Abstract
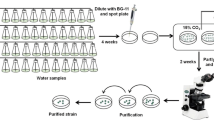
In order to explore the changes in the growth and protein contents of Spirulina and obtain a proper strain for the fixation of carbon dioxide (CO2) from flue gas, the strains isolated from the Spirulina farms and the strain 208 were cultured under different aeration conditions including no CO2, 10% CO2 and coal power plant flue gas supplements. The physiological indexes including filament length, biomass yield and chlorophyll a, soluble protein and phycocyanin contents were determined, respectively. When cultured without CO2 supplement, the strain 4–5 exhibited the highest biomass yield (1.880 g L−1) and a specific growth rate (0.367 d−1). However, the specific growth rate of all strains decreased significantly when they were cultured under 10% CO2 and unfiltered coal power plant flue gas supplements. Considerable differences were noted in the performance of the experimental microalgal strains under different contemporaneous conditions. The strain 7–8 achieved the highest biomass yield (1.603 g L−1) and relatively high phycocyanin content (7.1%) under 10% CO2 supplement. We noted that strain 4–5 had the highest specific growth rate (0.182 d−1) and biomass yield (0.43 g L−1) under coal power plant flue gas supplement. Strain 6–10 displayed the highest soluble protein content (66.02%), and strain 7–8 showed the highest phycocyanin content (9.28%) under coal power plant flue gas supplement.
Similar content being viewed by others
References
Aikawa, S., Izumi, Y., Matsuda, F., Hasunuma, T., Chang, J., and Kondo, A., 2012. Synergistic enhancement of glycogen production in Arthrospira platensis by optimization of light intensity and nitrate supply. Bioresource Technology, 108: 211–215.
Aslam, A., Thomas-Hall, S. R., Mughal, T. A., and Schenk, P. M., 2017. Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresource Technology, 233: 271–283.
Begum, H., Yusoff, F. M., Banerjee, S., Khatoon, H., and Shariff, M., 2016. Availability and utilization of pigments from microalgae. Critical Reviews in Food Science and Nutrition, 56(13): 2209–2222.
Bennett, A., and Bogorad, L., 1973. Complementary chromatic adaptation in a filamentous blue-green alga. The Journal of Cell Biology, 58: 419–35.
Braga, V. D. S., Mastrantonio, D. J. D. S., Costa, J. A. V., and Morais, M. G. D., 2018. Cultivation strategy to stimulate high carbohydrate content in Spirulina biomass. Bioresource Technology, 269: 221–226.
Braga, V. D. S., Moreira, J. B., Costa, J. A. V., and Morais, M. G. D., 2019. Potential of Chlorella fusca LEB 111 cultivated with thermoelectric fly ashes, carbon dioxide and reduced supply of nitrogen to produce macromolecules. Bioresource Technology, 277: 55–61.
Camargo, E. C., and Lombardi, A. T., 2018. Correction to: Effect of cement industry flue gas simulation on the physiology and photosynthetic performance of Chlorella sorokiniana. Journal of Applied Phycology, 30(2): 873.
Cardoso, L. G., Duarte, J. H., Andrade, B. B., Lemos, P. V. F., Costa, J. A. V., Druzian, J. I., et al., 2020. Spirulina sp. LEB 18 cultivation in outdoor pilot scale using aquaculture waste-water: High biomass, carotenoid, lipid and carbohydrate production. Aquaculture, 525: 735272.
Cheah, W. Y., Show, P. L., Chang, J., Ling, T. C., and Juan, J. C., 2015. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresource Technology, 184: 190–201.
Chen, C., Kao, P., Tan, C. H., Show, P. L., Cheah, W. Y., Lee, W., et al., 2016. Using an innovative pH-stat CO2 feeding strategy to enhance cell growth and C-phycocyanin production from Spirulina platensis. Biochemical Engineering Journal, 112: 78–85.
Chen, H., Wu, J., Wang, C., Fu, C., Shieh, C., Chen, C., et al., 2010. Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochemical Engineering Journal, 53(1): 52–56.
Cheng, J., Lu, H., He, X., Yang, W., Zhou, J., and Cen, K., 2017. Mutation of Spirulina sp. by nuclear irradiation to improve growth rate under 15% carbon dioxide in flue gas. Bioresource Technology, 238: 650–656.
Cheng, J., Guo, W., Ameer Ali, K., Ye, Q., **, G., and Qiao, Z., 2018. Promoting helix pitch and trichome length to improve biomass harvesting efficiency and carbon dioxide fixation rate by Spirulina sp. in 660 m2 raceway ponds under purified carbon dioxide from a coal chemical flue gas. Bioresource Technology, 261: 76–85.
Chiaramonti, D., Prussi, M., Casini, D., Tredici, M. R., Rodolfi, L., Bassi, N., et al., 2013. Review of energy balance in raceway ponds for microalgae cultivation: Re-thinking a traditional system is possible. Applied Energy, 102: 101–111.
