Abstract
Covid-19 pandemic has struck worldwide by end of 2019 and the use of various vaccine platforms was one of the main strategies to end this. To meet the needs for vaccine technology equality among many countries, we developed adenovirus-based Covid-19 vaccine candidate in Indonesia. SARS-CoV-2 Spike gene (S) was constructed into pAdEasy vector. The recombinant serotype 5 Adenovirus (AdV_S) genome was transfected into AD293 cells to produce recombinant adenovirus. Characterization using PCR confirmed the presence of spike gene. Transgene expression analysis showed the expression of S protein in AdV_S infected AD293 and A549 cells. Optimization of viral production showed the highest titer was obtained at MOI of 0.1 and 1 at 4 days. The in vivo study was performed by injecting Balb/c mice with 3.5 × 107 ifu of purified adenovirus. The result showed that S1-specific IgG was increased up to 56 days after single-dose administration of AdV_S. Interestingly, significant increase of S1 glycoprotein-specific IFN-γ ELISpot was observed in AdV_S treated Balb/c mice. In conclusion, the AdV_S vaccine candidate was successfully produced at laboratory scale, immunogenic, and did not cause severe inflammation in Balb/c mice. This study serves as initial step towards manufacturing of adenovirus-based vaccine in Indonesia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the 4th largest population in the world, Indonesia has about 270 million population according to the data in 2020 [1]. To cover vaccination against various infectious diseases, Indonesia should be able to produce enough vaccine doses on their own. In the late 2019, coronavirus pandemic known as Covid-19 has struck the world until now. One of the approaches to handle this pandemic is vaccination. To reach herd immunity against the pandemic, Indonesia needs enough doses to cover for 80% of about 270 million adult population. Until now, several Covid-19 vaccines have been approved for emergency use by the National Regulatory Agency. However, the dependency of the needed vaccines is still high since those vaccines were mainly produced abroad.
One of the platforms that was developed worldwide for Covid-19 was adenovirus-based vaccine. Several adenovirus-based Covid-19 vaccines had obtained their emergency use authorization. Vaxzevria was developed by Oxford-AstraZeneca using chimpanzee adenovirus (ChAdOx-1) and initially showed 70.4% efficacy given in two doses [2]. Single-dose of Ad26.COV2.S produced by Janssen Pharmaceuticals showed initial 66.9% efficacy [3], meanwhile single-dose of Convidecia (Ad5-nCoV) produced by Cansino Biologics showed 57.5% efficacy [4]. Combination of two types of human adenovirus, i.e. hAd26 and hAd5, was employed in Sputnik V and it showed 91.6% efficacy during the predominantly circulating wildtype SARS-CoV-2 [5].
The use of adenoviral vector as a platform for vaccine development has been explored for many infectious diseases due to strong induction of immune response. Earlier study explored the use of adenoviral vectors for the development of vaccines against Hepatitis B virus, Ebola virus, Rotavirus, Dengue, SARS-CoV and MERS-CoV [6]. Studies performed in animal models using adenovirus carrying spike gene of SARS-CoV demonstrated reduced pathogen infection in vaccinated mice and induced cellular immune response when given via intranasal route. Furthermore, an adenovirus-based vaccine candidate against MERS-CoV also induced neutralizing antibody and T cell response in animal study [7,8,9]. The use of adenoviral vector also overcome the needs for adjuvant, as in the use of protein subunit-based vaccine, or special formulation technology, as in the mRNA-based vaccine.
This study describes the first development of replication-defective adenovirus-based vaccine candidate against Covid-19 in Indonesia. The gene encoding for the SARS-CoV-2 spike protein was first codon-optimized and then sequentially subcloned into the Adeasy-1 plasmid. The recombinant adenovirus genome was then transfected to AD293 cells to produce the adenovirus carrying spike gene (AdV_S). The AdV_S production was optimized at various MOI and time of harvest. Further analyses on induced antibody titer, cellular immune response, preliminary toxicity studies in Balb/c mice were also investigated in this research.
Materials and Methods
Cells
AD293 cells (Agilent, RRID: CVCL_9804) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Lonza) supplemented with 10% fetal bovine serum (FBS, Gibco), 4mM L-glutamine, 4 mM sodium pyruvate (Lonza) and 100 U/ml penicillin, 100 µg/ml streptomycin and 0.25 µg/ml amphotericin B (Lonza) at 37 °C and 5% CO2. Human lung epithelial cells (A549, kind gift from Prof. Yoshiharu Matsuura, Osaka University, Japan, RRID: CVCL_0023) were grown in DMEM (Sigma-Aldrich) supplemented with 10% fetal bovine serum, 4 mM L-glutamine and 100 U/ml penicillin, 100 µg/ml streptomycin and 0.25 µg/ml amphotericin B at 37 °C and 5% CO2. Escherichia coli BJ5183 cells (Agilent) and E. coli DH5⍺ cells were cultivated in Luria–Bertani medium (1% tryptone, 0.5% yeast extract, 1% NaCl) containing suitable antibiotics (100 µg/ml ampicillin for E. coli BJ5183; 50 µg/ml kanamycin for E. coli DH5⍺-pShuttle-CMV and E. coli DH5⍺-pAdeasy_S).
Design of Spike Gene
The sequence of the SARS-CoV-2 spike gene was designed based on the genome sequence of hCoV-19/Wuhan/HB-WH4-206/2020 (GISAID EPI_ISL_454956). The original spike signal sequence was replaced with the signal sequence of tissue plasminogen activator (tPa). Kozak sequence was added, and spike gene was optimized according to the human codon usage using bioinformatic tools with targeted CAI > 0.7.
