol 20:766–784. https://doi.org/10.1038/s41580-019-0169-4
Skourti-Stathaki K, Kamieniarz-Gdula K, Proudfoot NJ (2014) R-loops induce repressive chromatin marks over mammalian gene terminators. Nature 516:436–439. https://doi.org/10.1038/nature13787
Ginno PA, Lim YW, Lott PL, Korf I, Chédin F (2013) GC Skew at the 5′ and 3′ ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome Res 23:1590–1600. https://doi.org/10.1101/gr.158436.113
** Z, Zinman L, Moreno D, Schymick J, Liang Y, Sato C, Zheng Y, Ghani M et al (2013) Hypermethylation of the CpG island near the G4C2 repeat in ALS with a C9orf72 expansion. Am J Hum Genet 92:981–989. https://doi.org/10.1016/j.ajhg.2013.04.017
Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K et al (2007) Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell 25:473–481. https://doi.org/10.1016/j.molcel.2007.01.017
Sollier J, Stork CT, García-Rubio ML, Paulsen RD, Aguilera A, Cimprich KA (2014) Transcription-coupled nucleotide excision repair factors promote R-loop-induced genome instability. Mol Cell 56:777–785. https://doi.org/10.1016/j.molcel.2014.10.020
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10(Suppl):S10-17. https://doi.org/10.1038/nm1066
Lin Y, Dent SYR, Wilson JH, Wells RD, Napierala M (2010) R Loops stimulate genetic instability of CTG.CAG repeats. Proc Natl Acad Sci USA 107:692–697. https://doi.org/10.1073/pnas.0909740107
Reddy K, Schmidt MHM, Geist JM, Thakkar NP, Panigrahi GB, Wang Y-H, Pearson CE (2014) Processing of double-R-loops in (CAG)·(CTG) and C9orf72 (GGGGCC)·(GGCCCC) repeats causes instability. Nucleic Acids Res 42:10473–10487. https://doi.org/10.1093/nar/gku658
Guler GD, Rosenwaks Z, Gerhardt J (2018) Human DNA helicase B as a candidate for unwinding secondary CGG repeat structures at the fragile X mental retardation gene. Front Mol Neurosci 11:138. https://doi.org/10.3389/fnmol.2018.00138
Voineagu I, Surka CF, Shishkin AA, Krasilnikova MM, Mirkin SM (2009) Replisome stalling and stabilization at CGG repeats, which are responsible for chromosomal fragility. Nat Struct Mol Biol 16:226–228. https://doi.org/10.1038/nsmb.1527
Brown RH, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. N Engl J Med 377:162–172. https://doi.org/10.1056/NEJMra1603471
Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, Sobue G (2022) Amyotrophic lateral sclerosis. Lancet Lond Engl 400:1363–1380. https://doi.org/10.1016/S0140-6736(22)01272-7
van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, van den Berg LH (2017) Amyotrophic lateral sclerosis. Lancet Lond Engl 390:2084–2098. https://doi.org/10.1016/S0140-6736(17)31287-4
Taylor JP, Brown RHJ, Cleveland DW (2016) Decoding ALS: from genes to mechanism. Nature 539:197–206. https://doi.org/10.1038/nature20413
Jaworska E, Kozlowska E, Switonski PM, Krzyzosiak WJ (2016) Modeling simple repeat expansion diseases with IPSC technology. Cell Mol Life Sci CMLS 73:4085–4100. https://doi.org/10.1007/s00018-016-2284-0
Gendron TF, Petrucelli L (2018) Disease mechanisms of C9ORF72 repeat expansions. Cold Spring Harb Perspect Med 8. https://doi.org/10.1101/cshperspect.a024224
Wang J, Haeusler AR, Simko EAJ (2015) Emerging role of RNA•DNA hybrids in C9orf72-linked neurodegeneration. Cell Cycle Georget Tex 14:526–532. https://doi.org/10.1080/15384101.2014.995490
