Abstract
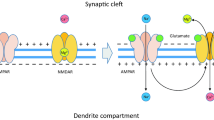
Glutamatergic chemical synapses mediate excitatory neurotransmission by the ion flow through α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-type glutamate receptors in the central nervous system (CNS). AMPA receptor-mediated synaptic transmission abnormalities may play a role in neurologic and neurodegenerative diseases, and compounds that can modulate AMPA receptor (AMPAR) signaling have been studied for decades as possible therapies for Alzheimer’s disease, Parkinson’s disease, depression, and epilepsy. Here, we aimed to determine the modulating effect of allosteric regulators on AMPA receptors by comparing their actions on AMPA-evoked currents, desensitization, and deactivation rate in human embryonic kidney cells (HEK293T) recombinant AMPAR subunits. In this study, patch-clamp electrophysiology was performed to examine how the AMPA subunit responded to benzodioxole (BDZ) derivatives. Our results showed that the BDZ derivatives affected AMPARs as negative modulators, particularly BDZs (8, 9, and 15), where they increased the desensitization rate and delayed the deactivation process. The BDZ compounds were utilized in this study as AMPA modulators to investigate fundamental and clinical AMPA receptor processes. We test BDZs as negative allosteric AMPAR modulators to reestablish glutamatergic synaptic transmission. These efforts have resulted in important molecules with neuroprotective properties on AMPA receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For a long time, benzodioxole (BDZ) derivatives have been the subject of numerous studies since they have a variety of biological activities like anticancer, antimicrobial, antioxidants, antiinflammatory, and antidepressants [1], [2] [3,4,5,6]. Benzodioxole has had a significant effect in the creation of antiepileptic pharmaceuticals since it seems to promise the manufacture of efficient antiepileptic medications due to its role in protecting epileptic convulsions by avoiding the constantly repetitive firing of a neuron [7, 8]. Epilepsy is a severe brain disease that could happen for various reasons, one of which is the pathological alterations in brain network signaling due to changes in synaptic plasticity mediated by α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) trafficking [9]. Accordingly, the importance of studying the effect of benzodioxole derivatives on the biophysical properties of AMPARs has been a significant focus of researchers. Rapid synaptic transmission in the central nervous system (CNS) is achieved by ionotropic glutamate receptors such as AMPA receptors [10],AMPARs contribute to functions in neuronal communication that determine learning and memory formation [11]. Overactivation of AMPA receptors (AMPARs) is involved in many neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, epilepsy, and strokes [9, 12,13,14]. As a result, investigating benzodioxole derivatives would pave the way to the possibility of develo** drugs to treat neurodegenerative diseases, where it would modulate AMPARs’ function by controlling their biophysical gating properties.
AMPA receptors are tetramers containing different combinations of four pore-forming homomeric or heteromeric subunits, which are as follows: GluA1, GluA2, GluA3, and GluA4 [15, 16]. Each GluA subunit consists of a large extracellular amino-terminal domain (ATD), three transmembrane domains (M1, M3, and M4), one reentrant loop (M2), a carboxyl-terminal domain (CTD), and a ligand-binding domain (LBD) consisting of two lobes (S1 and S2) where the neurotransmitter glutamate binds [17,18,19,20].
AMPARs are primed for activation by presynaptically released glutamate [21],their rapid kinetics specializes them in transmitting signals from the presynaptic neuron to the postsynaptic neuron [16, 22], and a very fast depolarization of the postsynaptic membrane is ensured by the influx of Na+ ions with K+ efflux [23]. Several studies have been conducted to clarify the biophysical gating properties (i.e., desensitization and deactivation parameters) of AMPA receptors [24, 25]. As a neuroprotective mechanism, AMPAR desensitization modulates synaptic responses by kee** the channel partially open, while glutamate remains bound to the receptor on a sub-millisecond time scale (τ des) [9, 47]. On the other hand, AMPAR deactivation is assessed when the receptor is unattached to glutamate (τ deact) [26]. Understanding both parameters (i.e., deactivation and desensitization) helped us determine the time of synaptic events, which paves the way for new regulatory mechanisms of AMPARs [27] and develo** AMPAR modulators to target these processes selectively. Controlling AMPAR activity using different competitive and non-competitive inhibitors would be a promising treatment for the pre-mentioned neurological diseases related to AMPARs. The ligand-binding domain can display different conformations based on interaction with other antagonists [28]. Several competitive antagonists of AMPARs have been synthesized, such as the quinoxalinedione derivatives 6-cyano-7-nitroquinoxaline2,3-dione (CNQX and the 6,7-dinitroquinoxaline-2,3-dione (DNQX,they were the first competitive antagonists of AMPARs [29] that stopped by clinical trials due to the lack of selectivity. As a result, many studies shift the need for non-competitive inhibitors to bind AMPA receptors allosterically and affect them [30], such as perampanel, which is the first highly selective non-competitive AMPA receptor antagonist to be approved for clinical use to treat epilepsy as a non-competitive AMPA receptor antagonist. Perampanel inhibited synaptic responses mediated by AMPA receptors but not by NMDA or kainate receptors [31, 32]. However, it was used in limited doses and had many side effects. Also, a promising non-competitive compound (i.e., 2,3-benzodiazepine) prepared in our lab had an inhibitory effect on AMPARs [33].