Construction of Recombinant Adenoviral Genome
The recombinant adenoviral genome was constructed using AdEasy vector system (Agilent). Initially, the spike gene was subcloned to pShuttle-CMV (RRID: Addgene_16403) using HindIII and SalI (ThermoFisher). Both fragments were ligated using T4 DNA ligase (Promega) overnight. The recombinant pShuttle_S was transformed into E. coli DH5⍺. The confirmed pShuttle_S plasmid was linearized with PmeI (ThermoFisher) and transformed into E. coli BJ5183 competent cells carrying AdEasy-1 plasmid (RRID: Addgene_16399) to generate recombinant adenoviral genome containing spike gene (pAdEasy_S). The confirmed pAdEasy_S was transformed into E. coli DH5⍺ for long term storage. The purified pAdEasy_S was characterized by PacI (ThermoFisher) digestion and PCR of the spike gene. Primer pair used to detect spike gene was 5′-CCATGAAACGGGGCCTGTGTTG-3′ and 5′-TTAGGTATAATGCAGCTTCACCCC-3′. PCR was performed using KOD-Plus-Neo DNA polymerase enzyme (Toyobo) and reactions were preliminary denaturation at 94 °C for 2 min followed by 25 cycles 98 °C for 10 s, 65 °C for 30 s and 68 °C for 4 min followed by 5 min final extension.
Production of Adenovirus
AD293 were plated at 3.5 × 105 cells per well in 6-well plate and transfection was performed by adding 2.5 µg PacI-linearized pAdEasy_S plasmid with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Cells were harvested and resuspended at 10 days post-transfection in sterile Dulbecco’s Phosphate Buffered Saline (DPBS, Lonza). The cells were lysed with four rounds of freeze–thaw cycles. Cell debris was removed by centrifugation at 5,000 rpm for 5 min at 6 °C. Collected supernatant was designated as the primary stock of Adenovirus containing spike (AdV_S).
Half of the initial AdV_S stock was used for the first-round (R1) virus propagation by infecting cultured AD293 cells for 9 days incubation. The second (R2) and third (R3) round of infection was each incubated for 4 days. The AdV_S from R3 was expanded to optimize round 4 (R4) amplification at indicated MOI and incubation times. The optimum condition was used for R4 virus production in several T75 flasks. The supernatant from R4 was then purified using AdEasy purification kit (Agilent). For all experiments, the spike gene confirmation was performed through PCR and viral titers were determined using AdEasy viral titer kit (Agilent), according to the manufacturer’s instruction.
Detection of Transgene Expression
AD293 and A549 cells were grown at 6 × 105 cells in its culture medium. Cultured cells were infected at MOI of 5 for AD293 cells and MOI of 500 for A549 cells. After 48 h of infection, cells were washed with DPBS and then lysed in 1 × protein sample buffer. The protein extract was collected for Western blot analysis. Protein samples were separated by SDS-PAGE and then transferred to PVDF membrane (Amersham) followed by blocking in 3% BSA/TBST. The membrane was blotted with either mouse anti-spike monoclonal antibody (1:2500; Sigma-Aldrich, RRID: AB_2893440) or mouse anti-beta actin (1:500; Sigma-Aldrich, RRID: AB_476692), followed by secondary antibody blotting using HRP-conjugated goat anti-mouse IgG (1:1000; Invitrogen, RRID: AB_92472) in 3% BSA/TBST. Membrane was developed using WesternSure® Premium substrate (Li-Cor) and then the signal was detected using Li-Cor CDigit instrument.
Animal Experiments
Approval of the animal use in this work was obtained from the research ethics committee of Padjadjaran University, Bandung, Indonesia with an ethical approval number: 641/UN6.KEP/EC/2021. 6-weeks-old male and female Balb/c mice at 25–30 g were randomly divided into three groups of five mice each. Mice were vaccinated with a single administration intramuscularly with 3.5 × 107 ifu of AdV_S or AdV_lacZ and 100 µl buffer. Mice were punctured at the retro-orbital sinus for blood collection before vaccination and every 2 weeks until week 8 after vaccination. The collected blood was then coagulated for 2 h at room temperature and centrifuged at 10,000 rpm for 10 min and sera were collected and stored at − 80 °C. On week 8, mice were sacrificed, and the organs were collected for histopathological examination. The splenocytes were harvested by pushing the spleen through a 70 µm cell strainer, followed by incubation with RBC lysis buffer. The collected splenocytes were frozen and stored in liquid nitrogen for ELISpot assay.
ELISA
ELISA indirect systems were used to perform SARS-CoV-2 S-specific IgG and IgM assays in mice. 96-well high binding ELISA plate (NEST) were coated with 1 µg/ml Spike (S1) SARS-CoV-2 (GenScript) in 50 mM carbonate-bicarbonate pH 9.6, overnight at 4 °C. Plates were then washed 3 times with PBS containing 0.1% Tween-20 (PBS-T), then blocked with 1% BSA (Merck) in PBS-T for 1 h at room temperature. Plates were washed twice with PBS, then mice serum at 1:50 dilution was added and incubated for 2 h at room temperature. After five times washes with PBS-T, secondary antibodies goat anti-mouse IgG HRP-conjugated (Merck) and goat anti-mouse IgM HRP-conjugated (Novusbio) at 1:10.000 were added at 100 µl/well for 1 h. The plate was washed five times with PBS-T to remove excess secondary antibody, then incubated with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Sigma-Aldrich) for 5 min. Finally, 1 M H2SO4 was used to terminate the reactions, and the absorbance was measured spectrophotometrically at 450 nm by a microplate reader (Biorad).
