Abstract
Rheumatic diseases are complex autoimmune diseases which include among others rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), and psoriatic arthritis (PsA). These diseases are characterized by prolonged and increased secretion of inflammatory factors, eventually leading to inflammation. This is often accompanied by persistent pain and stiffness in the joint and finally bone destruction and osteoporosis. These diseases can occur at any age, regardless of gender or origin. Autoimmune arthritis is admittedly associated with long-term treatment, and discontinuation of medication is associated with unavoidable relapse. Therefore, it is important to detect the disease at an early stage and apply appropriate preventative measures. During inflammation, pro-inflammatory factors such as interleukins (IL)-6, -17, -21, -22, and -23 are secreted, while anti-inflammatory factors including IL-10 are downregulated. Research conducted over the past several years has focused on inhibiting inflammatory pathways and activating anti-inflammatory factors to improve the quality of life of people with rheumatic diseases. The aim of this paper is to review current knowledge on stimulatory and inhibitory pathways involving the signal transducer and activator of transcription 3 (STAT3). STAT3 has been shown to be one of the crucial factors involved in inflammation and is directly linked with other pro-inflammatory factors and thus is a target of current research on rheumatoid diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoimmune diseases are a group of multifactorial disorders with complex and unclear etiology. Autoimmune rheumatic diseases are characterized by similar pathophysiological mechanisms and risk of systemic complications such as cardiovascular diseases, osteoporosis, and early death [1, 2]. Clinical syndromes are initiated by an abnormal immune response that incorrectly reads self antigens as foreign, attacking the body and leading to inflammation.
The immune system produces autoantibodies directed against self antigens. This process, called autoimmunity, is the basis for autoimmune diseases [3]. To date, two ways of autoimmunity development are known. Firstly, physiological autoimmunization may be characterized by a lack of clinical symptoms, i.e., the production of autoantibodies, and at this point the body’s homeostasis is maintained by elimination of degraded self and non-self antigens. The second process is associated with the occurrence of pathological autoimmune reactions that causes tissue damage. The occurrence of genetic predisposition and/or environmental factors (e.g., smoking, UV light, heavy metals) with increased activation of autoreactive T and B lymphocytes leads to tissue damage and loss of their functions [4, 3].
The occurrence of inflammation results from a complex relationship between genetics, hormonal, epigenetics, and environmental factors [1]. The association between the severity of rheumatoid arthritis and the expression of the major histocompatibility complex (MHC), known in humans as human leukocyte antigens (HLA), has been described. The HLA alleles that predispose to the disease, in addition to HLA alleles protecting against autoimmune diseases, are present [5].
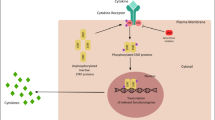
Hypothalamic-pituitary-adrenal (HPA) immune axis dysfunction could participate in autoimmune arthritis pathogenesis. During increased inflammation, there is not enough cortisol produced by the adrenal glands axis [6]. The pathomechanism of environmental factors, smoking [7], obesity [8], malnutrition with vitamin D deficiency, and environmental toxins such as heavy metals, infections, and drugs, on the occurrence of RA remains unclear [9, 8, 10]. Epigenetic disorders, related to changes in gene expression, may result from the environmental impact on humans. The epigenome is sensitive to environmental factors. Lowering of DNA methylation in T cells and peripheral blood mononuclear cells (PBMCs) or modification of histone proteins, described in detail by Araki and Mimura [11], may influence modifications of genes responsible for induction and maintenance of inflammatory processes in joints [12,13,98], and is produced by Th17 [56] and Th22 cells. The production of IL-22 is promoted by IL-17, IL-23, IL-1β, aryl-hydrocarbon receptors (AhR), and Notch signaling [99]. The IL-22R is a complex of IL-22R1 and IL-10R2 containing an intracellular, transmembrane, and extracellular signaling region. The cytokine binds to IL-22R1 leading to the formation of a complex. The IL-22/IL-22R1 complex changes conformation and allows association of IL-10R2, initiating the activation of tyrosine kinases 2 (TYK2) and JAK1, followed by phosphorylation of STAT3 on the tyrosine and serine residues, STAT1 and STAT5. It is also an activator of the MAPK pathways (ERK1/2, MEK1/2, c-Jun N-terminal kinase (JNK), and p38 kinase), which ultimately leads to antibacterial and inflammatory processes as well as tissue repair, depending on the environment in the organism in which the cytokine is expressed [100]. There is data on the duality of IL-22 activity in the literature which show the pro-inflammatory role of IL-22. On the other hand, there is also data on the protective role of IL-22 in controlling lung epithelial damage [101] or intestinal inflammation.
IL-22 levels are elevated in patients with rheumatoid arthritis and there is a relationship between its level and radiographic progression and disease activity [102, 103]. Researchers have shown that sulforaphane has an effect on increasing the levels of ROS in whole blood lymphocytes in RA patients. At the same time, reduced production of pro-inflammatory cytokines, i.e., IL-17A, IL-17F, and IL-22, has been demonstrated [69]. Studies conducted by Liu et al. [104] have shown that norepinephrine (NE), a neurotransmitter released from sympathetic nerves, inhibits the differentiation and function of Th17 cells by activating the β2-adrenergic receptor (β2-AR) on CD4+ T lymphocytes. The studies were conducted on CIA mice. This suggests that NE may have anti-inflammatory effects in CIA. A study was also carried out on rats suffering from pristane induced arthritis (PIA). Increased cytokines produced by Th17 (IL-17A, IL-21, IL-22), mainly IL-22 in the ratio of Th1 cytokines (TNF-α, INF-γ) and Th2 (IL-4, IL-10, TGFβ), have been shown in organs of immune rats (inguinal lymph nodes, spleen). Expression of IL-22 in synovium and serum correlated with the severity of PIA. The concentration of IL-21 was higher in PIA rats but was not significant compared to IL-22. In this study, IL-21 only supported Th17 differentiation and enhanced their response [99]. The same group showed that in PIA rats, the level of IL-22 expression was different in different phases of PIA. IL-22 levels increased in the spleen during the initial and chronic phase and in the synovium in the chronic phase. In contrast, no elevated levels of IL-22 were found in the acute phase of inflammation. In the acute phase, an increase in IL-17F and IFN-γ expression was observed in the synovial membrane of PIA rats [105]. Zhong et al. [106] reports that elevated IL-22+ T cells and IL-22 can promote RA development. Targeting Th22 and Th17 positively influences RA therapy. Patients were divided into two groups after basic treatments using conventional DMARDs, MTX, and leflunomide. The decreased plasma level of IL-22 correlated with a decreased level of Th22 and positively correlated with the reduction of DAS after treatment. The involvement of these cells in the pathogenesis of RA was previously demonstrated [107]. It has also been shown that treatment with MTX or ETA improves sleep efficiency because RA can cause sleep problems with a noted involvement of the HPA axis [108].