Da Silva Vaz, B., Costa, J. A. V., and de Morais, M. G., 2016. CO2 Biofixation by the cyanobacterium Spirulina sp. LEB 18 and the green alga Chlorella fusca LEB 111 grown using gas effluents and solid residues of thermoelectric origin. Applied Biochemistry and Biotechnology, 178(2): 418–429.
de Morais, M. G., and Costa, J. A. V., 2007. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. Journal of Biotechnology, 129(3): 439–445.
Duarte, J. H., de Morais, E. G., Radmann, E. M., and Costa, J. A. V., 2017. Biological CO2 mitigation from coal power plant by Chlorella fusca and Spirulina sp. Bioresource Technology, 234: 472–475.
Duarte, J. H., Fanka, L. S., and Costa, J. A. V., 2020. CO2 Biofixation via Spirulina sp. cultures: Evaluation of initial biomass concentration in tubular and raceway photobioreactors. Bio-Energy Research, 13(3): 939–943.
George, B., Pancha, I., Desai, C., Chokshi, K., Paliwal, C., Ghosh, T., et al., 2014. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus — A potential strain for bio-fuel production. Bioresource Technology, 171: 367–374.
Gordillo, F., Jiménez, C., Lopez Figueroa, F., and Niell, F., 1998. Effects of increased CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). Journal of Applied Phycology, 10: 461–469.
Hauck, J. T., Scierka, S. J., and Perry, M. B., 1996. Effects of simulated flue gas on growth of microalgae. Preprints of Papers American Chemical Society Division of Fuel Chemistry, 41: 1391–1396.
Hsieh-Lo, M., Castillo, G., Ochoa-Becerra, M. A., and Mojica, L., 2019. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Research, 42: 101600.
Jacob-Lopes, E., Scoparo, C. H. G., and Franco, T. T., 2008. Rates of CO2 removal by Aphanothece microscopica Nägeli in tubular photobioreactors. Chemical Engineering and Processing: Process Intensification, 47(8): 1365–1373.
Khan, S. A., Malla, F. A., Malav, L. C., Gupta, N., and Kumar, A., 2018. Potential of wastewater treating Chlorella minutissima for methane enrichment and CO2 sequestration of biogas and producing lipids. Energy, 150: 153–163.
Kumar, K., Banerjee, D., and Das, D., 2014. Carbon dioxide sequestration from industrial flue gas by Chlorella sorokiniana. Bioresource Technology, 152: 225–233.
Kumar, K., Dasgupta, C. N., Nayak, B., Lindblad, P., and Das, D., 2011. Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresource Technology, 102: 4945–4953.
Lee, J., Kim, D., Lee, J., Park, S., Koh, J., Cho, H., et al., 2002. Effects of SO2 and NO on growth of Chlorella sp. KR-1. Bioresource Technology, 82(1): 1–4.
Lichtenthaler, H. K., 1987. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods in Enzymology, 148: 350–382.
Mata, S. N., Santos, T. S., Cardoso, L. G., Andrade, B. B., Duarte, J. H., Costa, J. A., et al., 2020. Spirulina sp. LEB 18 cultivation in a raceway-type bioreactor using wastewater from desalination process: Production of carbohydrate-rich biomass. Bioresource Technology, 311: 123495.
Mcginn, P. J., Dickinson, K. E., Bhatti, S., Frigon, J., Guiot, S. R., and O Leary, S. J. B., 2011. Erratum to: Integration of microalgae cultivation with industrial waste remediation for biofuel and bioenergy production: Opportunities and limitations. Photosynthesis Research, 109(1): 249.
Mehar, J., Shekh, A., Nethravathy, M. U., Sarada, R., Chauhan, V. S., and Mudliar, S., 2019. Automation of pilot-scale open raceway pond: A case study of CO2-fed pH control on Spirulina biomass, protein and phycocyanin production. Journal of CO2 Utilization, 33: 384–393.
Molina Grima, E., Fernández, F. G. A., García Camacho, F., and Chisti, Y., 1999. Photobioreactors: Light regime, mass transfer, and scaleup. Journal of Biotechnology, 70(1): 231–247.
Ogbonda, K. H., Aminigo, R. E., and Abu, G. O., 2007. Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bioresource Technology, 98(11): 2207–2211.
Pavlik, D., Zhong, Y., Daiek, C., Liao, W., Morgan, R., Clary, W., et al., 2017. Microalgae cultivation for carbon dioxide sequestration and protein production using a high-efficiency photobioreactor system. Algal Research, 25: 413–420.
Pérez-López, P., de Vree, J. H., Feijoo, G., Bosma, R., Barbosa, M. J., Moreira, M. T., et al., 2017. Comparative life cycle assessment of real pilot reactors for microalgae cultivation in different seasons. Applied Energy, 205: 1151–1164.