ELISpot Assay
Following the manufacturer's protocols, splenocytes from vaccinated mice were tested for cytokine production using an ELISpot kit (RnDSystems). We examined immune responses from the splenocytes by observing the antigen-specific IFN-γ and IL-4 secreted cells. Cryopreserved splenocytes were grown overnight in RPMI 1640 (Sigma-Aldrich) medium supplemented with 10% FBS (Sigma-Aldrich), 1% Antibiotic–Antimycotic (ThermoFisher) and 50 µM 2-mercaptoethanol (Sigma-Aldrich) at 37 °C in 5% CO2 incubator. On the following day, 100 µl of 2.5 × 105 splenocytes were seeded in duplicate to a 96-well PVDF-backed microplate and stimulated with Spike (S1) SARS-CoV-2 (GenScript) 2 µg/ml; without spike (S1) as a negative control; and 2 µg/ml Concanavalin-A (Sigma-Aldrich) as a positive control. Splenocytes were then incubated in 5% CO2 incubator at 37 °C for 24 h. After removing the cells and washing the plate 4 times, 100 µl biotinylated anti-IFN-γ or anti-IL-4 was added to the plate and incubated at 4 °C overnight. The next day, plates were washed, then incubated with 100 µl of alkaline phosphatase-conjugated streptavidin at RT for 2 h. After rewashing the plates, 100 µl of BCIP/NBT substrates were added, and incubated at RT for 1 h. The substrate solution was decanted from the plate, rinsed with deionized water, then inverted to remove excess water, and dried for 90 min at room temperature. Develo** spots are counted using a dissecting microscope (Carl Zeiss). Statistical analysis was performed using two-way anova with Bonferroni posttest.
Preliminary Safety Study
The preliminary safety study of the injected vaccine candidate in mice was evaluated through several analyses. Local reactogenicity was observed at 1 h, 2 h, and 24 h post-injection on the site of injection with the draize scale on the appearance of edema and erythema. Body temperature was recorded 1 h, 2 h, and 24 h post-injection, while body weight was recorded every day until week 8 post injection. The organs, i.e. the kidney, heart, and liver, were collected at week 8 and further histopathological analysis was performed. After hematoxylin–eosin staining, the histopathological safety examination on the kidney, heart and liver was observed under a light microscope (Carl Zeiss, Germany) and then scored by 0 (normal), 1 (mild), 2 (moderate) and 3 (severe).
Results
To construct the adenoviral genome containing spike gene from SARS-CoV-2, the spike gene was codon optimized according to human codon preference. Codon optimization was performed with CAI index of 0.82 and 57%GC. This construct was generated synthetically with tPA leader sequence upstream of the spike gene. The synthesized construct was then subcloned into pShuttle-CMV. The recombinant pShuttle-CMV containing spike gene (pShuttle_S) was characterized using agarose gel electrophoresis. The result showed that migration distance of pShuttle_S was shorter than pShuttle (Fig. 1A). Restriction analysis of pShuttle_S using HindIII and SalI showed two bands with size of around 3800 bp, corresponding to the spike gene, and 7400 bp, corresponding to pShuttle-CMV (Fig. 1B). The confirmed pShuttle_S was then cut with PmeI and transformed into E. coli BJ5183 carrying the Adeasy-1 plasmid. The isolated recombinant Adeasy-1 plasmid was then characterized using restriction analysis and PCR. Adeasy-1 plasmid containing the spike gene (pAdeasy_S) was cut using PacI. The result showed two bands and the small band size was around 4500 bp (Fig. 1C). This band was known to occur when the recombination event happened at the ori sequence. The presence of spike gene was confirmed using PCR and the result showed one band with size of around 3800 bp (Fig. 1D). The recombinant pAdeasy_S was then used for AD293 cells transfection.
Characterization of recombinant shuttle plasmid and adenoviral genome. A, B pShuttle_S was characterized using 1% agarose gel electrophoresis in comparison to pShuttle (A) and cut with HindIII and SalI (B). C, D pAdeasy_S was characterized by restriction analysis using PacI (C) and characterized by PCR using primer specific for spike gene (D)
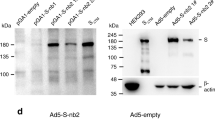
Production of recombinant adenovirus carrying spike gene (AdV_S) was carried out by transfection of pAdeasy_S into AD293 cells using Lipofectamine 2000. After 10 days incubation, virus was harvested, and the supernatant was used for sequential virus amplification from R1 to R4. At every round, virus harvest was characterized using PCR targeting the spike gene. The result showed that a PCR product band at the size of around 3800 bp was present at every round of virus amplification (Fig. 2A). The adenovirus produced from R3 was then used to infect AD293 and A549 cells for 48 h for spike protein expression analysis. It was found that AD293 cells infected with AdV_S at MOI of 5 expressed spike protein (Fig. 2B). In parallel, infection of A549 cells was performed at MOI of 500 and the presence of spike protein was also detected in these cells (Fig. 2B). Taken together, these results demonstrated that the spike protein was expressed in cells that supported the replication of adenovirus (AD293 cells) and in cells that did not support adenovirus replication (A549 cells).
Characterization of recombinant adenovirus. A AdV_S produced from round 1, 2 and 3 amplifications were analyzed by PCR using primer specific for spike gene. B Transgene expression of AD293 and A549 cells infected with AdV_lacZ and AdV_S were analyzed by Western blot using antibody specific for spike and beta-actin. The depicted results were representatives from three independent experiments
The produced AdV_S was then used for optimization of R4 virus production, prior to the immunogenicity study. To optimize the production of AdV_S, various MOI were used to infect AD293 cells for 4 days of incubation. The titer of harvested virus was measured using Adeasy Viral Titer Kit. It was found that MOI of 0.1, 1, 2, and 5 produced almost the equal amount of produced adenovirus (Fig. 3A). The next optimization was carried out using MOI 0.1 and 1 for 2–8 days incubation, with virus harvest performed every 2 days. The result showed that the virus titer peak was obtained at 4 days incubation, at 3.7 × 108 ifu/ml. The viral titer at 4 days harvest was similar between MOI of 0.1 and 1, but the produced virus was more consistent at the MOI of 1 (Fig. 3B). Therefore, production of AdV_S was carried out at MOI of 1 for 4 days using several flasks to prepare for the immunogenicity study.