In this investigation, we looked into the previously synthesized 15 benzodioxole derivatives by our team (shown in Scheme 1 and Fig. 1) (Hawash, Eid, et al., 2020; Hawash, [34] that were divided into two groups: benzodioxole aryl acetate (BDZ-1–7) and benzodioxole aryl acetic acid (BDZ-8–15). Both BDZ groups have similar structures, but the ester group (COOR) is found in the first group, while the aryl acetic acid group (COOH) is located in the second. As a result, they would have different effects on AMPA receptors. Because the COOH group is ionizable whereas the ester group is not, it is more reactive than the COOR group and may form electrostatic interactions with biological targets. Electrostatic interactions are essential in chemical binding to AMPA receptors because they may contribute to lobe 2 rigidity and more efficiently transmit agonist-binding energy to the ion channel linker. Peptide flip Asp residues and Lys 660 may encourage the rotation of the D655-S657 peptide bond and create two cross cleft H bonds, allowing for a more effective transfer of energy agonist-induced conformational changes to channel opening. [35].
This research examined how our benzodioxole derivatives affected the biophysical gating properties of AMPARs and the inhibitory effect using the patch-clamp electrophysiology technique. According to the available information on the structure–activity relationships (SARs) [3], we decided to study the benzodioxole effect by adding an electron-withdrawing group (i.e., a chlorine atom, bromine atom, and iodine atom) at the C-3 position vs. C-2 position or C-4 position of the phenyl ring (ortho-, meta-, and para-positions). The crystal structures of AMPAR do not give a comprehensive atomistic view of individual protein–ligand interactions or even an accurate orientation of the ligands at the binding pocket. Although the mechanics of non-competitive inhibition are not fully understood, inhibitors are considered to function by acting as “wedges” between the receptor’s transmembrane segments to disrupt gating motions required for channel opening. Because of the flexibility of the TMD-LBD linkers and pre-M1 helix, the binding pocket may adjust to promote the binding of different ligands in different orientations, indicating that an induced-fit process most likely performs binding of diverse ligands. In gating, these linkers undergo significant conformational changes, and their interaction with inhibitors implies that the inhibitors may maintain them in a closed state [36]. The study of these compounds aimed to answer the following questions: What is the significance of adding an electron-withdrawing group (i.e., a chlorine atom, bromine atom, and iodine atom) at the C-3 position vs. C-2 position/ or C-4 position of the phenyl ring? Would the ester group have the same effect as carboxylic acid? Answering these questions will help us evaluate their effect on the biophysical gating properties of the homomeric and heteromeric AMPA receptor subunits and guarantee better compounds and drugs to be synthesized to treat neurological diseases.
Methods
Chemicals and Reagents
Chemicals utilized in this study were purchased from Aldrich Chemical Company and were used as received: 3,4-(methylenedioxy)phenylacetic acid, acetic acid, oxalyl chloride, sodium bicarbonate, 2-chlorobenzoic acid, 3-chlorobenzoic acid, benzoic acid, 2,3-chlorobenzoic acid, 2-iodobenzoic acid, 3-iodobenzoic acid, 2-bromobenzoic acid, 3-bromobenzoic acid, phosphorus pentoxide, sodium hydroxide (NaOH), magnesium sulfate, hydrazine hydrate, methanol, ethanol, n-hexane, ethyl acetate, tetrahydrofuran (THF), and dichloromethane (DCM).
Preparation of the Benzodioxole Derivatives
The benzodioxole aryl acetate derivatives (BDZ-1–7) and acetic acid derivatives (BDZ-8–15) are synthesized as outlined in Scheme 1. The 3,4-(methylenedioxy)phenylacetic acid (1) (4.00 g, 22.20 mmol) was dissolved in 40 ml of methanol. After cooling in an ice bath at 0 °C, oxalyl chloride (2 ml, 23.4 mmol) was added dropwise to the reaction mixture, which was agitated for 30 min. The reaction mixture was evaporated to dryness under reduced pressure. After dissolving the dried residue in ethyl acetate, it was washed with saturated sodium bicarbonate (NaHCO3) and distilled water. The organic layer was separated and dried using a drying agent of sodium sulfate. It was then filtered and evaporated using a rota evaporator; the dried residue was purified by silica gel column chromatography using a 50:50 n-hexane: ethyl acetate mobile system, yielding compound (2), yellow oil with a 95% yield [33, 37].