Studies were carried out on FLS from RA patients treated with sodium nitroprusside, inducing apoptosis in the presence or absence of IL-22. IL-22 has been shown to increase the viability of RA-FLS and prevent apoptosis. STAT3 inhibitors reversed this process. Studies have shown that IL-22 protects against sodium nitroprusside-induced apoptosis in RA-FLS by activating STAT3 and the Bcl-2 gene [155].
References
Alam J, Jantan I, Bukhari SNA. Rheumatoid arthritis: recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed Pharmacother. 2017;92:615–33. https://doi.org/10.1016/j.biopha.2017.05.055.
Barut K, Adrovic A, Sahin S, Tarcin G, Tahaoglu G, Koker O, et al. Prognosis, complications and treatment response in systemic juvenile idiopathic arthritis patients: a single-center experience. Int J Rheum Dis. 2019;22(9):1661–9. https://doi.org/10.1111/1756-185x.13649.
Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–95. https://doi.org/10.1111/joim.12395.
Kochi Y, Suzuki A, Yamamoto K. Genetic basis of rheumatoid arthritis: a current review. Biochem Biophys Res Commun. 2014;452(2):254–62. https://doi.org/10.1016/j.bbrc.2014.07.085.
van Drongelen V, Holoshitz J. Human leukocyte antigen-disease associations in rheumatoid arthritis. Rheum Dis Clin North Am. 2017;43(3):363–76. https://doi.org/10.1016/j.rdc.2017.04.003.
Sattler J, Tu J, Stoner S, Li J, Buttgereit F, Seibel MJ, et al. Role of 11beta-HSD type 1 in abnormal HPA axis activity during immune-mediated arthritis. Endocr Connect. 2018;7(2):385–94. https://doi.org/10.1530/ec-17-0361.
Weng CH, Gupta S, Geraghty P, Foronjy R, Pernis AB. Cigarette smoke inhibits ROCK2 activation in T cells and modulates IL-22 production. Mol Immunol. 2016;71:115–22. https://doi.org/10.1016/j.molimm.2016.01.013.
Shoda H, Nagafuchi Y, Tsuchida Y, Sakurai K, Sumitomo S, Fujio K, et al. Increased serum concentrations of IL-1 beta, IL-21 and Th17 cells in overweight patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):111. https://doi.org/10.1186/s13075-017-1308-y.
Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):54–60. https://doi.org/10.1136/ard.2008.102962.
Yang TH, Yuan TH, Hwang YH, Lian IB, Meng M, Su CC. Increased inflammation in rheumatoid arthritis patients living where farm soils contain high levels of copper. J Formos Med Assoc. 2016;115(11):991–6. https://doi.org/10.1016/j.jfma.2015.10.001.
Araki Y, Mimura T. The histone modification code in the pathogenesis of autoimmune diseases. Mediators Inflamm. 2017;2017:2608605. https://doi.org/10.1155/2017/2608605.
Chen CJ, Hou JW, Chiang BL. The difference in immune response and IL-12p35 methylation between newborns and adults. J Biomed Sci. 2014;21:76. https://doi.org/10.1186/s12929-014-0076-0.
Meyer B, Chavez RA, Munro JE, Chiaroni-Clarke RC, Akikusa JD, Allen RC, et al. DNA methylation at IL32 in juvenile idiopathic arthritis. Scientific reports. 2015;5:11063. https://doi.org/10.1038/srep11063.
**a M, Liu J, Wu X, Liu S, Li G, Han C, et al. Histone methyltransferase Ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme A20. Immunity. 2013;39(3):470–81. https://doi.org/10.1016/j.immuni.2013.08.016.
Wu C, Goodall JC, Busch R, Gaston JS. Relationship of CD146 expression to secretion of interleukin (IL)-17, IL-22 and interferon-gamma by CD4(+) T cells in patients with inflammatory arthritis. Clin Exp Immunol. 2015;179(3):378–91. https://doi.org/10.1111/cei.12434.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. https://doi.org/10.1146/annurev.immunol.021908.132710.
Castro G, Liu X, Ngo K, De Leon-Tabaldo A, Zhao S, Luna-Roman R, et al. RORgammat and RORalpha signature genes in human Th17 cells. PloS one. 2017;12(8):e0181868. https://doi.org/10.1371/journal.pone.0181868.
Weijers L, Baerwald C, Mennini FS, Rodriguez-Heredia JM, Bergman MJ, Choquette D, et al. Cost per response for abatacept versus adalimumab in rheumatoid arthritis by ACPA subgroups in Germany, Italy, Spain. US and Canada. Rheumatol Int. 2017;37(7):1111–23. https://doi.org/10.1007/s00296-017-3739-9.
Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nature Reviews Rheumatology. 2019;15(1):9–17. https://doi.org/10.1038/s41584-018-0109-2.
Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein science : a publication of the Protein Society. 2018;27(12):1984–2009. https://doi.org/10.1002/pro.3519.
Ouyang W, O’Garra AJI. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. 2019;50(4):871-91.
Carbone G, Wilson A, Diehl SA, Bunn J, Cooper SM, Rincon M. Interleukin-6 receptor blockade selectively reduces IL-21 production by CD4 T cells and IgG4 autoantibodies in rheumatoid arthritis. Int J Biol Sci. 2013;9(3):279–88. https://doi.org/10.7150/ijbs.5996.
Reeh H, Rudolph N, Billing U, Christen H, Streif S, Bullinger E, et al. Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor alpha to gp130 expression: fusing experimental insights and dynamic modelling. Cell Commun Signal. 2019;17(1):46. https://doi.org/10.1186/s12964-019-0356-0.
Schinnerling K, Aguillon JC, Catalan D, Soto L. The role of interleukin-6 signalling and its therapeutic blockage in skewing the T cell balance in rheumatoid arthritis. Clin Exp Immunol. 2017;189(1):12–20. https://doi.org/10.1111/cei.12966.