Optimization of recombinant adenovirus production. A AdV_S production was analyzed upon infection at MOI of 0.1, 1, 2, and 5 after 4 days of infection. B AdV_S production was measured upon infection at MOI of 0.1 and 1 for 2–8 days. Adenoviral titer was measured using Adeasy Viral Titer Kit. Data plotted was mean ± SEM from three independent experiments
To analyze whether the produced AdV_S was able to induce immune response, the AdV_S was purified using Adeasy Viral Purification Kit. Three groups of Balb/c mice were given buffer, AdV_lacZ, and AdV_S, respectively. The blood samples were taken prior to vaccination and every 2 weeks until 8 weeks post vaccination. The antibody titer was then measured by ELISA using S1. The result showed that the IgG titer against S1 was increased in both male and female mice starting from week 2 until the end of experiment, in comparison to both control groups, buffer control and AdV_lacZ control. The antibody titer in male mice was slightly higher as compared to the antibody titer in female mice (Fig. 4A). Immunity induced by AdV_S against the SARS-CoV-2 S1 glycoprotein was measured by ex vivo IL-4 and IFN-γ ELISpot. AdV_S vaccine candidate induced significant SARS-CoV-2 S1 glycoprotein-specific IFNγ-ELISpot at day 56 post vaccination, compared to both controls (AdV_lacZ and buffer). Statistical analysis performed using two-way anova with Bonferroni posttest showed that in the male mice, AdV_S induced higher IFNγ when compared to AdV_lacZ control and buffer control groups, with p-value < 0.05. Additionally, in the female mice, AdV_S induced higher IFNγ when compared to AdV_lacZ control and buffer control groups, with p-value < 0.001 (Fig. 4B). However, the SARS-CoV-2 S1 glycoprotein-specific IL-4-ELISpot result was slightly increased in mice treated with AdV_S, as compared to both controls (Fig. 4B).
Immunogenicity and cellular immune responses of AdV_S in mice. A IgG titer measured before vaccination and every 2 weeks until week 8 after vaccination using S1-specific ELISA. Data shown were mean ± SEM from 5 animals per group. B IL-4 and IFN-γ ELISpot assays performed using ex vivo splenocytes obtained at week 8 after vaccination. Data shown were mean ± SEM from 5 animals per group. *p < 0.05, ***p < 0.001, two-way anova with Bonferroni posttest
Preliminary safety study was also performed for the AdV_S vaccine candidate, by measuring mice body temperature, weight, and analysis of the organs. During this study, the body temperature of mice ranged from 35–36 °C in all groups and there was no significant difference between the temperature of mice treated with AdV_S with the control groups, AdV_lacZ and buffer (Table S1). In terms of body weight, average mice weight gained continuously during the study, and there was no significant change between all groups on days 1, 7, and 28 (Table S2, Fig. S1). However, the weight gain of the AdV_S and AdV_lacZ mice group tended to be greater than the buffer control group. On day 56, there was no significant change in male body weight, but there was a significant change in female body weight. Additional analysis of the organ weight showed that there was no significant difference in the weight of the kidneys and heart. There was no significant difference in the liver of male mice, whereas there was a significant difference in liver weight of female mice. The female liver weight of mice was significantly higher than that of buffer control animals (Table S3).
The histopathological score of kidneys, heart, and liver from buffer control, AdV_lacZ, and AdV_S mice groups is shown in Table 1. There was inflammation and distorted glomerulus in some parts of the kidney of all mice groups (Fig. S2). There was also some inflamed part in the heart of all groups (Fig. S3). In addition, all groups had inflammation in some parts of liver tissue and binucleated hepatocyte cells (Fig. S4). However, there was no significant difference in the histopathological abnormalities of all organs. This result showed that AdV_S did not affect severe heart, kidney, or liver inflammation.
Discussion
To ending the Covid-19 pandemic, equitable access to safe and effective vaccines is very critical in all countries in the world. Conventional existing vaccines for Covid-19 were based on inactivated virus and recombinant protein subunit. While several conventional candidates have been developed, most recent Covid-19 vaccines were based on much newer platforms, such as adenoviral-based vector vaccine and mRNA vaccine. Some advantages of adenoviral-based vaccine were induction of strong immune responses, both humoral and cellular immune responses, and some of them can be given as single dose, for example the vaccine developed by Cansino Biologics and Janssen Pharmaceuticals.
In this study, the spike gene was initially modified by replacing the signal sequence using tPa signal sequence and by performing codon optimization. The expression of spike protein from this construct was in accordance with the previous research [10], where the use of tPa leader sequence and codon optimization improved the expression of spike protein. Other study also showed that the use of tPa leader sequence was one strategy to enhance the expression of spike protein [11]. In another vaccine candidates, the use of tPA leader sequence not only improved the antigen expression, but also elicited higher specific antibody responses [12, 13]. Production optimization was performed by variations of the infection MOIs. As reported previously, even MOI of less than 1 can be used to produce adenovirus [14, 15]. In this study, it was observed that the use of MOI less than one gave almost the same adenovirus yield as compared to higher MOI. However, the optimal MOI for adenoviral production was also dependent on the cell density and cell viability when the infection was performed [16].
Although the vaccine candidate was developed using the original sequence of Spike protein from the wildtype SARS-CoV-2, the current Covid-19 vaccines used for the third and fourth booster doses were also still employed the original Spike protein sequence. Decrease of neutralizing antibody capacity and vaccine efficacy against various SARS-CoV-2 variants were observed. The Ad26.COV2.S vaccine final vaccine efficacy data showed 69.7% against wildtype and alfa variants, while the efficacy against other variants were lower [17]. The Ad26.COV2.S vaccine neutralizing antibodies capacity was also reduced against the variants of concern (VOC) [18], however breakthrough infection after vaccination with this vaccine induced high neutralizing antibody titer against Omicron and other VOCs [19]. However, this vaccine showed 55% and 72% effectiveness against hospitalization during the circulation of Omicron variant in South Africa at 13 days and 1–2 months after the second dose, respectively [20]. Similarly, the ChAdOx-1 nCoV-19 vaccine showed reduced efficacy against symptomatic infection of VOCs [21] and during circulation of delta variant, the efficacy against symptomatic infection was reduced to 44.3% [22]. Meanwhile, the use of ChAdOx-1 nCoV-19 vaccine for the third dose vaccination showed ~ 60% efficacy against symptomatic infection by Omicron and ~ 80% efficacy against hospitalization in older adults [23]. Therefore, although the use of original Spike protein sequence showed decrease in efficacy against VOCs, the vaccine could still prevent severe/critical Covid-19 infection and hospitalization.