To a stirred solution of dichloromethane (20 ml) and intermediate compound (2) (333.0 mg, 1.71 mmol), benzoic acid derivatives (350.0 mg, 2.22 mmol) and phosphorus pentoxide (3.00 g) were added. Then, the mixture was agitated at room temperature for 16–20 h before being gently added to distilled water (30 ml) and extracted with ethyl acetate (2 × 40 ml). After separating the organic layer, it was treated with 1 M NaOH (30 ml), saturated sodium chloride (30 ml), and distilled water (2 × 40 ml). The organic layer was dried using sodium sulfate as the drying agent, filtered, and evaporated under reduced pressure, and then purified using silica gel column chromatography (n-hexane: ethyl acetate solvent system), yielding compounds (BDZ-1–7) with 60–96% yield (Jaradat, Nidal; Hawash, Mohammed; Abualhasan, 2020).
The benzodioxole aryl acetate derivatives (BDZ-1–7) (150.0 mg, 0.45 mmol) were dissolved in methanol/H2O/THF (11/11/11 ml), followed by the addition of NaOH (180.30 mg, 4.5 mmol). The solution was heated in an oil bath for 3 h and then cooled to room temperature. After the solution was evaporated, the residue was acidified by adding HCl 2 N dropwise (pH = 2). The precipitate was filtered and concentrated under a vacuum to obtain BDZ-8–15 with an 84–95% yield (Jaradat, Nidal; Hawash, Mohammed; Abualhasan, 2020). HRMS, IR, 1H-NMR, and 13C-NMR were used to characterize all evaluated compounds, and their spectrum data were recorded accordingly (Hawash, [34]).
Electrophysiological Recordings
The AMPA receptors used in our study were in the flip isoform. S.F. Heinemann (Salk Institute, La Jolla, CA) initially provided the templates of GluA1-3 (Q-form/flip). Wild-type AMPARs DNA was inserted into the pRK5 plasmid for expression in human embryonic kidney cells (HEK293) modified to produce a green fluorescent protein (pEGFP; Clontech, Palo Alto, CA) via a downstream internal ribosome entry site. The ratio of cotransfection was 1:9 (pEGFP-C1: GluA subunit). We used jetPRIME (Polyplus: New York, NY) to transfect HEK293T cells with the plasmid DNA transiently. JetPRIME (Polyplus: New York, NY) or Lipofectamine 2000 (Invitrogen; San Diego, CA) was utilized as transfection reagents. In 12-well plates, cells were cultured for 36 h following transfection. As explained in our previous work, the coverslips were then replated with laminin (1 mg/ml; Sigma, Germany) for electrophysiological recordings (Qneibi, Hamed, Fares, et al., 2019; Qneibi, Hamed, Natsheh, et al., 2019). Integrated patch clamp amplifiers with data acquisition system (IPA, Sutter Instruments, Novato, CA) and a rapid solution exchange system with a two-barrel theta glass pipette controlled by a piezoelectric translator were used to record whole-cell (patch-clamp) current recordings (Automate Scientific, Berkeley, CA). One barrel held the external solution (wash solution), which included 150 mM NaCl, 2.8 mM KCl, 0.5 mM MgCl2, 2 mM CaCl2, and 10 mM HEPES, and was adjusted to pH 7.4 with NaOH, while the chemical compound solution was in the other barrel; it was prepared by adding 100 mM of dimethylsulfoxide (DMSO) and washed solution to the benzodioxole derivatives to yield final concentrations of 0.2–200 μM with added glutamate (10 mM, unless otherwise noted). The pipette solution contained 110 mM CsF, 30 mM CsCl, 4 mM NaCl, 0.5 mM CaCl2, 10 mM EGTA, and 10 mM HEPES and adjusted to pH 7.2 with CsOH. Patch electrodes were made from borosilicate glass with a resistance of 2–4 MΩ. Typical 10–90% rise times were 200–300 us, measured from junction potentials at the patch pipette’s open tip after recordings. AMPAR-current deactivation (τw deact) and desensitization (τw des) were obtained after applying 10 mM of agonist (glutamate) for 500 ms for desensitization and 1 ms for deactivation, and the weighted tau (τw) was calculated as τw = (τf × af) + (τs × as), where af and as are the relative amplitudes of the fast (τf) and slow (τs) exponential component. Those measurements were taken after fitting the desensitization and deactivation currents with two exponentials fitting the current decay starting from 95% of the peak to the baseline current. All experiments were repeated in different cells obtained from at least 4–6 independent transfections (separated in time) at − 60 mV potential, pH 7.4, and room temperature (20–23 °C). Igor Pro7 (Wave Metrics, Inc.) was used for our data analysis. The total number of recorded whole-cell recordings and the detailed data analysis are provided in the supporting material.
Statistical Analysis
One-way analysis of variance (ANOVA) was utilized to compare the examined parameters. The data were presented as mean ± standard error of the mean. p values < 0.05 were regarded statistically significant, *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. GraphPad Prism statistical software was used for the statistical analysis. The n number reflected the total number of cells evaluated by each BDZ compound (n = 8–10); the number of cells was calculated depending on how each chemical impacted the cells’ health. The average inhibition by each substance on the studied cells was determined to increase the specificity of the findings.