Haeusler AR, Donnelly CJ, Periz G, Simko EAJ, Shaw PG, Kim M-S, Maragakis NJ, Troncoso JC et al (2014) C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 507:195–200. https://doi.org/10.1038/nature13124
** Z, Rainero I, Rubino E, Pinessi L, Bruni AC, Maletta RG, Nacmias B, Sorbi S et al (2014) Hypermethylation of the CpG-Island near the C9orf72 G4C2-repeat expansion in FTLD patients. Hum Mol Genet 23:5630–5637. https://doi.org/10.1093/hmg/ddu279
Russ J, Liu EY, Wu K, Neal D, Suh E, Irwin DJ, McMillan CT, Harms MB et al (2015) Hypermethylation of repeat expanded C9orf72 is a clinical and molecular disease modifier. Acta Neuropathol (Berl) 129:39–52. https://doi.org/10.1007/s00401-014-1365-0
Belzil VV, Bauer PO, Prudencio M, Gendron TF, Stetler CT, Yan IK, Pregent L, Daughrity L et al (2013) Reduced C9orf72 gene expression in C9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol (Berl) 126:895–905. https://doi.org/10.1007/s00401-013-1199-1
Vakoc CR, Mandat SA, Olenchock BA, Blobel GA (2005) Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol Cell 19:381–391. https://doi.org/10.1016/j.molcel.2005.06.011
Cohen S, Puget N, Lin Y-L, Clouaire T, Aguirrebengoa M, Rocher V, Pasero P, Canitrot Y et al (2018) Senataxin resolves RNA:DNA hybrids forming at DNA double-strand breaks to prevent translocations. Nat Commun 9:533. https://doi.org/10.1038/s41467-018-02894-w
Becherel OJ, Yeo AJ, Stellati A, Heng EYH, Luff J, Suraweera AM, Woods R, Fleming J et al (2013) Senataxin plays an essential role with DNA damage response proteins in meiotic recombination and gene silencing. PLoS Genet 9:e1003435. https://doi.org/10.1371/journal.pgen.1003435
Rawal CC, Zardoni L, Di Terlizzi M, Galati E, Brambati A, Lazzaro F, Liberi G, Pellicioli A (2020) Senataxin ortholog Sen1 limits DNA:RNA hybrid accumulation at DNA double-strand breaks to control end resection and repair fidelity. Cell Rep 31:107603. https://doi.org/10.1016/j.celrep.2020.107603
Walker C, Herranz-Martin S, Karyka E, Liao C, Lewis K, Elsayed W, Lukashchuk V, Chiang S-C et al (2017) C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat Neurosci 20:1225–1235. https://doi.org/10.1038/nn.4604
Moreira M-C, Klur S, Watanabe M, Németh AH, Le Ber I, Moniz J-C, Tranchant C, Aubourg P et al (2004) Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia-ocular apraxia 2. Nat Genet 36:225–227. https://doi.org/10.1038/ng1303
Chen Y-Z, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, Dierick I, Abel A et al (2004) DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am J Hum Genet 74:1128–1135. https://doi.org/10.1086/421054
Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S et al (1991) Identification of a Gene (FMR-1) Containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914. https://doi.org/10.1016/0092-8674(91)90397-h
Cabal-Herrera AM, Tassanakijpanich N, Salcedo-Arellano MJ, Hagerman RJ (2020) Fragile X-associated tremor/ataxia syndrome (FXTAS): pathophysiology and clinical implications. Int J Mol Sci 21. https://doi.org/10.3390/ijms21124391
Brouwer JR, Willemsen R, Oostra BA (2009) The FMR1 gene and fragile X-associated tremor/ataxia syndrome. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet 150B:782–798. https://doi.org/10.1002/ajmg.b.30910
Sidorov MS, Auerbach BD, Bear MF (2013) Fragile X mental retardation protein and synaptic plasticity. Mol Brain 6:15. https://doi.org/10.1186/1756-6606-6-15