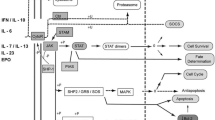
Eulenfeld R, Dittrich A, Khouri C, Müller PJ, Mütze B, Wolf A, et al. Interleukin-6 signalling: more than Jaks and STATs. European journal of cell biology. 2012;91(6-7):486–95. https://doi.org/10.1016/j.ejcb.2011.09.010.
Jung JY, Kim MY, Suh CH, Kim HA. Off-label use of tocilizumab to treat non-juvenile idiopathic arthritis in pediatric rheumatic patients: a literature review. Pediatric rheumatology online journal. 2018;16(1):79. https://doi.org/10.1186/s12969-018-0296-z.
Narazaki M, Kishimoto T. The two-faced cytokine IL-6 in host defense and diseases. Int J Mol Sci. 2018;19(11). 10.3390/ijms19113528.
**ng R, Yang L, ** Y, Sun L, Li C, Li Z, et al. Interleukin-21 induces proliferation and proinflammatory cytokine profile of fibroblast-like synoviocytes of patients with rheumatoid arthritis. Scand J Immunol. 2016;83(1):64–71. https://doi.org/10.1111/sji.12396.
Mease PJ, Gladman DD, Collier DH, Ritchlin CT, Helliwell PS, Liu L, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol. 2019;71(7):1112–24. https://doi.org/10.1002/art.40851.
Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130(1):27–33. https://doi.org/10.1016/j.clim.2008.08.018.
Anderson AE, Pratt AG, Sedhom MA, Doran JP, Routledge C, Hargreaves B, et al. IL-6-driven STAT signalling in circulating CD4+ lymphocytes is a marker for early anticitrullinated peptide antibody-negative rheumatoid arthritis. Ann Rheum Dis. 2016;75(2):466–73. https://doi.org/10.1136/annrheumdis-2014-205850.
Choy E, Caporali R, Xavier R, Fautrel B, Sanmarti R, Bao M, et al. Subcutaneous tocilizumab in rheumatoid arthritis: findings from the common-framework phase 4 study programme TOZURA conducted in 22 countries. Rheumatology (Oxford). 2018;57(3):499–507. https://doi.org/10.1093/rheumatology/kex443.
Mallalieu NL, Wimalasundera S, Hsu JC, Douglass W, Wells C, Penades IC, et al. Intravenous dosing of tocilizumab in patients younger than two years of age with systemic juvenile idiopathic arthritis: results from an open-label phase 1 clinical trial. Pediatric rheumatology online journal. 2019;17(1):57. https://doi.org/10.1186/s12969-019-0364-z.
Genovese MC, Fleischmann R, Kivitz A, Lee E-B, van Hoogstraten H, Kimura T et al. Efficacy and safety of sarilumab in combination with csDMARDs or as monotherapy in subpopulations of patients with moderately to severely active rheumatoid arthritis in three phase III randomized, controlled studies. Arthritis Res Ther. 2020;22(1):139-. 10.1186/s13075-020-02194-z.
Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet (London, England). 2013;381(9877):1541-50. 10.1016/s0140-6736(13)60250-0.
Boyapati A, Schwartzman S, Msihid J, Choy E, Genovese MC, Burmester GR, et al. Association of high serum interleukin-6 levels with severe progression of rheumatoid arthritis and increased treatment response differentiating sarilumab from adalimumab or methotrexate in a post hoc analysis. Arthritis Rheumatol. 2020;72(9):1456–66. https://doi.org/10.1002/art.41299.
Saito S, Suzuki K, Yoshimoto K, Kaneko Y, Matsumoto Y, Yamaoka K, et al. A new bioassay for measuring the strength of IL-6/STAT3 signal inhibition by tocilizumab in patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):231. https://doi.org/10.1186/s13075-017-1434-6.
Tormo AJ, Letellier MC, Sharma M, Elson G, Crabé S, Gauchat JF. IL-6 activates STAT5 in T cells. Cytokine. 2012;60(2):575–82. https://doi.org/10.1016/j.cyto.2012.07.002.
Machado SH, Xavier RM. Safety of tocilizumab in the treatment of juvenile idiopathic arthritis. Expert Opin Drug Saf. 2017;16(4):493–500. https://doi.org/10.1080/14740338.2017.1303479.
Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77(17):1865–79. https://doi.org/10.1007/s40265-017-0829-7.
Aletaha D, Bingham CO, 3rd, Tanaka Y, Agarwal P, Kurrasch R, Tak PP et al. Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): a randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study. Lancet (London, England). 2017;389(10075):1206-17. 10.1016/s0140-6736(17)30401-4.
Avci AB, Feist E, Burmester GR. Targeting IL-6 or IL-6 Receptor in rheumatoid arthritis: what’s the difference? BioDrugs. 2018;32(6):531–46. https://doi.org/10.1007/s40259-018-0320-3.
Takeuchi T, Tanaka Y, Yamanaka H, Harigai M, Nakano T, Akagi K, et al. Efficacy and safety of sirukumab in Japanese patients with moderate to severe rheumatoid arthritis inadequately controlled by disease modifying anti-rheumatic drugs: subgroup analysis of a phase 3 study. Mod Rheumatol. 2018;28(6):941–9. https://doi.org/10.1080/14397595.2018.1428929.
Takeuchi T, Yamanaka H, Harigai M, Tamamura R, Kato Y, Ukyo Y, et al. Sirukumab in rheumatoid arthritis refractory to sulfasalazine or methotrexate: a randomized phase 3 safety and efficacy study in Japanese patients. Arthritis Res Ther. 2018;20(1):42. https://doi.org/10.1186/s13075-018-1536-9.
Srenathan U, Steel K, Taams LS. IL-17+ CD8+ T cells: differentiation, phenotype and role in inflammatory disease. Immunol Lett. 2016;178:20–6. https://doi.org/10.1016/j.imlet.2016.05.001.
Tabarkiewicz J, Pogoda K, Karczmarczyk A, Pozarowski P, Giannopoulos K. The role of IL-17 and Th17 lymphocytes in autoimmune diseases. Arch Immunol Ther Exp (Warsz). 2015;63(6):435–49. https://doi.org/10.1007/s00005-015-0344-z.
Goepfert A, Lehmann S, Wirth E, Rondeau JM. The human IL-17A/F heterodimer: a two-faced cytokine with unique receptor recognition properties. Scientific reports. 2017;7(1):8906. https://doi.org/10.1038/s41598-017-08360-9.