Here we used the hAd5 as the backbone of the vaccine candidate. Previous studies have explored the use of hAd5, hAd26 and ChAdOx1 for Covid-19 vaccine. Although the use of hAd5 was thought to be hampered by pre-existing immunity, but it was also known that hAd5 has better immunogenicity than other serotypes [24, 25]. The Covid-19 vaccine using hAd5 also still showed similar efficacy at the similar dose to the vaccine using hAd26 [3, 4]. Minimizing pre-existing immunity could also be done using heterologous adenovirus serotype between prime and boost vaccine, as in the Sputnik vaccine, where hAd26 was used for prime dose and combinations of hAd26 and hAd5 were used for the booster dose [5]. Therefore, the hAd5 produced in this study could still be potentially used as single-dose prime vaccination or for heterologous booster vaccination together with other vaccine that used different serotypes.
Regarding the immune response induced in mice, the single-dose of the adenovirus Covid-19 vaccine candidate injected by intramuscular route induced the IgG titer at similar profile to the previous study [10]. In general, the induction of IgG titer response was similar towards the use of diverse adenovirus backbone, i.e. hAd5, hybrid hAd5 or ChAdOx-1 in mice or ferrets [10, 26, 27]. In terms of cellular immune response, IFN-γ was still observed at week 8 (day 56) after single dose immunization. High titer of IFN-γ was plausible related with high IgG titer in this study, because IFN-γ-produced by CD-4 + T cell, could induce B cell to switch antibody production to IgG2a [28]. In addition, there was a low number of IL-4-produced splenocytes in this experiment, which was probably related to high IFN-γ -produced splenocytes. Liew et al. (2019) shown that IFN-γ could probably suppress differentiation of Th2 cells which need IL-4 [29]. Even though IFN-γ was significantly increased in both male and female mice, there was possibility that the level of IFN-γ has already peaked at several weeks prior to week 8 (day 56) after immunization. This was previously observed that the highest level of IFN-γ was peaked at day 14 and slightly decrease at day 28 after vaccination of hAd5 Covid-19 vaccine in phase 1 clinical trial [30]. However, high level IFN-γ was still observed at day 28 after vaccination of hAd5 Covid-19 vaccine in human trial phase 1 and 2 [30, 31]. Study in BALB/c mice shown that single immunization with Chimpanzee Adenovirus based vaccine (AdC68-19S) could increase CD8 + and CD4 + T cells producing IFN-γ that also imply the induction of strong cellular immune response 1 week after immunization by IM route [27]. Study of vaccination with a single dose of ChAdOx1 nCoV-19 in clinical trial phase 1/2 demonstrated that IFN-γ production by CD4 + T cells was also observed at day 14 and slightly decrease at day 28 and 56 [32]. The higher increase of IFN-γ compared to IL-4 in ELISpot assay of splenocytes from mice treated with AdV_S was consistent with previous study where Ad5 vector induced more cellular-immune response [28, 33]. Liu et al. (2008) showed that Ad5 vector was able to induce CD4 and high CD8 T cells in Rhesus Monkeys [34]. Further study on the CD8 and CD4 specific responses were still needed to be explored for our developed adenovirus-based Covid-19 vaccine candidate.
In the preliminary safety study of the vaccine candidate, slight increase of body temperature and body weight gain was observed in most of the adenovirus treated mice. However, treatment with adenovirus did not raise the body temperature more than 2 °C, which were the limit of fever indication in mice [35]. Meanwhile, the slight increase of mice body weight was probably related to the effect of hAd5, which was used as a vector carrying the SARS-CoV2 spike gene. It seemed that the increase in body weight of the animals vaccinated with AdV_S was affected by hAd5 vector as the backbone of AdV_S. So et.al. (2005) demonstrated that hAd5 can cause an increase in body weight in mice due to the increase of mice appetite and/or satiety [36]. Moreover, Ad5 vector also leads to increase body adiposity in mice. Weight gains due to hAd5 was also found in previous study [37]. The effect of Ad5 might be the same as hAd36. hAd36 upregulated the proliferation, adipogenic commitment, and differentiation of adult adipose tissue-derived stem cells and other adipogenic progenitors, leading to an increase in the number of fat cells [38]. Moreover, hAd36 seemed to increase the lipid content of these fat cells by promotion of lipid uptake or cellular entry of glucose, which increases cellular lipids by stimulation of de novo lipogenesis. However, in this study, the hAd5 lacks E1/E3 and unable to reproduce, therefore the effect of hAd5 on weight gain might not be as high as replication-competent hAd5.