Richter JD, Zhao X (2021) The molecular biology of FMRP: new insights into fragile X syndrome. Nat Rev Neurosci 22:209–222. https://doi.org/10.1038/s41583-021-00432-0
Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DBJ, Moine H, Kooy RF, Tassone F, Gantois I et al (2017) Fragile X syndrome. Nat Rev Dis Primer 3:17065. https://doi.org/10.1038/nrdp.2017.65
Hagerman RJ, Hagerman P (2016) Fragile X-associated tremor/ataxia syndrome — features, mechanisms and management. Nat Rev Neurol 12:403–412. https://doi.org/10.1038/nrneurol.2016.82
Yousuf A, Ahmed N, Qurashi A (2022) Non-canonical DNA/RNA structures associated with the pathogenesis of fragile X-associated tremor/ataxia syndrome and fragile X syndrome. Front Genet 13:866021. https://doi.org/10.3389/fgene.2022.866021
Kumari D, Usdin K (2014) Polycomb group complexes are recruited to reactivated FMR1 alleles in fragile X syndrome in response to FMR1 transcription. Hum Mol Genet 23:6575–6583. https://doi.org/10.1093/hmg/ddu378
Skourti-Stathaki K, Torlai Triglia E, Warburton M, Voigt P, Bird A, Pombo A (2019) R-loops enhance polycomb repression at a subset of developmental regulator genes. Mol Cell 73:930-945.e4. https://doi.org/10.1016/j.molcel.2018.12.016
Loomis EW, Sanz LA, Chédin F, Hagerman PJ (2014) Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet 10:e1004294. https://doi.org/10.1371/journal.pgen.1004294
Di Ruscio A, Ebralidze AK, Benoukraf T, Amabile G, Goff LA, Terragni J, Figueroa ME, De Figueiredo Pontes LL et al (2013) DNMT1-interacting RNAs block gene-specific DNA methylation. Nature 503:371–376. https://doi.org/10.1038/nature12598
Kraan CM, Godler DE, Amor DJ (2019) Epigenetics of fragile X syndrome and fragile X-related disorders. Dev Med Child Neurol 61:121–127. https://doi.org/10.1111/dmcn.13985
Iwahashi CK, Yasui DH, An H-J, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB et al (2006) Protein composition of the intranuclear inclusions of FXTAS. Brain J Neurol 129:256–271. https://doi.org/10.1093/brain/awh650
Walker FO (2007) Huntington’s disease. Lancet Lond Engl 369:218–228. https://doi.org/10.1016/S0140-6736(07)60111-1
Tabrizi SJ, Flower MD, Ross CA, Wild EJ (2020) Huntington disease: new insights into molecular pathogenesis and therapeutic opportunities. Nat Rev Neurol 16:529–546. https://doi.org/10.1038/s41582-020-0389-4
Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F et al (1993) The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat Genet 4:398–403. https://doi.org/10.1038/ng0893-398
Finkbeiner S (2011) Huntington’s Disease. Cold Spring Harb Perspect Biol 3. https://doi.org/10.1101/cshperspect.a007476
Pradhan S, Gao R, Bush K, Zhang N, Wairkar YP, Sarkar PS (2022) Polyglutamine expansion in Huntingtin and mechanism of DNA damage repair defects in Huntington’s disease. Front Cell Neurosci 16:837576. https://doi.org/10.3389/fncel.2022.837576
Gao R, Chakraborty A, Geater C, Pradhan S, Gordon KL, Snowden J, Yuan S, Dickey AS et al (2019) Mutant Huntingtin impairs PNKP and ATXN3, disrupting DNA repair and transcription. eLife 8. https://doi.org/10.7554/eLife.42988
Enokido Y, Tamura T, Ito H, Arumughan A, Komuro A, Shiwaku H, Sone M, Foulle R et al (2010) Mutant Huntingtin impairs Ku70-mediated DNA repair. J Cell Biol 189:425–443. https://doi.org/10.1083/jcb.200905138
Seeberg E, Eide L, Bjørås M (1995) The base excision repair pathway. Trends Biochem Sci 20:391–397. https://doi.org/10.1016/s0968-0004(00)89086-6