Tu JF, Pan HY, Ying XH, Lou J, Ji JS, Zou H. Mast cells comprise the major of interleukin 17-producing cells and predict a poor prognosis in hepatocellular carcinoma. Medicine (Baltimore). 2016;95(13):e3220. https://doi.org/10.1097/md.0000000000003220.
Monin L, Gaffen SL. Interleukin 17 family cytokines: signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harbor perspectives in biology. 2018;10(4). 10.1101/cshperspect.a028522.
O'Shea JJ, Gadina M, Siegel RM. 9 - Cytokines and cytokine receptors. In: Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM, editors. Clinical Immunology (Fifth Edition). London: Elsevier; 2019. p. 127-55.e1.
Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282(18):13447–55. https://doi.org/10.1074/jbc.M700499200.
Juszczak M, Glabinski A. [Th17 cells in the pathogenesis of multiple sclerosis]. Postepy Hig Med Dosw (Online). 2009;63:492-501.
Ito H, Yamada H, Shibata TN, Mitomi H, Nomoto S, Ozaki S. Dual role of interleukin-17 in pannus growth and osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther. 2011;13(1):R14. https://doi.org/10.1186/ar3238.
Kim EK, Kwon JE, Lee SY, Lee EJ, Kim DS, Moon SJ, et al. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017;8(1):e2565. https://doi.org/10.1038/cddis.2016.490.
Pickens SR, Volin MV, Mandelin AM 2nd, Kolls JK, Pope RM, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol. 2010;184(6):3233–41. https://doi.org/10.4049/jimmunol.0903271.
Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11(7):415–29. https://doi.org/10.1038/nrrheum.2015.53.
Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7(4):R784–95. https://doi.org/10.1186/ar1733.
Lee S-Y, Kwok S-K, Son H-J, Ryu J-G, Kim E-K, Oh H-J et al. IL-17-mediated Bcl-2 expression regulates survival of fibroblast-like synoviocytes in rheumatoid arthritis through STAT3 activation. 2013;15(1):1-10.
Koga T, Otomo K, Mizui M, Yoshida N, Umeda M, Ichinose K, et al. Calcium/calmodulin-dependent kinase IV facilitates the recruitment of interleukin-17-producing cells to target organs through the CCR6/CCL20 axis in Th17 cell-driven inflammatory diseases. Arthritis Rheumatol. 2016;68(8):1981–8. https://doi.org/10.1002/art.39665.
Kawashiri SY, Kawakami A, Iwamoto N, Fujikawa K, Aramaki T, Tamai M, et al. Proinflammatory cytokines synergistically enhance the production of chemokine ligand 20 (CCL20) from rheumatoid fibroblast-like synovial cells in vitro and serum CCL20 is reduced in vivo by biologic disease-modifying antirheumatic drugs. The Journal of rheumatology. 2009;36(11):2397–402. https://doi.org/10.3899/jrheum.090132.
Kageyama Y, Kobayashi H, Kato N. Infliximab treatment reduces the serum levels of interleukin-23 in patients with rheumatoid arthritis. Mod Rheumatol. 2009;19(6):657–62. https://doi.org/10.1007/s10165-009-0217-6.
Clanchy FIL, Williams RO. Ibudilast inhibits chemokine expression in rheumatoid arthritis synovial fibroblasts and exhibits immunomodulatory activity in experimental arthritis. Arthritis Rheumatol. 2019;71(5):703–11. https://doi.org/10.1002/art.40787.
Silva Rodrigues JF, Silva ESC, Franca Muniz T, de Aquino AF, Neuza da Silva Nina L, Fialho Sousa NC et al. Sulforaphane modulates joint inflammation in a murine model of complete Freund’s adjuvant-induced mono-arthritis. Molecules. 2018;23(5). 10.3390/molecules23050988.
Kong JS, Yoo SA, Kim HS, Kim HA, Yea K, Ryu SH, et al. Inhibition of synovial hyperplasia, rheumatoid T cell activation, and experimental arthritis in mice by sulforaphane, a naturally occurring isothiocyanate. Arthritis Rheum. 2010;62(1):159–70. https://doi.org/10.1002/art.25017.
Fragoulis A, Laufs J, Muller S, Soppa U, Siegl S, Reiss LK, et al. Sulforaphane has opposing effects on TNF-alpha stimulated and unstimulated synoviocytes. Arthritis Res Ther. 2012;14(5):R220. https://doi.org/10.1186/ar4059.
Choi YJ, Lee WS, Lee EG, Sung MS, Yoo WH. Sulforaphane inhibits IL-1beta-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of MMPs, COX-2, and PGE2. Inflammation. 2014;37(5):1496–503. https://doi.org/10.1007/s10753-014-9875-4.
Hung CN, Huang HP, Wang CJ, Liu KL, Lii CK. Sulforaphane inhibits TNF-alpha-induced adhesion molecule expression through the Rho A/ROCK/NF-kappaB signaling pathway. J Med Food. 2014;17(10):1095–102. https://doi.org/10.1089/jmf.2013.2901.
Liu J, Wada Y, Katsura M, Tozawa H, Erwin N, Kapron CM, et al. Rho-associated coiled-coil kinase (ROCK) in molecular regulation of angiogenesis. Theranostics. 2018;8(21):6053–69. https://doi.org/10.7150/thno.30305.
Liang J, Jahraus B, Balta E, Ziegler JD, Hubner K, Blank N, et al. Sulforaphane inhibits inflammatory responses of primary human T-cells by increasing ROS and depleting glutathione. Front Immunol. 2018;9:2584. https://doi.org/10.3389/fimmu.2018.02584.
Davidson RK, Jupp O, de Ferrars R, Kay CD, Culley KL, Norton R, et al. Sulforaphane represses matrix-degrading proteases and protects cartilage from destruction in vitro and in vivo. Arthritis Rheum. 2013;65(12):3130–40. https://doi.org/10.1002/art.38133.
Davidson R, Gardner S, Jupp O, Bullough A, Butters S, Watts L, et al. Isothiocyanates are detected in human synovial fluid following broccoli consumption and can affect the tissues of the knee joint. Scientific reports. 2017;7(1):3398. https://doi.org/10.1038/s41598-017-03629-5.
