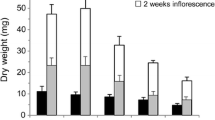
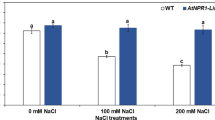
Abstract
The Arabidopsis thaliana NOK2 accession displays salt tolerance compared to more commonly known A. thaliana accessions, such as Col-0, but the basis of this phenotypic feature is unknown. This work was focused on determining whether salt tolerance in NOK2 plants is affected by calcium supplementation to the growth medium. A. thaliana seedlings were grown in pots containing a mixture of sand and peat under controlled conditions in a low-level Ca(NO3)2 medium supplemented with 0 or 50 mM NaCl with and without amendment with two higher levels of Ca(NO3)2. Calcium amendment was beneficial for salt-treated NOK2 plants, as shown by the increase in dry weight of NOK2 plants with and without NaCl, but had no impact on Col-0 biomass. Sodium accumulation decreased as a function of calcium amendment in NOK2, while Col-0 maintained its high Na levels under these conditions. Leaf K+ content, K+ uptake, and Ca content decreased in NOK2 and Col-0 plants growing in the low-level Ca medium when NaCl was added, but rose in leaves of both accessions with calcium amendment, although K remained low in both accessions in the absence of NaCl. K+/Na+ selectivity increased preferentially in NOK2 with increasing calcium in the presence of NaCl, but when Na was restricted and not under any conditions in Col-0. Preferential effects of calcium were not observed on the transcript accumulation of seven Na+, K+ or Ca2+ transport genes for either of the accessions, except for increased transcription of the CAX4 gene in NOK2 leaves at the highest calcium concentration used (5 mM). Leaf membrane leakage, which increased two-fold higher in Col-0 under salt application compared with the increase in NOK2, declined for both accessions in response to calcium supplementation, and in NOK2 this decline reached no salt levels when Ca2+ amendments were highest. Chlorophyll and carotenoid content dropped two-fold in Col-0 in response to salt, but were unchanged in NOK2 under these conditions. In contrast, leaf anthocyanins, which were normally tenfold higher in Col-0 than in NOK2 in the low-level Ca2+ medium, declined in Col-0 plants as a function of Ca2+ supplementation, but were maintained at low levels in NOK2 leaves regardless of salinity and calcium. In conclusion, NOK2 plants responded positively to calcium supplementation by improving biomass yield during salinity treatment, whereas this amendment only affected Col-0 by reducing its permeability and anthocyanin titre. K+/Na+ selectivity appeared to be an important characteristic of NOK2 response to calcium. The regulation of this response may involve the CAX4 Ca2+/H+ vacuolar transport gene, but does not appear to involve six other common ion transporters.



Similar content being viewed by others
Abbreviations
- DW:
-
Dry weight
References
Ahmad S, Wahid A, Rasul E, Wahid A (2005) Comparative morphological and physiological responses of green gram genotypes to salinity applied at different growth stages. Bot Bull Acad Sin 6:35–42
Al Harbi AR (1995) Growth and nutrient composition of tomato and cucumber seedlings as affected by sodium chloride salinity and supplemental calcium. J Plant Nutr 18:1403–1416
Albrecht V, Weinl S, Blazevic D, D’Angelo C, Batistic O, Kolukisaoglu U, Bock R, Schulz B, Harter K, Kudla J (2003) The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J 36:457–470
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Balakumar T, Hani V, Vincent B, Paliwal K (1993) On the interaction of UV-B radiation (280–315 nm) with water stress in crop plants. Physiol Plant 87:217–222
Batistic O, Kudla J (2008) Plant calcineurin B-like proteins and their interacting protein kinases. Biochim Biophys Acta 1793:985–992