In summary, the adenovirus-based Covid-19 candidate that was produced demonstrated immunogenicity in mice, eliciting a cellular immune response as determined by IFN-γ ELISPOT assay, and did not cause severe inflammation in mice. This milestone in the development of a locally-made adenoviral vaccine candidate represents a significant step towards the creation of viral vector-based vaccines in Indonesia. However, in order to fully assess the safety and efficacy of the vaccine candidate, a challenge study and complete toxicity study in a preclinical setting are still necessary prior to its clinical study. Additionally, the vaccine candidate will be utilized as a model for the development of a bioprocess to locally manufacture adenoviral-based vaccines. By optimizing the bioprocess development and scaling it up, either upstream or downstream processes, this could potentially be advantageous for commercializing adenovirus-based vaccines. It is crucial to overcome the challenges in bioprocess development for adenovirus-based vaccines because the same process could be applied to various adenovirus-based vaccine candidates. Therefore, ensuring equitable access to safe and effective vaccines in all countries, in preparation for possible future pandemics, is imperative.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Statistik, B. P. (2021). 2020 Population Census. Berita Resmi Statistik No. 7/01/Th. XXIV. Retrieved April 26, 2022, from https://www.bps.go.id/pressrelease/2021/01/21/1854/hasil-sensus-penduduk-2020.html
Voysey, M., Clemens, S. A. C., Madhi, S. A., Weckx, L. Y., Folegatti, P. M., Aley, P. K., Angus, B., Baillie, V. L., Barnabas, S. L., Bhorat, Q. E., Bibi, S., Briner, C., Cicconi, P., Collins, A. M., Colin-Jones, R., Cutland, C. L., Darton, T. C., Dheda, K., & Zuidewind, P. (2021). Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet, 397(10269), 99–111. https://doi.org/10.1016/S0140-6736(20)32661-1
Sadoff, J., Gray, G., Vandebosch, A., Cárdenas, V., Shukarev, G., Grinsztejn, B., Goepfert, P. A., Truyers, C., Fennema, H., Spiessens, B., Offergeld, K., Scheper, G., Taylor, K. L., Robb, M. L., Treanor, J., Barouch, D. H., Stoddard, J., Ryser, M. F., Marovich, M. A., ENSEMBLE Study Group. (2021). Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. The New England journal of medicine. https://doi.org/10.1056/NEJMoa2101544
Halperin, S. A., Ye, L., MacKinnon-Cameron, D., Smith, B., Cahn, P. E., Ruiz-Palacios, G. M., Ikram, A., Lanas, F., Lourdes Guerrero, M., Muñoz Navarro, S. R., Sued, O., Lioznov, D. A., Dzutseva, V., Parveen, G., Zhu, F., Leppan, L., Langley, J. M., Barreto, L., Gou, J., & Zubkova, T. (2022). Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-cont. The Lancet, 399(10321), 237–248. https://doi.org/10.1016/S0140-6736(21)02753-7
Logunov, D. Y., Dolzhikova, I. V., Shcheblyakov, D. V., Tukhvatulin, A. I., Zubkova, O. V., Dzharullaeva, A. S., Kovyrshina, A. V., Lubenets, N. L., Grousova, D. M., Erokhova, A. S., Botikov, A. G., Izhaeva, F. M., Popova, O., Ozharovskaya, T. A., Esmagambetov, I. B., Favorskaya, I. A., Zrelkin, D. I., Voronina, D. V., & Gintsburg, A. L. (2021). Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet, 397(10275), 671–681. https://doi.org/10.1016/S0140-6736(21)00234-8
Tatsis, N., & Ertl, H. C. J. (2004). Adenoviruses as vaccine vectors. Molecular Therapy, 10(4), 616–629. https://doi.org/10.1016/j.ymthe.2004.07.013
Padron-Regalado, E. (2020). Vaccines for SARS-CoV-2: Lessons from other coronavirus strains. Infectious Diseases and Therapy, 9(2), 255–274. https://doi.org/10.1007/s40121-020-00300-x
Guo, X., Deng, Y., Chen, H., Lan, J., Wang, W., Zou, X., Hung, T., Lu, Z., & Tan, W. (2015). Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology, 145(4), 476–484. https://doi.org/10.1111/imm.12462
Kim, E., Okada, K., Kenniston, T., Raj, V. S., AlHajri, M. M., Farag, E. A. B. A., AlHajri, F., Osterhaus, A. D., Haagmans, B. L., & Gambotto, A. (2014). Immunogenicity of an adenoviral-based Middle East Respiratory Syndrome coronavirus vaccine in BALB/c mice. Vaccine, 32(45), 5975–5982. https://doi.org/10.1016/j.vaccine.2014.08.058
Wu, S., Zhong, G., Zhang, J., Shuai, L., Zhang, Z., Wen, Z., Wang, B., Zhao, Z., Song, X., Chen, Y., Liu, R., Fu, L., Zhang, J., Guo, Q., Wang, C., Yang, Y., Fang, T., Lv, P., Wang, J., & Chen, W. (2020). A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nature Communications. https://doi.org/10.1038/s41467-020-17972-1
Mercado, N. B., Zahn, R., Wegmann, F., Loos, C., Chandrashekar, A., Yu, J., Liu, J., Liu, J., McMahan, K., McMahan, K., He, X., Martinez, D. R., Rutten, L., Bos, R., van Manen, D., Vellinga, J., Custers, J., Langedijk, J. P., & Barouch, D. H. (2020). Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature, 586(7830), 583–588. https://doi.org/10.1038/s41586-020-2607-z
Luo, M., Tao, P., Li, J., Zhou, S., Guo, D., & Pan, Z. (2008). Immunization with plasmid DNA encoding influenza A virus nucleoprotein fused to a tissue plasminogen activator signal sequence elicits strong immune responses and protection against H5N1 challenge in mice. Journal of Virological Methods, 154(1–2), 121–127. https://doi.org/10.1016/j.jviromet.2008.08.011