Kamel KM, Gad AM, Mansour SM, Safar MM, Fawzy HM. Novel anti-arthritic mechanisms of polydatin in complete Freund’s adjuvant-induced arthritis in rats: involvement of IL-6, STAT-3, IL-17, and NF-small ka. CyrillicB. Inflammation. 2018;41(5):1974–86. https://doi.org/10.1007/s10753-018-0841-4.
Kim SE, Lee JY, Shim KS, Lee S, Min K, Bae JH, et al. Attenuation of inflammation and cartilage degradation by sulfasalazine-containing hyaluronic acid on osteoarthritis rat model. Int J Biol Macromol. 2018;114:341–8. https://doi.org/10.1016/j.ijbiomac.2018.03.059.
Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, et al. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther. 2018;20(1):115. https://doi.org/10.1186/s13075-018-1582-3.
Oike T, Kanagawa H, Sato Y, Kobayashi T, Nakatsukasa H, Miyamoto K, et al. IL-6, IL-17 and Stat3 are required for auto-inflammatory syndrome development in mouse. Scientific reports. 2018;8(1):15783. https://doi.org/10.1038/s41598-018-34173-5.
van der Heijde D, Mease PJ, Landewé RBM, Rahman P, Tahir H, Singhal A, et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatology (Oxford). 2020;59(6):1325–34. https://doi.org/10.1093/rheumatology/kez420.
Deodhar A, Gladman DD, McInnes IB, Spindeldreher S, Martin R, Pricop L et al. Secukinumab immunogenicity over 52 weeks in patients with psoriatic arthritis and ankylosing spondylitis. 2020;47(4):539-47. 10.3899/jrheum.190116 %J The Journal of Rheumatology.
Tahir H, Deodhar A, Genovese M, Takeuchi T, Aelion J, Van den Bosch F, et al. Secukinumab in active rheumatoid arthritis after anti-TNFα therapy: a randomized, double-blind placebo-controlled phase 3 study. Rheumatology and therapy. 2017;4(2):475–88. https://doi.org/10.1007/s40744-017-0086-y.
Combe B, Rahman P, Kameda H, Cañete JD, Gallo G, Agada N et al. Safety results of ixekizumab with 1822.2 patient-years of exposure: an integrated analysis of 3 clinical trials in adult patients with psoriatic arthritis. Arthritis Res Ther. 2020;22(1):14-. 10.1186/s13075-020-2099-0.
Sekhon S, Jeon C, Nakamura M, Yan D, Afifi L, Bhutani T, et al. Clinical utility of ixekizumab in the treatment of moderate-to-severe plaque psoriasis. Psoriasis (Auckl). 2017;7:65–72. https://doi.org/10.2147/PTT.S129792.
Glatt S, Strimenopoulou F, Vajjah P, Shaw S, Ionescu L, Popa S et al. OP0108 Bimekizumab, a monoclonal antibody that inhibits both IL-17A and IL-17F, produces a profound response in both skin and joints: results of an early-phase, proof-of-concept study in psoriatic arthritis. 2016;75(Suppl 2):95-6. 10.1136/annrheumdis-2016-eular.2952 %J Annals of the Rheumatic Diseases.
Glatt S, Helmer E, Haier B, Strimenopoulou F, Price G, Vajjah P, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83(5):991–1001. https://doi.org/10.1111/bcp.13185.
Glatt S, Taylor PC, McInnes IB, Schett G, Landewe R, Baeten D, et al. Efficacy and safety of bimekizumab as add-on therapy for rheumatoid arthritis in patients with inadequate response to certolizumab pegol: a proof-of-concept study. Ann Rheum Dis. 2019;78(8):1033–40. https://doi.org/10.1136/annrheumdis-2018-214943.
Pavelka K, Chon Y, Newmark R, Lin SL, Baumgartner S, Erondu N. A study to evaluate the safety, tolerability, and efficacy of brodalumab in subjects with rheumatoid arthritis and an inadequate response to methotrexate. The Journal of rheumatology. 2015;42(6):912–9. https://doi.org/10.3899/jrheum.141271.
Jung SM, Kim Y, Kim J, Jung H, Yi H, Rim YA, et al. Sodium chloride aggravates arthritis via Th17 polarization. Yonsei Med J. 2019;60(1):88–97. https://doi.org/10.3349/ymj.2019.60.1.88.
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–22. https://doi.org/10.1038/nature11868.
**ng R, ** Y, Sun L, Yang L, Li C, Li Z, et al. Interleukin-21 induces migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Clin Exp Immunol. 2016;184(2):147–58. https://doi.org/10.1111/cei.12751.
Wang Y, Jiang X, Zhu J, Dan Y, Zhang X, Wang X, et al. IL-21/IL-21R signaling suppresses intestinal inflammation induced by DSS through regulation of Th responses in lamina propria in mice. Scientific reports. 2016;6:31881. https://doi.org/10.1038/srep31881.
Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408(6808):57–63. https://doi.org/10.1038/35040504.
Sglunda O, Mann HF, Hulejova H, Pecha O, Plestilova L, RuZickova O, et al. Decrease in serum interleukin-21 levels is associated with disease activity improvement in patients with recent-onset rheumatoid arthritis. Physiol Res. 2014;63(4):475–81.
Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13(5):379–95. https://doi.org/10.1038/nrd4296.
Li J, Shen W, Kong K, Liu Z. Interleukin-21 induces T-cell activation and proinflammatory cytokine secretion in rheumatoid arthritis. Scand J Immunol. 2006;64(5):515–22. https://doi.org/10.1111/j.1365-3083.2006.01795.x.
Vollmer TL, Liu R, Price M, Rhodes S, La Cava A, Shi FD. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J Immunol. 2005;174(5):2696–701. https://doi.org/10.4049/jimmunol.174.5.2696.
Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L, et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 2007;56(4):1152–63. https://doi.org/10.1002/art.22452.
**ng R, Sun L, Wu D, ** Y, Li C, Liu X, et al. Autoantibodies against interleukin-21 correlate with disease activity in patients with rheumatoid arthritis. Clinical rheumatology. 2018;37(1):75–80. https://doi.org/10.1007/s10067-017-3862-8.
Sakuraba K, Oyamada A, Fujimura K, Spolski R, Iwamoto Y, Leonard WJ, et al. Interleukin-21 signaling in B cells, but not in T cells, is indispensable for the development of collagen-induced arthritis in mice. Arthritis Res Ther. 2016;18:188. https://doi.org/10.1186/s13075-016-1086-y.
