Abstract
The amorphous Co3O4 nanostructure, which adopted sodium hexametaphosphate as structure-directing agent, has been successfully synthesized in large scale via two steps: preparation of the precursor and the calcination process. The results of X-ray diffraction indicate that the prepared materials are mainly composed of Co3O4; the formless Co3O4 nanoplate with loose structures is observed by scanning electron microscopy. Cyclic voltammetry, chronopotentiometry, and electrochemical impedance measurements are applied in a mild aqueous electrolyte (2 mol L−1 KOH) to investigate the performance of the Co3O4, which show a high specific capacitance (SC) of 482.61 F g−1 at 5 mA cm−2. Besides, the SC degradation is only 10.05 % after 250 continuous charge–discharge cycles at 5 mA cm−2, indicating an excellent electrochemical stability. The improved performance is reasonably ascribed to their irregular structure for ionic transport during the electrochemical reaction, which presents as promising candidates for supercapacitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Supercapacitors, as one class of energy storage devices, have attracted extensive attention because of their highly improved power density, long cycle life, and short charge time, and are thus one of the potential options in tackling the energy and environmental challenges emerging to accompany with the rapid developments of modern society [1–3]. On the basis of the electrode materials and charge storage mechanisms, electrochemical supercapacitors can be classified as electrical double-layer capacitors [4–6] and Faradic redox reaction pseudocapacitors [7–9]. To date, carbon-related materials have been widely utilized as building blocks to construct electrodes for double-layer capacitors due to their good processing ability, large surface area porosity, good cycle life, and low cost [10, 11]. However, the devices fabricated suffer from their relatively low capacity in storing charge [11]. In contrast, the Faradic redox reaction pseudocapacitors made with transition metal oxides have drawn extensive research attention due to their improved specific capacitances through tuning of the microstructures of these materials [12–16]. Supercapacitors made with RuO2 materials show excellent performances with a specific capacitance; however, the practical applications of supercapacitors fabricated with RuO2-based materials are hindered due to their high cost and the difficulty in accessing these materials. Toward the end of commercializing electrochemical capacitors in applications, diverse efforts have thus focused on low-cost transition metal oxide materials such as MnO2, V2O5, NiO, and Co3O4, which are a group of very promising supercapacitor electrode materials as substitute to the expensive and toxic RuO2. Cobalt oxide, as typical transition metal oxide, has been utilized in designing and fabricating catalysts [17], biosensors [18], field emitters [19], and Li batteries [20], which is a kind of promising electrode material with the consideration of low cost, natural abundance, and environmental safety for pseudocapacitors [21, 22]. Furthermore, for cobalt oxide electrode, its difficulty for electrolyte penetration and poor electronic conductivity result in a relatively low specific capacitance and ineffective utilization of the active material as supercapacitors. For instance, various nanostructured Co3O4 powders including nanoparticles [22], nanowalls [23], nanowires [24], nanorods [25], nanotubes [26], nanoboxes [27], polyhedrons [28], and hierarchical microspheres [29] have already been synthesized successfully with different methods. Novel supercapacitors with excellent performances have been fabricated with sheets of Co3O4 nanostructures [30, 31]. Recently, it was found that Co3O4 polyhedrons prepared with organic solvents such as citric acid and polyvinyl alcohol show fine capacitance [32]. There are very few studies in the literature that show how inorganic solvents affect the formation of Co3O4 microstructure in terms of growth mechanism and property.
Herein, we report a simple and facile approach to prepare Co3O4 polyhedrons without any solvent through a two-step synthetic method. After adopting sodium hexametaphosphate calgon [(NaPO3)6] as inorganic structure-directing agent in the liquid phase reaction, we get the formless Co3O4 nanoplates with loose structures which perform excellent pseudocapacitive characteristics and good stability. The result indicates that the structure-directing agent plays an important role in the process of precursor formation.
Experimental
All of the reagents were analytically pure, purchased from Tian** Chemical Industrial Co. Ltd. (Tian**, China), and used without further purification.
Sample preparation
Typically, 1.19 g of CoCl2 · 6H2O and 0.119 g of (NaPO3)6 together with 0.3 g cetyltrimethylammonium bromide were dissolved in 30 mL deionized water to form a homogeneous solution in the open beaker under continuous magnetic stirring. Then, the solution was poured into three flasks and was heated up to 60 °C by condensing water bath. Next, 1.46 mL of hydrazine hydrate (N2H4 · H2O) 30 mL aqueous solution was added. The mixed solution was kept at 60 °C for 2 h. After the solution was cooled down to room temperature naturally, the precipitate was collected by vacuum filtration and rinsed with deionized water and ethanol for several times, and dried in an oven at 80 °C for 12 h to get the pink powders of precursor. Finally, the sample was calcined in a muffle furnace at a temperature of 350 °C for 4 h, and then cooled to room temperature. The rubricans powder of the final product was collected for the characterization and applications.