Results
Subjecting AMPA Receptors to the BDZ Compounds Reduced AMPA Receptor Currents with Low Micromolar Concentrations
Whole-cell patch-clamp techniques were used to examine the inhibitory effects of our 15 BDZ compounds on homomeric and heteromeric AMPA-type receptors (GluA1, GluA1/2, GluA2, and GluA2/3) in transfected HEK293T cells under different BDZ solutions. At 10 mM glutamate concentration and a 500 ms protocol, approximately 95% of the AMPA receptors channels were open. A stands for normalized current readings, while AI indicates the inhibited current following BDZ compound exposure, as BDZ compounds affect AMPA receptors by reducing the current of AMPA-type subunits, as shown in Fig. 2. As the concentration increases, BDZs continue to block AMPA receptors, as illustrated in Fig. S1, until a plateau is established at a concentration of 16 µM. Our previous experience with BDZ compounds led us to choose this concentration range [33]. BDZ-15 was shown to be the most effective inhibitor of homomeric and heteromeric AMPA receptor subunits since it reduced the cell currents by ninefold. On the other hand, BDZ-8 and BDZ-9 were potent in inhibiting AMPA receptors by sevenfold and eightfold, respectively. While BDZ-2, 3, and 4 decreased AMPA receptor cell currents, they were insufficient to be considered AMPA receptor inhibitors. Our results also showed that increasing glutamate concentrations in all AMPAR-type subunits did not change current amplitudes (Fig. S2), and glutamate concentration effects plateaued at 2 µM onwards, suggesting that BDZ compounds function as non-competitive inhibitors.
Different AMPA-type subunits are inhibited by the BDZ compounds. Whole-cell recordings of amplitude (a, c, e, and h) from HEK239-expressing AMPAR cells after treating the cell with 10 mM glutamate alone (black) and with Glu + BDZ compounds (white) were obtained. Inhibition tests on GluA1, GluA2, GluA1/2, and GluA2/3 are shown in b, d, f, and h. At − 60 mV, pH 7.4, and 22 °C, the whole-cell current recording was conducted. One-way analysis of variance (ANOVA) was used for comparison: *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. Several tested cells for each BDZ compound (n = 8–10). All values are presented as mean ± SEM
Moreover, the IC50 calculations for the 15 BDZ compounds (Table S16), calculated using the GraphPad Prism software to confirm the BDZs effectiveness in modulating AMPA receptors, supported the results presented. BDZ-15 suppressed GluA2, GluA1, GluA1/2, and GluA2/3 at low micromolar concentrations (3.73 µM, 4.15 µM, 3.93 µM, and 4.02 µM, respectively), and BDZ-15 revealed the most substantial inhibitory influence on AMPA receptor currents. The IC50 for GluA2 was estimated to be 3.19 µM when BDZ-9 was used and 4.11 µM when BDZ-8 was used, as shown in the dose–response curve (Fig. 3). The IC50 values for all BDZs are included in Table S16.
Dose–response curve of AMPAR subunits. a–d A total of 3 to 4 separate experiments were done at − 60 mV, pH 7.4, and 22 °C to evaluate the IC50 values of BDZ compounds on whole-cell normalized peak amplitude in HEK239T cells using GraphPad Prism. After 500 ms of exposure to BDZ, the cell was rinsed with glutamate for 20 s, and lastly, glutamate alone (10 mM) was administered, and the instantaneous evoked current was recorded. Error bars represent the standard error of the means
BDZ Compounds Act as Negative Modulators of AMPA Receptor Desensitization and Deactivation in HEK293T Cells
Following the BDZ compound’s inhibitory effect, we aimed to test their potential as drugs by assessing their impact on the biophysical gating properties of AMPA receptors, as AMPA receptor activation is heavily dependent on the desensitization and deactivation processes. Glutamate exposure for 500 ms causes AMPARs in HEK293T cells to desensitize, and our data reveal that BDZ-8, BDZ-9, and BDZ-15 had the most influence on the GluA1 receptor as the (τw des) values post-BDZ rinse decreased by about threefold. Moreover, BDZ-8, BDZ-9, and BDZ-15 reduced GluA1/2 (τw des) levels by approximately twofold in HEK293T cells. There was an approximately threefold drop-in (τw des) for GluA2 and GluA2/3 after rinsing with BDZ-8 and BDZ-9, whereas BDZ-15 reduced (τw des) for GluA2 and GluA2/3 by fivefold (Fig. 4). The results showed that BDZ-8, BDZ-9, and BDZ-15 had the most significant influence on desensitization for all AMPA receptor subunits, owing to that to the LBD rearrangements that allow the base of the D2 lobes to take positions comparable to those of the inactivated state, reducing the stress produced by the M3-S2 linkers on the M3 helix induced by glutamate binding. On the other hand, BDZ-8, BDZ-9, and BDZ-15 significantly impacted AMPA receptor deactivation by increasing (τw deact) by approximately fourfold and fivefold for all AMPA receptor subunits (Fig. 5). In general, we found that the BDZ compounds had a significant effect on the desensitization and deactivation mechanisms of AMPA receptors.