**ng R, Zhang Y, Li C, Sun L, Yang L, Zhao J, et al. Interleukin-21 promotes osteoclastogenesis in RAW264.7 cells through the PI3K/AKT signaling pathway independently of RANKL. Int J Mol Med. 2016;38(4):1125–34. https://doi.org/10.3892/ijmm.2016.2722.
Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC. IL-TIF/IL-22: genomic organization and map** of the human and mouse genes. Genes Immun. 2000;1(8):488–94. https://doi.org/10.1038/sj.gene.6363716.
Wang B, Zhao P, Zhou Y, Meng L, Zhu W, Jiang C, et al. Increased expression of Th17 cytokines and interleukin-22 correlates with disease activity in pristane-induced arthritis in rats. PloS one. 2017;12(11):e0188199. https://doi.org/10.1371/journal.pone.0188199.
Perusina Lanfranca M, Lin Y, Fang J, Zou W, Frankel T. Biological and pathological activities of interleukin-22. Journal of molecular medicine (Berlin, Germany). 2016;94(5):523-34. 10.1007/s00109-016-1391-6.
Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem. 2012;287(12):8816–29. https://doi.org/10.1074/jbc.M111.304758.
da Rocha LF Jr, Duarte AL, Dantas AT, Mariz HA, Pitta Ida R, Galdino SL, et al. Increased serum interleukin 22 in patients with rheumatoid arthritis and correlation with disease activity. The Journal of rheumatology. 2012;39(7):1320–5. https://doi.org/10.3899/jrheum.111027.
Leipe J, Schramm MA, Grunke M, Baeuerle M, Dechant C, Nigg AP, et al. Interleukin 22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann Rheum Dis. 2011;70(8):1453–7. https://doi.org/10.1136/ard.2011.152074.
Liu Y, Rui XX, Shi H, Qiu YH, Peng YP. Norepinephrine inhibits Th17 cells via beta2-adrenergic receptor (beta2-AR) signaling in a mouse model of rheumatoid arthritis. Med Sci Monit. 2018;24:1196-204. 10.12659/msm.906184.
Hou W, Wang B, Zhou Y, Xu K, Meng L, Zhu W, et al. IL22 expression is increased variedly in the initial phase, onset and chronic phase of a pristaneinduced arthritis rat model. Mol Med Rep. 2017;16(2):1109–16. https://doi.org/10.3892/mmr.2017.6739.
Zhong W, Zhao L, Liu T, Jiang Z. IL-22-producing CD4+T cells in the treatment response of rheumatoid arthritis to combination therapy with methotrexate and leflunomide. Scientific reports. 2017;7:41143. https://doi.org/10.1038/srep41143.
Zhao L, Jiang Z, Jiang Y, Ma N, Zhang Y, Feng L, et al. IL-22+ CD4+ T cells in patients with rheumatoid arthritis. Int J Rheum Dis. 2013;16(5):518–26. https://doi.org/10.1111/1756-185x.12099.
Straub RH, Detert J, Dziurla R, Fietze I, Loeschmann PA, Burmester GR, et al. Inflammation is an important covariate for the crosstalk of sleep and the HPA axis in rheumatoid arthritis. Neuroimmunomodulation. 2017;24(1):11–20. https://doi.org/10.1159/000475714.
Zhao M, Li Y, **ao W. Anti-apoptotic effect of interleukin-22 on fibroblast-like synoviocytes in patients with rheumatoid arthritis is mediated via the signal transducer and activator of transcription 3 signaling pathway. Int J Rheum Dis. 2017;20(2):214–24. https://doi.org/10.1111/1756-185x.12939.
Zhu J, Jia E, Zhou Y, Xu J, Feng Z, Wang H, et al. Interleukin-22 secreted by NKp44+ natural killer cells promotes proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Medicine (Baltimore). 2015;94(52):e2137. https://doi.org/10.1097/md.0000000000002137.
Miyazaki Y, Nakayamada S, Kubo S, Nakano K, Iwata S, Miyagawa I, et al. Th22 cells promote osteoclast differentiation via production of IL-22 in rheumatoid arthritis. Front Immunol. 2018;9:2901. https://doi.org/10.3389/fimmu.2018.02901.
Kim KW, Kim HR, Park JY, Park JS, Oh HJ, Woo YJ, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64(4):1015–23. https://doi.org/10.1002/art.33446.
Wen H, Liu Y, Li J, Wei D, Liu D, Zhao F. Inhibitory effect and mechanism of 1,25-dihydroxy vitamin D3 on RANKL expression in fibroblast-like synoviocytes and osteoclast-like cell formation induced by IL-22 in rheumatoid arthritis. Clin Exp Rheumatol. 2018;36(5):798–805.
Cardoso PRG, Matias KA, Dantas AT, Marques CDL, Pereira MC, Duarte A, et al. Losartan, but not enalapril and valsartan, inhibits the expression of IFN-gamma, IL-6, IL-17F and IL-22 in PBMCs from rheumatoid arthritis patients. Open Rheumatol J. 2018;12:160–70. https://doi.org/10.2174/1874312901812010160.
Perez-Vazquez F, Bäck M, Chavarria-Avila E, Gomez-Bañuelos E, Ramos-Becerra CG, Pizano-Martínez Ó, et al. Enalapril influence on arterial stiffness in rheumatoid arthritis women: a randomized clinical trial. Frontiers in medicine. 2019;6:341. https://doi.org/10.3389/fmed.2019.00341.
Bloch Y, Bouchareychas L, Merceron R, Skladanowska K, Van den Bossche L, Detry S et al. Structural activation of pro-inflammatory human cytokine IL-23 by cognate IL-23 receptor enables recruitment of the shared receptor IL-12Rbeta1. Immunity. 2018;48(1):45-58.e6. 10.1016/j.immuni.2017.12.008.
Yago T, Nanke Y, Kawamoto M, Kobashigawa T, Yamanaka H, Kotake S. IL-23 and Th17 disease in inflammatory arthritis. Journal of clinical medicine. 2017;6(9). 10.3390/jcm6090081.
Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–7. https://doi.org/10.1084/jem.20030896.
Zaky DS, El-Nahrery EM. Role of interleukin-23 as a biomarker in rheumatoid arthritis patients and its correlation with disease activity. Int Immunopharmacol. 2016;31:105–8. https://doi.org/10.1016/j.intimp.2015.12.011.