Berezin I, Mizrachy-Dagry T, Brook E, Mizrahi K, Elazar M, Zhuo S, Saul-Tcherkas V, Shaul O (2008) Overexpression of AtMHX in tobacco causes increased sensitivity to Mg2+, Zn2+, and Cd2+ ions, induction of V-ATPase expression, and a reduction in plant size. Plant Cell Rep 27:939–949
Berthomieu P, Conejero G, Nublat A et al (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22:2004–2014
Buchanan BB, Gruissem W, Jones R (2000) Biochemistry and molecular biology of plants Maryland. Am Soc Plant Physiol
Cachorro P, Ortiz A, Cerda A (1994) Implications of calcium nutrition on the response of Phaseolus vulgaris L. to salinity. Plant Soil 159:205–212
Carden DE, Walker DJ, Flowers TJ, Miller AJ (2003) Single cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol 131:676–683
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9
Chan CWM, Schorrak LM, Smith RK, Bent AF, Sussman MR (2003) A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol 132:728–731
Cheng NH, Pittman JK, Shigaki T, Hirschi KD (2002a) Characterization of CAX4, an Arabidopsis H+/Cation Antiporter. Plant Physiol 128:1245–1254
Cheng NH, Pittman JK, Shigaki T, Hirschi KD (2002b) Characterization of CAX4, an Arabidopsis H+/Ca2+ antiporter. Plant Physiol 128:1245–1254
Close DC, McArthor C (2002) Rethinking the role of many plant phenolics—protection against photodamage not herbivores? OIKOS 99:166–172
Cramer GR, Lynch J, Lauchli A, Epstein E (1987) Influx of Na+ K+ and Ca2+ into roots of salt stressed cotton seedlings: effects of supplemental Ca2+. Plant Physiol 83:510–516
D’Angelo C, Weinl S, Batistic O, Pandey G, Cheong Y, Schu¨ltke S, Albrecht V, Ehlert B, Schulz B, Harter K et al (2006) Alternative complex formation of the Ca2+-regulated protein kinase CIPK1 controls abscisic acid-dependent and independent stress responses in Arabidopsis. Plant J 48:857–872
Davenport RJ, Munoz-Mayor A, Jha D, Essah PA, Rus A, Tester M (2007) The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ 30:497–507
Davenport RJ, Tester M (2000) A weakly voltage-dependent, non-selective cation channel mediates toxic sodium influx in wheat. Plant Physiol 122:823–834
Boer De (1999) Potassium translocation into the root xylem. Plant Biol 1:36–45
De Pascale S, Maggio A, Fogliano V, Ambrosino P, Ritieni A (2001) Irrigation with saline water improves carotenoids content and antioxidant activity of tomato. J Hort Sci Biotechnol 76:447–453
Deeken R, Sanders C, Ache P, Hedrich R (2000) Developmental and light dependent regulation of a phloem localised K+ channel of Arabidopsis thaliana. Plant J 23:285–290
Demidchik V, Davenport RJ, Tester M (2002) Nonselective cation channels in plants. Annu Rev Plant Biol 53:67–107
Demidchik V, Tester M (2002) Sodium fluxes through nonselective cation channels in the plasma membrane of protoplast from Arabidopsis roots. Plant Physiol 128:379–387
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Foot JP, Caporn SJM, Lee JA, Ashenden TW (1996) The effect of long-term ozone fumigation on the growth, physiology and frost sensitivity of Calluna vulgaris. New Phytol 133:503–511
Gay AP, Hauck B (1994) Acclimation of Lolium temulentum to enhanced carbon dioxide concentration. J Exp Bot 45:1133–1141
Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux Ferriere N, Thibaud JB, Sentenac H (1998) Identification and disruption of a plant shakerlike outward channel involved in K+ release into the xylem sap. Cell 94:647–655
Geisler M, Frangne N, Gomes E, Martinoia E, Palmgren MG (2000) The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol 124:1814–1827
Ghars MA, Parre E, Debez A, Bordenave M, Richard L, Leport L, Bouchereau A, Savouré A, Abdelly C (2008) Comparative salt tolerance analysis between Arabidopsis thaliana and Thellungiella halophila, with special emphasis on K+/Na+ selectivity and proline accumulation. J Plant Physiol 165:588–599
Gobert A, Park G, Amtmann A, Sanders D, Maathuis FJ (2006) Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J Exp Bot 57:791–800