BDZ compounds affect AMPAR desensitization weighted time constants (τw des) in whole-cell patched HEK293T expressing AMPAR subunits (a, c, e, and g). Desensitization time constants fluctuate when glutamate (Glu) alone or Glu + BDZ compounds are applied to distinct AMPAR-type subunits. b, d, f, and h show how the traces were affected by BDZ-8, BDZ-9, and BDZ-15 compounds that had the best effect on AMPA receptor subunits. One-way analysis of variance (ANOVA) was used for comparison: *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. The number of tested cells for each BDZ compound (n = 8–10). All values are presented as mean ± SEM
BDZ compounds affect AMPAR deactivation weighted time constants (τw deact) in whole-cell patched HEK293T expressing AMPAR subunits (a, c, e, and g). Deactivation time constants fluctuate when glutamate (Glu) alone or Glu + BDZ compounds are applied to distinct AMPAR-type subunits. b, d, f, and h show how the traces were affected by BDZ-8, BDZ-9, and BDZ-15 compounds that had the best effect on AMPA receptor subunits. One-way analysis of variance (ANOVA) was used for comparison: *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. The number of tested cells for each BDZ compound (n = 8–10). All values are presented as mean ± SEM
Discussion
Our study seeks to get further insight into the AMPAR biophysical gating and negative allosteric modulator processes by studying the effect of BDZ drugs on diverse homomeric and heteromeric subunit configurations of AMPARs. Because AMPARs play an essential role in neuronal development and the progression of neurodegenerative disorders, we wanted to test whether the BDZ derivatives might effectively reduce and relieve AMPAR overactivation and excitotoxicity.
Receptor functions can vary depending on how the AMPAR subunits are assembled into homomeric or heteromeric tetramers. Most central nervous system AMPARs form heteromers with GluA2; for example, in the forebrain, including the hippocampus and cerebral neocortex, the primarily expressed subunits are GluA1 and GluA2, with low levels of GluA3 and GluA4 [38]. Mammalian AMPAR function is controlled mainly by the GluA2 subunit. Aside from the kinetics, single-channel conductance, and Ca2+ permeability, this subunit is the most tightly regulated of the glutamate receptor subunits, with specific regulatory processes at the level of gene expression and RNA editing receptor assembly and trafficking. GluA1 is the second most abundant subunit occupying the A/C positions of the LBD, and it is often present in matured neurons. As a result, neurons that lack or have a reduced number of GluA2 and GluA1 subunits display severe effects, such as increased Ca2+ concentrations and the development of diseases. The other primary heteromer in principal cortical cells has been speculated to be GluA2/3. GluA3 occurs less often in co-assembly with the GluA2 subunit, but they play an essential role in AMPAR trafficking [39, 40].
Receptor deactivation or desensitization occurs after AMPA receptor activation, depending on the nature of the ligand unbinding or channel closure. Rapid desensitization of AMPA receptors occurs on a millisecond time scale. The postsynaptic response frequency is also influenced by how the AMPA receptors recover from desensitization. AMPA receptor desensitization is a direct result of the tetrameric receptor complex’s conformational state, and it is mediated by intersubunit rearrangements that disconnect the action of the S1–S2 ligand-binding core closure from the ion channel gate closure [41]. We found that BDZ compounds make AMPA receptors less sensitive to glutamate by decreasing their deactivation and increasing their return to the unbound state. We hypothesize that BDZs have a negative allosteric effect, reducing glutamatergic receptor synaptic efficacy, where they act as a non-competitive and selective AMPA receptor antagonist. A previous study on AMPA receptor allosteric modulation discovered that hetero-oligomerization of recombinant AMPA receptors with the GluA2 subunit increased modulator potency, suggesting that GluA2 has unique pharmacological activities in addition to unique permeability qualities [42].
Neuronal trauma, ischemia, and other related diseases benefit from AMPA receptor non-competitive inhibitors. The open or closed state of the receptor complex does not affect the inhibition of AMPA receptors, which is mainly voltage-independent. By not competing directly with endogenous neurotransmitters, negative allosteric regulation of the AMPA receptor can adjust the excitatory neural field frequency and amplitude regardless of the concentration of neurotransmitters or the polarization state of the synaptic membrane [43].
The mechanism of gating on AMPA receptors can be better understood by understanding how BDZ compounds act by attaching to an allosteric site and negatively modulating AMPA receptors. Glutamate hyperactivity-related illnesses might benefit from the negative allosteric effects of BDZ compounds that bind beyond the orthostatic binding site and block the receptor through non-competitive mechanisms. Negative allosteric modulation of AMPARs is helpful in the treatment of neurologic illnesses connected to glutamatergic hyperfunction; this includes 2,3-benzodiazepines, quinazolinones, and pyridines [44,45,46,47].
The benzodioxole moiety is considered a suitable group to bind the allosteric sites of AMPA receptors. The oxygen atoms in benzene as methoxy groups or as ether in benzodioxole have crucial work in the possible binding interaction with the allosteric binding site [48]. However, many structures with a benzodioxole moiety showed potent activities on AMPA receptors, which makes this group promising for these biological targets [49], as well as the presence of halogens as Cl atom was considered an important group to improve the biological activity on AMPA receptors [33].