Radmann, E. M., Camerini, F. V., Santos, T. D., and Costa, J. A V., 2011. Isolation and application of SOX and NOX resistant microalgae in biofixation of CO2 from thermoelectricity plants. Energy Conversion and Management, 52(10): 3132–3136.
Rafiqul, I. M., Hassan, A., Sulebele, G., Orosco, C. A., Roustaian, P., and Jalal, K. C. A., 2003. Salt stress culture of blue-green algae Spirulina fusiformis. Pakistan Journal of Biological Sciences, 6: 648–650.
Rathnasamy, S., and Debora, J., 2014. Extraction and purification of C-phycocyanin from Spirulina platensis using aqueous two phase extraction and its applications. Asian Journal of Chemistry, 26: 3729–3732.
Shurair, M., Almomani, F., Judd, S., Bhosale, R., and Kumar, A., 2016. Potential for green algae Spirulina to capture carbon dioxide from gas stream. Proceedings of TechConnect World Innovation Conference & Expo., Materials for Energy, Efficiency and Sustainability. Washington DC, USA, 141–143.
Singh, J., and Dhar, D. W., 2019. Overview of carbon capture technology: Microalgal biorefinery concept and state-of-the-art. Frontiers in Marine Science, 6: 29.
Tan, Y., Fang, M., **, L., Zhang, C., Li, H., and **ng, X., 2015. Culture characteristics of the atmospheric and room temperature plasma-mutated Spirulina platensis mutants in CO2 aeration culture system for biomass production. Journal of Bioscience and Bioengineering, 120(4): 438–443.
Tang, D., Han, W., Li, P., Miao, X., and Zhong, J., 2011. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresource Technology, 102(3): 3071–3076.
Thomas, D. M., Mechery, J., and Paulose, S. V., 2016. Carbon dioxide capture strategies from flue gas using microalgae: A review. Environmental Science and Pollution Research, 23(17): 16926–16940.
Wang, B., Li, Y., Wu, N., and Lan, C. Q., 2008. CO2 bio-mitigation using microalgae. Applied Microbiology and Biotechnology, 79(5): 707–718.
Wang, X., Liang, J., Luo, C., Chen, C., and Gao, Y., 2014. Biomass, total lipid production, and fatty acid composition of the marine diatom Chaetoceros muelleri in response to different CO2 levels. Bioresource Technology, 161: 124–130.
Wang, X., Miao, J., Pan, L., Li, Y., Lin, Y., and Wu, J., 2019. Toxicity effects of p-choroaniline on the growth, photosynthesis, respiration capacity and antioxidant enzyme activities of a diatom, Phaeodactylum tricornutu. Ecotoxicology and Environmental Safety, 169: 654–661.
Wiechelman, K. J., Braun, R. D., and Fitzpatrick, J. D., 1988. Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for color formation. Analytical Biochemistry, 175(1): 231–237.
Wu, H., Wang, G., **ang, W., Li, T., and He, H., 2016. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. International Journal of Food Properties, 19(10): 2349–2362.
**ong, J., Kurade, M. B., Abou-Shanab, R. A. I., Ji, M., Choi, J., Kim, J. O., et al., 2016. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresource Technology, 205: 183–190.
Yi, Q., Li, W., Feng, J., and **e, K., 2015. Carbon cycle in advanced coal chemical engineering. Chemical Society Reviews, 44(15): 5409–5445.
Yong, S. K., and Lee, S. H., 2018. Quantitative analysis of Spirulina platensis growth with CO2 mixed aeration. Environmental Engineering Research, 23(2): 216–222.
Zarrouk, C., 1966. Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthèse de Spirulina maxima (Setch. et Garndner) Geitler. PhD thesis. Faculte des Sciences, Universite de Paris.
Zhao, B., Su, Y., Zhang, Y., and Cui, G., 2015. Carbon dioxide fixation and biomass production from combustion flue gas using energy microalgae. Energy, 89: 347–357.
Zhu, B., Shen, H., Li, Y., Liu, Q., **, G., Han, J., et al., 2020. Large-scale cultivation of Spirulina for biological CO2 mitigation in open raceway ponds using purified CO2 from a coal chemical flue gas. Frontiers in Bioengineering and Biotechnology, 7: 441.
Zhu, B., Sun, F., Yang, M., Lu, L., Yang, G., and Pan, K., 2014. Large-scale biodiesel production using flue gas from coal-fired power plants with Nannochloropsis microalgal biomass in open raceway ponds. Bioresource Technology, 174: 53–59.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2016YFB0601 001). We also thank the reviewers for their valuable and constructive comments and all the staff at the Laboratory of Applied Microalgae Biology for their help during the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Q., **, G., Liu, Q. et al. Tolerance Comparison Among Selected Spirulina Strains Cultured Under High Carbon Dioxide and Coal Power Plant Flue Gas Supplements. J. Ocean Univ. China 20, 1567–1577 (2021). https://doi.org/10.1007/s11802-021-4783-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-021-4783-3