Chatterjee N, Walker GC (2017) Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen 58:235–263. https://doi.org/10.1002/em.22087
Maiuri T, Suart CE, Hung CLK, Graham KJ, Barba Bazan CA, Truant R (2019) DNA damage repair in Huntington’s disease and other neurodegenerative diseases. Neurother J Am Soc Exp Neurother 16:948–956. https://doi.org/10.1007/s13311-019-00768-7
Iyer RR, Pluciennik A, Napierala M, Wells RD (2015) DNA triplet repeat expansion and mismatch repair. Annu Rev Biochem 84:199–226. https://doi.org/10.1146/annurev-biochem-060614-034010
Nakamori M, Panigrahi GB, Lanni S, Gall-Duncan T, Hayakawa H, Tanaka H, Luo J, Otabe T et al (2020) A slipped-CAG DNA-binding small molecule induces trinucleotide-repeat contractions in vivo. Nat Genet 52:146–159. https://doi.org/10.1038/s41588-019-0575-8
Perego MGL, Taiana M, Bresolin N, Comi GP, Corti S (2019) R-Loops in Motor Neuron Diseases. Mol Neurobiol 56:2579–2589. https://doi.org/10.1007/s12035-018-1246-y
Jangi M, Fleet C, Cullen P, Gupta SV, Mekhoubad S, Chiao E, Allaire N, Bennett CF et al (2017) SMN deficiency in severe models of spinal muscular atrophy causes widespread intron retention and DNA damage. Proc Natl Acad Sci U S A 114:E2347–E2356. https://doi.org/10.1073/pnas.1613181114
Cuartas J, Gangwani L (2022) R-loop mediated DNA damage and impaired DNA repair in spinal muscular atrophy. Front Cell Neurosci 16:826608. https://doi.org/10.3389/fncel.2022.826608
Ma D, Tan YJ, Ng ASL, Ong HL, Sim W, Lim WK, Teo JX, Ng EYL et al (2020) Association of NOTCH2NLC repeat expansions with Parkinson disease. JAMA Neurol 77:1559–1563. https://doi.org/10.1001/jamaneurol.2020.3023
Shi C-H, Fan Y, Yang J, Yuan Y-P, Shen S, Liu F, Mao C-Y, Liu H et al (2021) NOTCH2NLC intermediate-length repeat expansions are associated with Parkinson disease. Ann Neurol 89:182–187. https://doi.org/10.1002/ana.25925
Klockgether T, Mariotti C, Paulson HL (2019) Spinocerebellar ataxia. Nat Rev Dis Primer 5:24. https://doi.org/10.1038/s41572-019-0074-3
Seidel K, Siswanto S, Brunt ERP, den Dunnen W, Korf H-W, Rüb U (2012) Brain pathology of spinocerebellar ataxias. Acta Neuropathol (Berl) 124:1–21. https://doi.org/10.1007/s00401-012-1000-x
Dueñas AM, Goold R, Giunti P (2006) Molecular pathogenesis of spinocerebellar ataxias. Brain J Neurol 129:1357–1370. https://doi.org/10.1093/brain/awl081
Rüb U, Schöls L, Paulson H, Auburger G, Kermer P, Jen JC, Seidel K, Korf H-W et al (2013) Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol 104:38–66. https://doi.org/10.1016/j.pneurobio.2013.01.001
McLoughlin HS, Moore LR, Paulson HL (2020) Pathogenesis of SCA3 and implications for other polyglutamine diseases. Neurobiol Dis 134:104635. https://doi.org/10.1016/j.nbd.2019.104635
Tian Y, Wang J-L, Huang W, Zeng S, Jiao B, Liu Z, Chen Z, Li Y et al (2019) Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am J Hum Genet 105:166–176. https://doi.org/10.1016/j.ajhg.2019.05.013
Sun Q-Y, Xu Q, Tian Y, Hu Z-M, Qin L-X, Yang J-X, Huang W, Xue J et al (2020) Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain J Neurol 143:222–233. https://doi.org/10.1093/brain/awz372
Liufu T, Zheng Y, Yu J, Yuan Y, Wang Z, Deng J, Hong D (2022) The polyG diseases: a new disease entity. Acta Neuropathol Commun 10:79. https://doi.org/10.1186/s40478-022-01383-y