Wallace, A., West, K., Rothman, A., Ennis, F. A., Lu, S., & Wang, S. (2013). Post-translational intracellular trafficking determines the type of immune response elicited by DNA vaccines expressing Gag antigen of Human Immunodeficiency Virus Type 1 (HIV-1). Human Vaccines and Immunotherapeutics, 9(10), 2095–2102. https://doi.org/10.4161/hv.26009
Yamada, K., Morishita, N., Katsuda, T., Kubo, S., Gotoh, A., & Yamaji, H. (2009). Adenovirus vector production using low-multiplicity infection of 293 cells. Cytotechnology, 59(3), 153–160. https://doi.org/10.1007/s10616-009-9208-x
da Chen, K., Wu, X. X., Yu, D. S., Ou, H. L., Li, Y. H., Zhou, Y. Q., & Li, L. J. (2018). Process optimization for the rapid production of adenoviral vectors for clinical trials in a disposable bioreactor system. Applied Microbiology and Biotechnology, 102(15), 6469–6477. https://doi.org/10.1007/s00253-018-9091-5
Joe, C. C. D., Jiang, J., Linke, T., Li, Y., Fedosyuk, S., Gupta, G., Berg, A., Segireddy, R. R., Mainwaring, D., Mainwaring, D., Cashen, P., Rees, B., Chopra, N., Nestola, P., Humphreys, J., Davies, S., Smith, N., Bruce, S., & Douglas, A. D. (2022). Manufacturing a chimpanzee adenovirus-vectored SARS-CoV-2 vaccine to meet global needs. Biotechnology and bioengineering, 119(1), 48–58. https://doi.org/10.1002/bit.27945
Sadoff, J., Gray, G., Vandebosch, A., Cárdenas, V., Shukarev, G., Grinsztejn, B., Goepfert, P. A., Truyers, C., Van Dromme, I., Spiessens, B., Vingerhoets, J., Custers, J., Scheper, G., Robb, M. L., Treanor, J., Ryser, M. F., Barouch, D. H., Swann, E., & Douoguih, M. (2022). Final analysis of efficacy and safety of single-dose Ad26.COV2.S. New England Journal of Medicine, 386(9), 847–860. https://doi.org/10.1056/nejmoa2117608
Zhang, G. F., Meng, W., Chen, L., Ding, L., Feng, J., Perez, J., Ali, A., Sun, S., Liu, Z., Huang, Y., Guo, H., & Gao, S. J. (2022). Neutralizing antibodies to SARS-CoV-2 variants of concern including Delta and Omicron in subjects receiving mRNA-1273, BNT162b2, and Ad26.COV2.S vaccines. Journal of Medical Virology, 94(12), 5678–5690. https://doi.org/10.1002/jmv.28032
Kitchin, D., Richardson, S. I., van der Mescht, M. A., Motlou, T., Mzindle, N., Moyo-Gwete, T., Ayres, F., Manamela, N. P., Spencer, H., Lambson, B., Oosthuysen, B., Kaldine, H., du Pisanie, M., Mennen, M., Skelem, S., Williams, N., Ntusi, N. A. B., Burgers, W. A., & Moore, P. L. (2022). Ad26.COV2.S breakthrough infections induce high titers of neutralizing antibodies against Omicron and other SARS-CoV-2 variants of concern. Cell Reports Medicine. https://doi.org/10.1016/j.xcrm.2022.100535
Gray, G., Collie, S., Goga, A., Garrett, N., Champion, J., Seocharan, I., Bamford, L., Moultrie, H., & Bekker, L.-G. (2022). Effectiveness of Ad26.COV2.S and BNT162b2 Vaccines against Omicron Variant in South Africa. New England Journal of Medicine, 386(23), 2243–2245. https://doi.org/10.1056/nejmc2202061
Nasreen, S., Chung, H., He, S., Brown, K. A., Gubbay, J. B., Buchan, S. A., Fell, D. B., Austin, P. C., Schwartz, K. L., Sundaram, M. E., Sundaram, M. E., Chen, B., Tadrous, M., Wilson, K., Wilson, S. E., & Kwong, J. C. (2022). Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nature Microbiology, 7(3), 379–385. https://doi.org/10.1038/s41564-021-01053-0
Andrews, N., Tessier, E., Stowe, J., Gower, C., Kirsebom, F., Simmons, R., Gallagher, E., Thelwall, S., Groves, N., Dabrera, G., Myers, R., Campbell, C. N. J., Amirthalingam, G., Edmunds, M., Edmunds, M., Zambon, M., Brown, K., Hopkins, S., & Lopez Bernal, J. (2022). Duration of protection against mild and severe disease by Covid-19 Vaccines. New England Journal of Medicine, 386(4), 340–350. https://doi.org/10.1056/nejmoa2115481
Kirsebom, F. C. M., Andrews, N., Sachdeva, R., Stowe, J., Ramsay, M., & Lopez Bernal, J. (2022). Effectiveness of ChAdOx1-S COVID-19 booster vaccination against the Omicron and Delta variants in England. Nature Communications, 13(1), 7688. https://doi.org/10.1038/s41467-022-35168-7
Abbink, P., Lemckert, A. A. C., Ewald, B. A., Lynch, D. M., Denholtz, M., Smits, S., Holterman, L., Damen, I., Vogels, R., Thorner, A. R., O’Brien, K. L., Carville, A., Mansfield, K. G., Goudsmit, J., Havenga, M. J., & Barouch, D. H. (2007). Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. Journal of Virology, 81(9), 4654–4663. https://doi.org/10.1128/jvi.02696-06
Dicks, M. D. J., Spencer, A. J., Coughlan, L., Bauza, K., Gilbert, S. C., Hill, A. V. S., & Cottingham, M. G. (2015). Differential immunogenicity between HAdV-5 and chimpanzee adenovirus vector ChAdOx1 is independent of fiber and penton RGD loop sequences in mice. Scientific Reports, 5(July), 1–15. https://doi.org/10.1038/srep16756
Lambe, T., Spencer, A. J., Thomas, K. M., Gooch, K. E., Thomas, S., White, A. D., Humphries, H. E., Wright, D., Belij-Rammerstorfer, S., Thakur, N., Conceicao, C., Watson, R., Alden, L., Allen, L., Aram, M., Bewley, K. R., Brunt, E., Brown, P., & Gilbert, S. C. (2021). ChAdOx1 nCoV-19 protection against SARS-CoV-2 in rhesus macaque and ferret challenge models. Communications Biology, 4(1), 1–12. https://doi.org/10.1038/s42003-021-02443-0