The BDZs structures comprise the key pharmacophore groups (carbonyl, heteroatoms, and halogenated aromatic or heterocyclic ring) that may participate in the stated binding interactions, such as hydrogen, hydrophobic, and π-π interactions with the AMPA receptor amino acids [33, 50]. Furthermore, the carbonyl functional group and/or heteroatoms such as oxygen, as well as aromatic or heterocyclic rings, may generate hydrogen bonds and hence produce hydrophobic interactions [50].
The presence of halogen atoms such as Cl or Br was beneficial for AMPA receptor inhibitory activities [33, 51]. It was clear that compounds containing Cl atom are better than compounds containing Br or I atoms [51], and this is due to the stronger electron-withdrawing effects of Cl atom than the other atoms (H, Br, or I), which can improve the π-π binding interactions, as seen in BDZ-15, which has two Cl atoms, which make the phenyl ring more electron deficient than the other compounds.
Previously, our research focused on the impact of core heterocyclic structures on AMPA receptors, such as benzodiazepine or curcumin moieties [33, 52]. According to a literature review, almost no previous study has evaluated molecules, including carboxylic acid derivatives, on the molecular level of the heteromeric and homomeric AMPAR. As a consequence of these findings, our research into these sorts of chemical structures has grown more intriguing.
The carboxylic acid in BDZ-8–15 has a higher degree of reactivity than the ester group in (BDZ-1–7), which may indicate a possible ionic interaction between the carboxylic acid and several amino acid residues that line the binding pocket and are likely to contribute to non-competitive inhibitor binding, as demonstrated by our results. The amino acids that may bind the compounds can be found in the receptor’s pre-M1, M3, M4, and S2-M4 linkers, between the TMD and LBD [36]. One of the most potent inhibitors of both homomeric and heteromeric AMPAR subunits was the BDZ-15 derivative, which contained two Cl-atoms (in ortho and para positions) and was ninefold more effective than the other compounds. Higher effectiveness against AMPAR is thus linked to increased electron-withdrawal ability. The reduced activity of other BDZ derivatives may be due to the size constraint, despite their great electron-withdrawal capabilities (bromine has a bigger atomic radius than chlorine).
Conclusions
To better understand the biophysical gating features of AMPA receptors and the efficacy of potential excitotoxic therapies, 15 BDZ compounds were used in experiments. Allosteric modulators like BDZ-8, BDZ-9, and BDZ-15, which function as negative allosteric modulators (NAMs), significantly impact AMPAR deactivation and desensitization rates. It was found that amplitude inhibition was not dependent on glutamate levels to support the non-competitive nature of the BDZ compounds. Moreover, GluA2 was the most affected subunit by all the tested BDZs. In disorders associated with glutamate hyperactivity, NAMs of AMPA receptors are a great therapeutic promise. For the future design of NAMs, this study gives a further molecular understanding of how NAMs interact with the AMPA receptor.
Data Availability
All data generated or analyzed during this study are available upon request.
References
Annas D, Cheon SY, Yusuf M, Bae SJ, Ha KT, Park KH (2020) Synthesis and initial screening of lactate dehydrogenase inhibitor activity of 1,3-benzodioxole derivatives. Sci Rep 10(1):1–9. https://doi.org/10.1038/s41598-020-77056-4
Hawash M, Eid AM, Jaradat N, Abualhasan M, Amer J, Zaid AN, Draghmeh S, Daraghmeh D, Daraghmeh H et al (2020) Synthesis and biological evaluation of benzodioxole derivatives as potential anticancer and antioxidant agents. Heterocycl Commun 157–167
Lai YY, Lien HM, Kuo PT, Huang CL, Kao JY, Lin H, Yang DY (2011) Study of the anti-proliferative activity of 5-substituted 4,7-dimethoxy-1,3-benzodioxole derivatives of sy-1 from Antrodia camphorata on human COLO 205 colon cancer cells. Evid-Based Complement Alternat Med. https://doi.org/10.1093/ecam/nep230
Leite ACL, Peixoto Da Silva K, De Souza IA, Magali De Araújo J, Brondani DJ (2004) Synthesis, antitumour and antimicrobial activities of new peptidyl derivatives containing the 1,3-benzodioxole system. Eur J Med Chem 39(12):1059–1065. https://doi.org/10.1016/J.EJMECH.2004.09.007
Li S, Wang C, Li W, Koike K, Nikaido T, Wang MW (2007) Antidepressant-like effects of piperine and its derivative, antiepilepsirine. J Asian Nat Prod Res 9(5):421–430. https://doi.org/10.1080/10286020500384302