Golldack D, Quigley F, Michalowski CB, Kamasani UR, Bohnert HJ (2003) Salinity stress tolerant and sensitive rice (Oryza sativa L.) regulate AKT1 type potassium channel transcripts differently. Plant Mol Biol 51:71–81
Gould KS, Markham KR, Smith RH, Goris JJ (2000) Functional role of anthocyanins in the leaves of Quintinia serrata A Cunn. J Exp Bot 51:1107–1115
Guo KM, Babourina O, Christopher DA, Borsics T, Rengel Z (2008) The cyclic nucleotide-gated channel, AtCNGC 10, influences salt tolerance in Arabidopsis. Physiol Plant 134:499–507
Harper JF, Harmon A (2005) Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol 6:555–566
Hasegawa PM, Bressan RA, Zhu JK, Bonhert HJ (2000) Plant cellular and molecular responses to high salinity. Ann Rev Plant Physiol Plant Mol Biol 51:463–499
Hirschi KD (1999) Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11:2113–2122
Hirschi KD (2004) The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol 136:2438–2442
Kaddour R, Nasri N, M’rah S, Berthomieu P, Lachaâl M (2009) Comparative effect of potassium on K and Na uptake and transport in two accessions of Arabidopsis thaliana during salinity stress. C R Biol 332:784–794
Kaplan B, Sherman T, Fromm H (2007) Cyclic nucleotide-gated channels in plants. FEBS Lett 581:2237–2246
Kaya C, Ak BE, Higgs D, MurilloAmador B (2002) Influence of foliar applied calcium nitrate on strawberry plants grown under salt stress conditions. Aust J Exp Agric 42:631–636
Korenkov V, Hirschi K, Crutchfield JD, Wagner GJ (2007) Enhancing tonoplast Cd/H antiporter activity increases Cd, Zn and Mn tolerance, and impacts root/shoot Cd partitioning in Nicotiana tabacum L. Planta 226(6):1379–1387
Krol M, Gray GR, Hurry VM, O’quist G, Malek L, Huner NPA (1995) Low-temperature stress and photoperiod affect an increased tolerance to photoinhibition in Pinus banksiana seedlings. Can J Bot 73:1119–1127
Kudla J, Xu Q, Harter K, Gruissem W, Luan S (1999) Genes for calcineurin B like proteins in Arabidopsis are differentially regulated by stress signals. PNAS 96:4718–4723
Labidi N, Lachaâl M, Chibani F, Grignon C, Hajji M (2002) Variability of the response to NaCl of eight ecotypes of Arabidopsis thaliana. J Plant Nutr 25:2627–2638
Lecourieux D, Ranjeva R, Pugin A (2006) Calcium in plant defence signalling pathways. New Phytol 171:249–269
Li XL, Borsics T, Harrington HM, Christopher DA (2005) Arabidopsis AtCNGC10 rescues potassium channel mutants of E. coli, yeast and Arabidopsis and is regulated by calcium/calmodulin and cyclic GMP in E. coli. Funct Plant Biol 32:643–653
Lichtenthaler HK (1988) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enz 148:350–383
Luan S (2009) The CBL–CIPK network in plant calcium signaling. Trends Plant Sci 14:37–42
Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W (2002) Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 14(Suppl):S389–S400
Luan S, Lan W, Lee SC (2009) Potassium nutrition, sodium toxicity, and calcium signaling: connections through the CBL–CIPK network. Curr Opin Plant Biol 12:339–346
Lutts S, Kinet JM, Bouharmont J (1996) NaCl induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Maathuis FJ, Sanders D (2001a) Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol 127:1617–1625
Maathuis FJ, Sanders D (2001b) Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol 127:1617–1625
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Marten I, Hoth S, Deeken R, Ache P, Ketchum KA, Hoshi T, Hedrich R (1999) AKT3 a phloemlocalized K+ channel is blocked by protons. Proc Natl Acad Sci USA 96:7581–7586
Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D et al (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126:1646–1667
Mengel K, Kirkby EA (2001) Principles of plant nutrition. Kluwer, Dordrecht
Moller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 21:2163–2178
Murray JR, Hackett WP (1991) Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedera helix L. Plant Physiol 97:343–351