Guo W, Yu D, Wang X, Luo C, Chen Y, Lei W et al. Anti-inflammatory effects of interleukin-23 receptor cytokine-binding homology region rebalance T cell distribution in rodent collagen-induced arthritis. Oncotarget. 2016;7(22):31800-13. 10.18632/oncotarget.9309.
Ganesan R, Rasool M. Interleukin 17 regulates SHP-2 and IL-17RA/STAT-3 dependent Cyr61, IL-23 and GM-CSF expression and RANKL mediated osteoclastogenesis by fibroblast-like synoviocytes in rheumatoid arthritis. Mol Immunol. 2017;91:134–44. https://doi.org/10.1016/j.molimm.2017.09.003.
Raychaudhuri SK, Abria C, Raychaudhuri SP. Regulatory role of the JAK STAT kinase signalling system on the IL-23/IL-17 cytokine axis in psoriatic arthritis. Ann Rheum Dis. 2017;76(10):e36. https://doi.org/10.1136/annrheumdis-2016-211046.
Pfeifle R, Rothe T, Ipseiz N, Scherer HU, Culemann S, Harre U, et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol. 2017;18(1):104–13. https://doi.org/10.1038/ni.3579.
Andersen T, Hvid M, Johansen C, Stengaard-Pedersen K, Hetland ML, Horslev-Petersen K, et al. Interleukin-23 in early disease development in rheumatoid arthritis. Scand J Rheumatol. 2015;44(6):438–42. https://doi.org/10.3109/03009742.2015.1033007.
Cornelissen F, Asmawidjaja PS, Mus AM, Corneth O, Kikly K, Lubberts E. IL-23 dependent and independent stages of experimental arthritis: no clinical effect of therapeutic IL-23p19 inhibition in collagen-induced arthritis. PloS one. 2013;8(2):e57553. https://doi.org/10.1371/journal.pone.0057553.
Helliwell PS, Gladman DD, Chakravarty SD, Kafka S, Karyekar CS, You Y, et al. Effects of ustekinumab on spondylitis-associated endpoints in TNFi-naïve active psoriatic arthritis patients with physician-reported spondylitis: pooled results from two phase 3, randomised, controlled trials. RMD Open. 2020;6(1):e001149. https://doi.org/10.1136/rmdopen-2019-001149.
Krizova L, Kuchar M, Petrokova H, Osicka R, Hlavnickova M, Pelak O, et al. p19-targeted ABD-derived protein variants inhibit IL-23 binding and exert suppressive control over IL-23-stimulated expansion of primary human IL-17+ T-cells. Autoimmunity. 2017;50(2):102–13. https://doi.org/10.1080/08916934.2016.1272598.
Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (London, England). 2020;395(10230):1115-25. 10.1016/s0140-6736(20)30265-8.
Khatri A, Suleiman AA, Polepally AR, Othman AA. Exposure-response relationships for efficacy and safety of risankizumab in phase II and III trials in psoriasis patients. Clin Pharmacol Ther. 2019. https://doi.org/10.1002/cpt.1594.
Du J, Wang X, Tan G, Liang Z, Zhang Z, Yu HJCI. The association between genetic polymorphisms of interleukin 23 receptor gene and the risk of rheumatoid arthritis: an updated meta-analysis. 2020;210:108250.
Floss DM, Moll JM, Scheller J. IL-12 and IL-23-close relatives with structural homologies but distinct immunological functions. Cells. 2020;9(10). 10.3390/cells9102184.
Malemud CJJTaimd. The role of the JAK/STAT signal pathway in rheumatoid arthritis. 2018;10(5-6):117-27.
Vogel TP, Milner JD, Cooper MA. The Ying and Yang of STAT3 in human disease. J Clin Immunol. 2015;35(7):615–23. https://doi.org/10.1007/s10875-015-0187-8.
Goropevšek A, Holcar M. Avčin TJCria, immunology. The role of STAT signaling pathways in the pathogenesis of systemic lupus erythematosus. 2017;52(2):164–81.
Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125(4):591–9. https://doi.org/10.1182/blood-2014-09-602763.
Niu Q, Huang ZC, Wu XJ, ** YX, An YF, Li YM, et al. Enhanced IL-6/phosphorylated STAT3 signaling is related to the imbalance of circulating T follicular helper/T follicular regulatory cells in patients with rheumatoid arthritis. Arthritis Res Ther. 2018;20(1):200. https://doi.org/10.1186/s13075-018-1690-0.
Oike T, Sato Y, Kobayashi T, Miyamoto K, Nakamura S, Kaneko Y, et al. Stat3 as a potential therapeutic target for rheumatoid arthritis. Scientific reports. 2017;7(1):10965. https://doi.org/10.1038/s41598-017-11233-w.
Altman R, Hochberg M, Gibofsky A, Jaros M, Young C. Efficacy and safety of low-dose SoluMatrix meloxicam in the treatment of osteoarthritis pain: a 12-week, phase 3 study. Current medical research and opinion. 2015;31(12):2331–43. https://doi.org/10.1185/03007995.2015.1112772.
Wen HL, Yang G, Dong QR. Ellipticine inhibits the proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes via the STAT3 pathway. Immunopharmacol Immunotoxicol. 2017;39(4):219–24. https://doi.org/10.1080/08923973.2017.1327963.
Gao W, McCormick J, Connolly M, Balogh E, Veale DJ, Fearon U. Hypoxia and STAT3 signalling interactions regulate pro-inflammatory pathways in rheumatoid arthritis. Ann Rheum Dis. 2015;74(6):1275–83. https://doi.org/10.1136/annrheumdis-2013-204105.
Chang L, Feng X, Gao W. Proliferation of rheumatoid arthritis fibroblast-like synoviocytes is enhanced by IL-17-mediated autophagy through STAT3 activation. Connect Tissue Res. 2019;60(4):358–66. https://doi.org/10.1080/03008207.2018.1552266.
You H, Xu D, Zhao J, Li J, Wang Q, Tian X, et al. JAK inhibitors: prospects in connective tissue diseases. Clinical Reviews in Allergy & Immunology. 2020. https://doi.org/10.1007/s12016-020-08786-6.
Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet (London, England). 2019;393(10188):2344-58. 10.1016/s0140-6736(19)30546-x.
Wollenhaupt J, Lee EB, Curtis JR, Silverfield J, Terry K, Soma K et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21(1):89. 10.1186/s13075-019-1866-2.