Sudjarwo SA (2005) The potency of piperine as antiinflammatory and analgesic. Measurement 41(3):190–194
Kumar S (2013) A review on anticonvulsant activity of 1, 3-benzodioxole ring system based compounds Sagar Kumar S.D. College of Pharmacy and Vocational Studies, Muzaffarnagar, Uttar Pradesh, India. 4(9), 3296–3303. https://doi.org/10.13040/IJPSR.0975-8232.4(9).3296-03
Rollas SX, Küçükgüzel, SG (2007) Biological activities of hydrazone derivatives. 1910–1939
Hanada T (2020) Ionotropic glutamate receptors in epilepsy: a review focusing on AMPA and NMDA receptors
Bredt DS, Nicoll RA (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40:361–379
Riedel G, Platt B, Micheau J (2003) Glutamate receptor function in learning and memory. 140
Chang PKY, Verbich D, Mckinney RA (2012) AMPA receptors as drug targets in neurological disease - advantages, caveats, and future outlook. Eur J Neurosci 35(12):1908–1916
Qu W, Yuan B, Liu J, Liu Q, Zhang X, Cui R, Yang W, Li B (2021) Emerging role of AMPA receptor subunit GluA1 in synaptic plasticity: implications for Alzheimer's disease. August 2020, 1–13. https://doi.org/10.1111/cpr.12959
Yamada KA (1998) Modulating excitatory synaptic neurotransmission: potential treatment for neurological disease ? 80, 67–80
Greger IH, Ziff EB, Penn AC (2007) Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci 30(8):407–416. https://doi.org/10.1016/J.TINS.2007.06.005
Lambolez B (1997) Subunit composition, kinetic, and permeation properties of AMPA receptors in single neocortical nonpyramidal cells. J Neurosci 17(17):6685–6696. https://doi.org/10.1523/jneurosci.17-17-06685.1997
Armstrong N, Gouaux E (2000) Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron 28(1):165–181. https://doi.org/10.1016/S0896-6273(00)00094-5
Sobolevsky AI, Rosconi MP, Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462(7274):745–756
Trusseli L, Zhang S, Ramant IM (1993) Desensitization of AMPA receptors upon multiquantal neurotransmitter release. 10, 1185–1196
Twomey EC, Maria V (2017) Channel opening and gating mechanism in AMPA-subtype glutamate receptors. Nat Publ Group 549(7670):60–65. https://doi.org/10.1038/nature23479
Nicoll RA (2014) Auxiliary subunits assist AMPA-type. 1253(2006). https://doi.org/10.1126/science.1123339
Greger IH, Watson JF, Cull-Candy SG (2017) Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron 94(4):713–730. https://doi.org/10.1016/j.neuron.2017.04.009
Singer JH, Diamond JS (2006) Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. 443(October), 705–708https://doi.org/10.1038/nature05123
Menuz K, Brien JLO, Karmizadegan S, Bredt DS, Nicoll RA (2008) TARP redundancy is critical for maintaining AMPA receptor function. 28(35), 8740–8746.https://doi.org/10.1523/JNEUROSCI.1319-08.2008
Robert A, Howe JR (2003) How AMPA receptor desensitization depends on receptor occupancy. J Neurosci 23(3):847–858
Lett N, Seeburg PH, Ruppersberg JP (1994) A molecular determinant for submillisecond desensitization in glutamate receptors 266(November):10–13
Chen S, Zhao Y, Wang Y, Shekhar M, Tajkhorshid E, Gouaux E, Chen S, Zhao Y, Wang Y, Shekhar M, Tajkhorshid E, Gouaux E (2017) Activation and desensitization mechanism of AMPA article activation and desensitization mechanism of AMPA receptor-TARP complex by Cryo-EM. Cell 170(6):1234-1237.e14. https://doi.org/10.1016/j.cell.2017.07.045
Bei Y (2017) 乳鼠心肌提取 HHS public access. Physiol Behav 176(3):139–148. https://doi.org/10.1126/science.1256508.Structure
Catarzi D, Colotta V, Varano F (2006) Competitive AMP receptorantagonists 27(2):239–278. https://doi.org/10.1002/med.20084
Russo E, Gitto R, Citraro R, Chimirri A, Sarro G De (2012) New AMPA antagonists in epilepsy. 11–15
Plosker GL (2012) Perampanel: as adjunctive therapy in patients with partial-onset seizures. CNS Drugs 26(12):1085–1096. https://doi.org/10.1007/s40263-012-0021-2
Rogawski MA, Hanada T (2013) Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurologica Scandinavica 127(SUPPL.197), 19–24
Qneibi M, Jaradat N, Hawash M, Olgac A, Emwas N (2020) Ortho versus meta chlorophenyl-2,3-benzodiazepine analogues: synthesis, molecular modeling, and biological activity as AMPAR antagonists. ACS Omega 5(7):3588–3595
Jaradat N, Hawash M, Abualhasan M (2020) Synthesis and biological evaluation of benzodioxol derivatives as cyclooxygenase inhibitors. Lett Drug Des Discovery 17
Holley SM, Ahmed AH, Srinivasan J, Murthy SE, Weiland GA, Oswald RE, Nowak LM (2012) The loss of an electrostatic contact unique to AMPA receptor ligand binding domain 2 slows channel activation. Biochemistry 51(19):4015–4027. https://doi.org/10.1021/bi3001837