Nakamura Y, Tanaka K, Ohta E, Sakata M (1990) Protective effect of external Ca2+ on elongation and the intracellular concentration of K+ in intact mung bean roots under high NaCl stress. Plant Cell Physiol 31:815–821
Nedjimi B, Daoud Y (2009) Ameliorative effect of CaCl2 on growth, membrane permeability and nutrient uptake in Atriplex halimus subsp. schweinfurthii grown at high (NaCl) salinity. Desal 249:163–166
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742
Patel NT, Vaghela PM, Patel AD, Pandey AN (2011) Implications of calcium nutrition on the response of Caesalpinia crista (Fabaceae) to soil salinity. Acta Ecol Sin 31:24–30
Perez Prat E, Narashimhan ML, Binzel ML, Botella MA, Chen Z, Valpuesta V, Bressan RA, Hasegawa PM (1992) Induction of a putative Ca2+-ATPase mRNA in NaCl adapted cells. Plant Physiol 100:1471–1478
Qi Z, Spalding EP (2004) Protection of plasma membrane K+ transport by the salt overly sensitive Na+/H+ antiporter during salinity stress. Plant Physiol 136:2548–2555
Rajendran L, Ravishankar GA, Venkataraman LV, Prathiba KR (1992) Anthocyanin production in callus cultures of Daucus carota as influence by nutrient stress and osmoticum. Biotech Lett 14:707–712
Roberts SK, Snowman BN (2000) The effects of ABA on channel-mediated K+ transport across higher plant roots. J Exp Bot 51:1585–1594
Schmidt C, He T, Cramer GR (1993) Supplemental calcium does not improve growth of salt stressed Brassicas. Plant Soil 155(156):415–418
Shalata A, Neumann PM (2001) Exogenous ascorbic acid (Vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52(364):2207–2211
Shi HZ, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long distance Na+ transport in plants. Plant Cell 4:465–477
Shi J, Kim K-N, Ritz O, Albrecht V, Gupta R, Harter K, Luan S, Kudla J (1999) Novel protein kinase associated with calcineurin B like calcium sensors in Arabidopsis. Plant Cell 11:2393–2405
Snedden WA, Fromm H (2001) Calmodulin as a versatile calcium signal transducer in plants. New Phytol 151:35–66
Steyn WJ, Wand SJE, Holcroft DM, Jacobs G (2002) Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol 155:349–361
Tuna AL, Kaya C, Ashraf M et al (2007) The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Env Exp Bot 59:173–178
White PJ (1997) Cation channels in the plasma membrane of rye roots. J Exp Bot 48:499–514
White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM (2002) Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochem Biophys Acta 1564:299–309
White PJ, Davenport RJ (2002) The voltage-independent cation channel in the plasma membrane of wheat roots is permeable to divalent cations and may be involved in cytosolic Ca2+ homeostasis. Plant Physiol 130:1386–1395
Winkel Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Wyatt SE, Tsou P-L, Robertson D (2002) Expression of the high-capacity calcium-binding domain of calreticulin increases bioavailable calcium stores in plants. Transgenic Res 11:1–10
Acknowledgments
This work was supported by the Tunisian-French CMCU (network 02F/924).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Hajduch.
R. Kaddour and H. Mahmoudi have equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. Representative semi-quantitative RT-PCR analysis of transcript accumulation for three ion transporters in NOK2 and Col-0 plants in response to NaCl treatment and calcium supplementation. Calcium (0.5, 2.5 and 5 mM) and NaCl (0 or 50 mM) were applied to 23 day-old plants for 16 days. Amplification conditions were as described in “Materials and methods”. EF-1α was used as the reference gene.
Rights and permissions
About this article
Cite this article
Kaddour, R., Mahmoudi, H., Baâtour, O. et al. Physiological and molecular responses of two Arabidopsis accessions to calcium amendment and salt constraint. Acta Physiol Plant 34, 439–450 (2012). https://doi.org/10.1007/s11738-011-0840-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0840-7