Lee SH, Park JS, Byun JK, Jhun J, Jung K, Seo HB, et al. PTEN ameliorates autoimmune arthritis through down-regulating STAT3 activation with reciprocal balance of Th17 and Tregs. Scientific reports. 2016;6:34617. https://doi.org/10.1038/srep34617.
Deng J, Fan C, Gao X, Zeng Q, Guo R, Wei Y, et al. Signal transducer and activator of transcription 3 hyperactivation associates with follicular helper T cell differentiation and disease activity in rheumatoid arthritis. Front Immunol. 2018;9:1226. https://doi.org/10.3389/fimmu.2018.01226.
Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–63. https://doi.org/10.1074/jbc.C600321200.
Tan S, Xu J, Lai A, Cui R, Bai R, Li S, et al. Curculigoside exerts significant antiarthritic effects in vivo and in vitro via regulation of the JAK/STAT/NFkappaB signaling pathway. Mol Med Rep. 2019;19(3):2057–64. https://doi.org/10.3892/mmr.2019.9854.
Malemud CJ. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10(5-6):117–27. https://doi.org/10.1177/1759720X18776224.
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(5):521–46. https://doi.org/10.1007/s40265-017-0701-9.
Deng XM, Yan SX, Wei W. IL-21 acts as a promising therapeutic target in systemic lupus erythematosus by regulating plasma cell differentiation. Cellular & molecular immunology. 2015;12(1):31–9. https://doi.org/10.1038/cmi.2014.58.
Shabgah AG, Navashenaq JG, Shabgah OG, Mohammadi H, Sahebkar A. Interleukin-22 in human inflammatory diseases and viral infections. Autoimmunity reviews. 2017;16(12):1209–18. https://doi.org/10.1016/j.autrev.2017.10.004.
Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. The Journal of investigative dermatology. 2009;129(6):1339–50. https://doi.org/10.1038/jid.2009.59.
Cantini F, Niccoli L, Nannini C, Cassarà E, Kaloudi O, Giulio Favalli E, et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Seminars in arthritis and rheumatism. 2017;47(2):183–92. https://doi.org/10.1016/j.semarthrit.2017.03.008.
Zavvar M, Assadiasl S, Soleimanifar N, Pakdel FD, Abdolmohammadi K, Fatahi Y, et al. Gene therapy in rheumatoid arthritis: strategies to select therapeutic genes. Journal of cellular physiology. 2019;234(10):16913–24. https://doi.org/10.1002/jcp.28392.
Correll CK, Bullock DR, Cafferty RM, Vehe RK. Safety of weekly adalimumab in the treatment of juvenile idiopathic arthritis and pediatric chronic uveitis. Clinical rheumatology. 2018;37(2):549–53. https://doi.org/10.1007/s10067-017-3890-4.
Baeten D, Østergaard M, Wei JC-C, Sieper J, Järvinen P, Tam L-S, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018;77(9):1295–302. https://doi.org/10.1136/annrheumdis-2018-213328.
Burmester GR, Strand V, Rubbert-Roth A, Amital H, Raskina T, Gómez-Centeno A et al. Safety and efficacy of switching from adalimumab to sarilumab in patients with rheumatoid arthritis in the ongoing MONARCH open-label extension. RMD Open. 2019;5(2):e001017-e. 10.1136/rmdopen-2019-001017.
Smolen JS, Agarwal SK, Ilivanova E, Xu XL, Miao Y, Zhuang Y, et al. A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann Rheum Dis. 2017;76(5):831–9. https://doi.org/10.1136/annrheumdis-2016-209831.
de la Varga MR, Rodriguez-Bayona B, Anez GA, Medina Varo F, Perez Venegas JJ, Brieva JA, et al. Clinical relevance of circulating anti-ENA and anti-dsDNA secreting cells from SLE patients and their dependence on STAT-3 activation. Eur J Immunol. 2017;47(7):1211–9. https://doi.org/10.1002/eji.201646872.
Smolen JS, Genovese MC, Takeuchi T, Hyslop DL, Macias WL, Rooney T, et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. The Journal of rheumatology. 2019;46(1):7–18. https://doi.org/10.3899/jrheum.171361.
El Sayed A, Abd Hilal E-M, Abogamal A, Labeeb A, Abdel Hamid A, El Gerby A et al. Clinical efficacy and safety of leflunomide in Egyptian patients with active rheumatoid arthritis: CLEAR interim results. 2018;12(1).
Emery P, Vencovsky J, Sylwestrzak A, Leszczynski P, Porawska W, Baranauskaite A, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):51–7. https://doi.org/10.1136/annrheumdis-2015-207588.
Fox RJ, Coffey CS, Conwit R, Cudkowicz ME, Gleason T, Goodman A, et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N Engl J Med. 2018;379(9):846–55. https://doi.org/10.1056/NEJMoa1803583.
Daneshtalab N, Lewanczuk RZ, Russell AS, Jamali F. Drug-disease interactions: losartan effect is not downregulated by rheumatoid arthritis. J Clin Pharmacol. 2006;46(11):1344–55. https://doi.org/10.1177/0091270006292163.
Pahor M, Anton SD, Beavers DP, Cauley JA, Fielding RA, Kritchevsky SB, et al. Effect of losartan and fish oil on plasma IL-6 and mobility in older persons. The ENRGISE Pilot Randomized Clinical Trial. J Gerontol A Biol Sci Med Sci. 2019;74(10):1612–9. https://doi.org/10.1093/gerona/gly277.
Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe DJ, Bombardier C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis: a network meta-analysis. Cochrane Database Syst Rev. 2016(8):Cd010227. 10.1002/14651858.CD010227.pub2.
Zhu T, Moy S, Valluri U, Cao Y, Zhang W, Sawamoto T, et al. Investigation of potential drug-drug interactions between peficitinib (ASP015K) and methotrexate in patients with rheumatoid arthritis. Clinical drug investigation. 2020. https://doi.org/10.1007/s40261-020-00937-z.
Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012;64(9):2824–35. https://doi.org/10.1002/art.34498.
Acknowledgements
The authors would like to thank prof. David Aebisher for proofreading and English language correction.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Woś, I., Tabarkiewicz, J. Effect of interleukin-6, -17, -21, -22, and -23 and STAT3 on signal transduction pathways and their inhibition in autoimmune arthritis. Immunol Res 69, 26–42 (2021). https://doi.org/10.1007/s12026-021-09173-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-021-09173-9