Narangoda C, Sakipov SN, Kurnikova MG (2019) AMPA receptor noncompetitive inhibitors occupy a promiscuous binding site. ACS Chem Neurosci 10(11):4511–4521
Parasuraman J, Roy S, Chakrabotry J B, Choudhury I, Mahato K, Rakshit S, Mandal L, Ganguly D, Paul K, Pal C (2016) ( 12 ) United States Patent ( 10 ) Patent No:2(12)
Isaac JTR, Ashby MC, Mcbain CJ (2007) Review the role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. 859–871. https://doi.org/10.1016/j.neuron.2007.06.001
Henley JM, Wilkinson KA (2016) Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci 17(6):337–350. https://doi.org/10.1038/nrn.2016.37
Zhao Y, Chen S, Swensen AC, Qian W-J, Gouaux E (2019) Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science 364(6438):355–362
Jones V, Westbrook GL, Westbrook GL (1991) The impact of receptor desensitization fast synaptic transmission. 96–101
Cotton JLS, Partin KM (2000) The contributions of GluR2 to allosteric modulation of AMPA receptors. Neuropharmacology 39(1):21–31. https://doi.org/10.1016/S0028-3908(99)00105-7
Bigge CF, Nikam SS (1997) Review central & peripheral nervous systems AMPA receptor agonists , antagonists and modulators: their potential for clinical utility. 1099–1114
Bonaventura, Di C, Labate A, Maschio M, Meletti S (2017) perspectives ce pt t. In Expert Opinion on Pharmacotherapy (Vol. 0, Issue 0). Taylor & Francis. https://doi.org/10.1080/14656566.2017.1392509
Lazzaro, J. T, Paternain, AV Lerma J, Chnard BL, Ewing FE, Huang J (2002) Functional characterization of CP-465, 022, a selective, non-competitive AMPA receptor antagonist. 42, 143–153
Sólyom S, Tarnawa I (2002) Non-competitive AMPA Antagonists of 2 , 3-Benzodiazepine Type. 913–939.
Zwart R, Sher E, **, X., **, X., Sims, JR, Chappell AS, Gleason, SD, Hahn PJ, Gardinier K, Gernert DL, Hobbs J, Smith JL, Valli SN, Witkin JM, Lilly E, Indiana JRS (2014) Perampanel, an antagonist of a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, for the treatment of epilepsy: studies in human epileptic brain and nonepileptic brain and in rodent models. October, 124–133
Horva K, Horva EJ, So  (2000) Anxiolytic 2, 3-benzodiazepines, their speci ® c binding to the basal ganglia. Prog Neurobiol60
Zappalà M, Pellicanò A, Micale N, Menniti FS, Ferreri G, De Sarro G, Grasso S, De Micheli C (2006) New 7,8-ethylenedioxy-2,3-benzodiazepines as non-competitive AMPA receptor antagonists. Bioorg Med Chem Lett 16(1):167–170. https://doi.org/10.1016/j.bmcl.2005.09.029
El-Helby AGA, Ayyad RRA, El-Adl K, Elwan A (2017) Quinoxalin-2(1H)-one derived AMPA-receptor antagonists: design, synthesis, molecular docking and anticonvulsant activity. Med Chem Res 26(11):2967–2984
Jaradat N, Hawash M, Qneibi M, Shtayeh T, Sobuh S, Arar M, Bdir S (2022) The effect of novel negative allosteric 2, 3-benzodiazepine on glutamate AMPA receptor and their cytotoxicity. J Mol Struct 1261:132936
Qneibi M, Hamed O, Jaradat N, Hawash M, Al-kerm R, Al-kerm R, Sobuh S, Tarazi S (2021) Bioorganic chemistry the AMPA receptor biophysical gating properties and binding site: focus on novel curcumin-based diazepines as non-competitive antagonists. Bioorg Chem 116:105406
Hawash M, Jaradat N, Hameedi S, Mousa A (2020) Design, synthesis and biological evaluation of novel benzodioxole derivatives as COX inhibitors and cytotoxic agents. BMC Chemistry 1–9
Stern-bach Y, Russo S, Neuman M, Rosenmund C (1998) A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. 21, 907–918
Acknowledgements
The authors would like to thank An-Najah National University for funding this study [grant number (ANNU-2021-Sc004)], the Dean of Scientific Research.
Funding
This work was supported by a grant from the An-Najah National University (Grant number: ANNU-2021-Sc004).
Author information
Authors and Affiliations
Contributions
Mohammad Qneibi: conceptualization, methodology, software, formal analysis, validation, investigation, resources, data curation, writing–original draft, writing–review and editing, visualization, supervision, project administration, and funding acquisition. Mohammed Hawash: validation, investigation, writing–review and editing. Nidal Jaradat: validation, writing–review and editing. Sosana Bdir: writing– review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors have given their consent for publication.
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qneibi, M., Hawash, M., Jaradat, N. et al. Affecting AMPA Receptor Biophysical Gating Properties with Negative Allosteric Modulators. Mol Neurobiol 59, 5264–5275 (2022). https://doi.org/10.1007/s12035-022-02913-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02913-4