Oh J, Jia T, Xu J, Chong J, Dervan PB, Wang D (2022) RNA polymerase II trapped on a molecular treadmill: structural basis of persistent transcriptional arrest by a minor groove DNA binder. Proc Natl Acad Sci USA 119. https://doi.org/10.1073/pnas.2114065119
Escudé C, Nguyen CH, Kukreti S, Janin Y, Sun JS, Bisagni E, Garestier T, Hélène C (1998) Rational design of a triple helix-specific intercalating ligand. Proc Natl Acad Sci U S A 95:3591–3596. https://doi.org/10.1073/pnas.95.7.3591
Arya DP (2011) New approaches toward recognition of nucleic acid triple helices. Acc Chem Res 44:134–146. https://doi.org/10.1021/ar100113q
Shaw NN, Arya DP (2008) Recognition of the unique structure of DNA:RNA hybrids. Biochimie 90:1026–1039. https://doi.org/10.1016/j.biochi.2008.04.011
Groh M, Gromak N (2014) Out of balance: R-loops in human disease. PLoS Genet 10:e1004630. https://doi.org/10.1371/journal.pgen.1004630
Barbieri CM, Li T-K, Guo S, Wang G, Shallop AJ, Pan W, Yang G, Gaffney BL (2003) Aminoglycoside complexation with a DNA.RNA hybrid duplex: the thermodynamics of recognition and inhibition of RNA processing enzymes. J Am Chem Soc 125:6469–6477. https://doi.org/10.1021/ja021371d
Shaw NN, ** H, Arya DP (2008) Molecular recognition of a DNA:RNA hybrid: sub-nanomolar binding by a neomycin-methidium conjugate. Bioorg Med Chem Lett 18:4142–4145. https://doi.org/10.1016/j.bmcl.2008.05.090
Lee H-G, Imaichi S, Kraeutler E, Aguilar R, Lee Y-W, Sheridan SD, Lee JT (2023) Site-specific R-loops induce CGG repeat contraction and fragile X gene reactivation. Cell 186:2593-2609.e18. https://doi.org/10.1016/j.cell.2023.04.035
Hensel N, Detering NT, Walter LM, Claus P (2020) Resolution of pathogenic R-loops rescues motor neuron degeneration in spinal muscular atrophy. Brain J Neurol 143:2–5. https://doi.org/10.1093/brain/awz394
Jauregui-Lozano J, Escobedo S, Easton A, Lanman NA, Weake VM, Hall H (2022) Proper control of R-loop homeostasis is required for maintenance of gene expression and neuronal function during aging. Aging Cell 21:e13554. https://doi.org/10.1111/acel.13554
Hashizume A, Fischbeck KH, Pennuto M, Fratta P, Katsuno M (2020) Disease mechanism, biomarker and therapeutics for spinal and bulbar muscular atrophy (SBMA). J Neurol Neurosurg Psychiatry 91:1085–1091. https://doi.org/10.1136/jnnp-2020-322949
Funding
This work was supported by the Excellent Youth Foundation of Hunan Province (No. 2023JJ10098), National Natural Science Foundation of China (No. 82071437), the Natural Science Foundations of Hunan Province (No. 2021JJ31115), the National Key Research and Development Program of China (No.2021YFC2501200), and the Project Program of National Clinical Research Center for Geriatric Disorders (**angya Hospital) (No.2021KFJJ10).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Qian Xu conceived the study. Material preparation, data collection, and analysis were performed by Yiting Wu and Tingwei Song. The first draft of the manuscript was written by Yiting Wu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This is a review. The ethics approval is not required.
Consent to Participate
Not applicable.
Consent for Publication
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1 and 2.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Song, T. & Xu, Q. R-LOOPs on Short Tandem Repeat Expansion Disorders in Neurodegenerative Diseases. Mol Neurobiol 60, 7185–7195 (2023). https://doi.org/10.1007/s12035-023-03531-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03531-4