Abstract
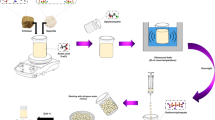
Pb(II)-imprinted pectin-carboxymethyl chitosan-BADGE or Pb(II)-CPB was synthesized by mixed pectin (Pec) and carboxymethyl chitosan (CC) and then crosslinked with a crosslinking agent bisphenol A diglycidyl ether (BADGE) to form a stable adsorbent and resistant to acidic media. As a first step, the synthesis was performed by reacting the –OH group among Pec and CC with BADGE to form CPB. The Pb(II) ion was then imprinted with the Pec–CC gel and formed Pb(II)-CPB. Furthermore, the release of Pb(II) ions from the adsorbent was performed using the chelating agent, \(\hbox {Na}_{{2}}\)EDTA. The kinetic and thermodynamic equilibrium of the batch sorption of Pb(II) onto Pb(II)-CPB were investigated. The results of this study showed that the adsorption process of the Pb(II)-CPB adsorbent could be well described by the pseudo-second order model. The thermodynamic biosorbent in adsorption of the Pb(II) ion follows Freundlich isotherm. The adsorption energy of the adsorbent was 15.59 kJ \(\hbox {mol}^{-1}\). This proves that the mechanism process of the adsorbent to Pb(II) ions occurs by physical adsorption. The Pb(II)-CPB adsorbent shows a significantly higher capacity compared to Pec and chitosan. Adsorption capacity of Pb(II)-CPB was \(664.44 \times 10^{-3}\). The Pb(II)-CPB adsorbent has higher selectivity on \(\hbox {Pb}^{2+}\) ions compared to Pec and CC with the adsorption selectivity order Pb(II)/Zn(II)\( \, < \, \)Pb(II)/Cu(II).




Similar content being viewed by others
References
Fu F and Wang Q 2011 J. Environ. Manage. 92 407
Gottipati R and Mishra S 2010 Chem. Eng. J. 160 99
Sölener M, Tunali S, Özcan A S, Özcan A and Gedikbey T 2008 Desalination 223 308
Renard C M G C, Crepeau M J and Thibault J F 1995 Carbohydr. Res. 275 155
Mata Y N, Blázquez M L, Ballester A F and González Muñoz J A 2009 Chem. Eng. J. 150 289
Fares M M, Tahboub Y R, Khatatbeh S T and Abul-Haija Y M 2011 J. Polym. Environ. 19 431
Schiewer S and Iqbal M 2010 J. Hazard. Mater. 177 899
Kupchik L A, Kartel N T, Bogdanov E S, Bogdanov O V and Kupchik M P 2006 J. Macromol. Chem. Polym. Mater. 79 457
Oshita K, Oshima M, Gao Y, Lee K H and Motomizu S 2002 J. Anal. Sci. 18 1121
Hastuti B, Siswanta D, Mudasir and Triyono 2015 Indo. J. Chem. 15 248
Hastuti B, Siswanta D, Mudasir and Triyono 2017 Oriental. J. Chem. 33 148
Buhani, Suharso and Aprilia L 2012 Indo. J. Chem. 12 94
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hastuti, B., Siswanta, D., Mudasir et al. Kinetic and thermodynamic biosorption of Pb(II) by using a carboxymethyl chitosan–pectin–BADGE–Pb(II)-imprinted ion polymer adsorbent. Bull Mater Sci 42, 143 (2019). https://doi.org/10.1007/s12034-019-1831-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-019-1831-3