Li, M., Guo, J., Lu, S., Zhou, R., Shi, H., Shi, X., Cheng, L., Liang, Q., Liu, H., Wang, P., Wang, N., Wang, Y., Fu, L., **ng, M., Wang, R., Ju, B., Liu, L., Lau, S. Y., & Zhang, L. (2021). Single-Dose immunization with a chimpanzee adenovirus-based vaccine induces sustained and protective immunity against SARS-CoV-2 infection. Frontiers in Immunology, 12(June), 1–14. https://doi.org/10.3389/fimmu.2021.697074
Yan, L., Zhao, Z., Xue, X., Zheng, W., Xu, T., Liu, L., Tian, L., Wang, X., He, H., & Zheng, X. (2020). A bivalent human adenovirus Type 5 vaccine expressing the rabies virus glycoprotein and canine distemper virus hemagglutinin protein confers protective immunity in mice and foxes. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2020.01070
Liew, M. F., Chan, A., & Lim, H. F. (2019). House-dust mite Immunotherapy in asthma: Uncertainties and therapeutic strategies. Current Treatment Options in Allergy, 6(4), 363–376. https://doi.org/10.1007/s40521-019-00236-9
Zhu, F. C., Li, Y. H., Guan, X. H., Hou, L. H., Wang, W. J., Li, J. X., Wu, S. P., Wang, B. S., Wang, Z., Wang, L., Jia, S. Y., Jiang, H. D., Wang, L., Jiang, T., Hu, Y., Gou, J. B., Xu, S. B., Xu, J. J., Wang, X. W., & Chen, W. (2020). Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet, 6736(20), 1–10. https://doi.org/10.1016/S0140-6736(20)31208-3
Zhu, F. C., Guan, X. H., Li, Y. H., Huang, J. Y., Jiang, T., Hou, L. H., Li, J. X., Li, J. X., Wang, L., Wang, W. J., Wu, S. P., Wang, Z., Wu, X. H., Xu, J. J., Zhang, Z., Jia, S. Y., Wang, B. S., Hu, Y., & Chen, W. (2020). Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet, 396(10249), 479–488. https://doi.org/10.1016/S0140-6736(20)31605-6
Ewer, K. J., Barrett, J. R., Belij-Rammerstorfer, S., Sharpe, H., Makinson, R., Morter, R., Flaxmanm, A., Wright, D., Bellamy, D., Bittaye, M., Dold, C., Provine, N. M., Aboagye, J., Silk, S. E., Alderson, J., Aley, P. K., Angus, B., Berrie, E., & Stafford, E. (2021). T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nature Medicine, 27(2), 270–278. https://doi.org/10.1038/s41591-020-01194-5
Kanagavelu, S., Termini, J. M., Gupta, S., Raffa, F. N., Fuller, K. A., Rivas, Y., Philip, S., Kornbluth, R. S., & Stone, G. W. (2014). HIV-1 adenoviral vector vaccines expressing multi-trimeric BAFF and 4–1BBL enhance t cell mediated anti-viral immunity. PLoS ONE. https://doi.org/10.1371/journal.pone.0090100
Liu, J., Ewald, B. A., Lynch, D. M., Denholtz, M., Abbink, P., Lemckert, A. A. C., Carville, A., Mansfield, K. G., Havenga, M. J., Goudsmit, J., & Barouch, D. H. (2008). Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. Journal of Virology, 82(10), 4844–4852. https://doi.org/10.1128/jvi.02616-07
Lee, C. T., Zhong, L., Mace, T. A., & Repasky, E. A. (2012). Elevation in body temperature to fever range enhances and prolongs subsequent responsiveness of macrophages to endotoxin challenge. PLoS ONE. https://doi.org/10.1371/journal.pone.0030077
So, P.-W., Herlihy, A. H., & Bell, J. D. (2005). Adiposity induced by adenovirus 5 inoculation. International Journal of Obesity, 29(6), 603–606. https://doi.org/10.1038/sj.ijo.0802917
Montes-Galindo, D. A., Espiritu-Mojarro, A. C., Melnikov, V., Moy-López, N. A., Soriano-Hernandez, A. D., Galvan-Salazar, H. R., Guzman-Muñiz, J., Martinez-Fierro, M. L., Martinez-Fierro, M. L., Paz-Michel, B., Zaizar-Fregoso, S. A., Sanchez-Ramirez, C. A., Ramirez-Flores, M., Delgado-Enciso, I., & Delgado-Enciso, I. (2019). Adenovirus 5 produces obesity and adverse metabolic, morphological, and functional changes in the long term in animals fed a balanced diet or a high-fat diet: A study on hamsters. Archives of Virology, 164(3), 775–786. https://doi.org/10.1007/s00705-018-04132-6
Dhurandhar, N. V. (2011). A framework for identification of infections that contribute to human obesity. The Lancet Infectious Diseases, 11(12), 963–969. https://doi.org/10.1016/S1473-3099(11)70274-2
Acknowledgements
This research was funded by Yayasan Solidarity Forever, Indonesia. The authors also thank Prof. Yoshiharu Matsuura and Dr. David Chen for kindly giving the A549 cells and to Orca Bioteknologi Nusantara for the donation of some laboratory consumables.
Funding
Yayasan Solidarity Forever
Author information
Authors and Affiliations
Contributions
Conceptualization and Study design: AA, EAG-R, MIT, and DN; Methodology: AA, EAG-R, MIT, TH, IAS, NAH; Formal analysis and investigation: TH, IAS, NAH; Writing—original draft preparation: AA, TH, MIT; Writing—review and editing: AA, EAG-R, MIT, and DN; Funding acquisition: AA, EAG-R, MIT, DSR and DN; Supervision: AA, EAG-R, MIT, and DN.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
Approval of the animal use was obtained from the research ethics committee of Padjadjaran University, Bandung, Indonesia with an ethical approval number: 641/UN6.KEP/EC/2021.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Artarini, A., Hadianti, T., Giri-Rachman, E.A. et al. Development of Adenovirus-Based Covid-19 Vaccine Candidate in Indonesia. Mol Biotechnol 66, 222–232 (2024). https://doi.org/10.1007/s12033-023-00749-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00749-4