Characterization
The morphologies and structures of the sample were examined by scanning electron microscopy (SEM,S-4800, Germany), the crystallographic phases of the as-prepared sample were investigated by D/MAX-2400X X-ray diffractometer with Cu-Kα radiation (λ = 0.154056 nm), employing a scanning rate of 10° min−1 in the 2θ range of 10°–90°.
Electrochemical studies were carried out in a three-electrode system. A piece of nickel foam mesh coated with freshly prepared Co3O4 nanoarchitectures, a platinum electrode, and a saturated calomel electrode (SCE) were used as working electrode, counter electrode, and reference electrode, respectively. The working electrode was composed of active material (Co3O4, 75 wt.%, 1 mg cm−2), graphite(5 wt.%), conductive material (acetylene carbon black, ATB, 5 wt.%) and binder (polytetrafluoroethylene, PTFE, 10 wt.%), and 5 % ethyl alcohol. The mixture of electrode materials was ground for 10 min and coated onto the surface of nickel foam meshes (1 × 1 cm2). Then the meshes were dried at 80 °C for 12 h and then pressed under 10 MPa to obtain a working electrode. The electrochemical measurements were evaluated by the means of cyclic voltammetry (CV), chronopotentiometry (CP), and electrochemical impedance spectroscopy (EIS) in a beaker cell with 2 mol L−1 KOH aqueous solution as the electrolyte. The specific capacitance (SC) values determined from the CP curves were calculated according to the following equation:
where Cs, I, t, m, and V are SC (F g−1), the discharge current (mA), the discharging time (s), the mass of the electroactive material (mg), and the potential interval of the discharge (V), respectively. All of the above electrochemical measurements were evaluated on a CHI660B electrochemical workstation (Chenhua, Shanghai, China).
Results and discussion
XRD patterns of Co3O4
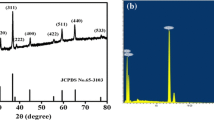
Figure 1 shows the XRD patterns of Co3O4. It is indicated that all of the diffraction peaks in the XRD profile of the product are well indexed into the cubic Co3O4 (JCPDS card no. 42-1467, a=0.8037) with sharp and slender peaks, which implies the excellent degree of crystallinity. Finally, no other peaks were detected, which indicate its high purity.
SEM images of Co3O4
The scanning electron microscope images of the Co3O4 prepared by hydrothermal synthesis in Fig. 2 show that the features of Co3O4 are graduating from eight-side cube to loosen the flake structure to amorphous fleecinesses as the content of the hexametaphosphate calgon increases, which may result from the important role of the structure-directing agent. Under normal circumstances, there are three ways how crystal structure control agents influence on Co3O4 [33] from the point view of crystallography [34]: the exposure to atomic density and symmetrical characteristic of different directions which could lead the diversity of electronic structure, the binding, and the surface energy together with chemical activity on distinct lattice plane. It seems that the inorganic ligands have selective adsorption on some crystal face along with crystal nucleation growth. The hexametaphosphate anions and hydrazine hydrate ions may function as linkers connecting to Co2+, which affect the structure during the process of particle growth and can change the speed of growth owing to the specific adsorption of inorganic matter on <111> crystal face; so particles with morphology different from the crystal balance morphology are formed. After washing and calcination, inorganic ligands were removed, which lead to the special morphology.
Electrochemical measurement of Co3O4
Figure 3 shows the CV curves of Co3O4 nanoarchitectures tested in an aqueous electrolyte of 2 mol/L KOH from −0.1 to 0.5 V (vs. SCE) with the scan rates between 5 and 50 mV s−1. It is well accepted that the pseudocapacitive process is associated with two redox couples [35] which are reflected in the equation below. The first redox couple A1/B1 corresponds to the conversion between CoOOH and Co3O4 as illustrated as below and the second redox couple A2/B2 is attributed to the change between CoOOH and CoO2, which are well-defined good pseudocapacitive characteristics of the final product of Co3O4 caused by fast and reversible faradaic redox reaction of the materials. As the scan rate increases, the anodic peaks shift toward high potential and the cathodic peaks move toward negative potential simultaneously, respectively; and the broad and cathodic peaks become overlapped, which may be attributed to the polarization caused by the high scan rate of the redox peaks [36]:
Figure 4 reveals the galvanostatic CP curves of the Co3O4 electrode at various current densities in the potential window of −0.1 ~ 0.4 V, in which the electrode exhibited the excellent capacitor ability due to its amorphous porous structure. In Fig. 4, the electrode potential is not in a linear relationship with time, and the charge–discharge curves do not exhibit pure double-layer capacitance behavior, which are in accordance with the cyclic voltammetry tests. It can be seen that the curves were composed of two parts; the voltage exhibited a good linear relationship with time in the range of −0.1 ~ 0.25 V, which present the ideal behavior of the electrical double-layer capacitor. Besides, the capacitance of the electrode consisted of the electrolyte ionic charge and the generation of aggregates in the liquid interface. The potential is nonlinear with time in the range of 0.25 ~ 0.4 V. It is suspected that the exhibited pseudocapacitance is from the interface between the electrode and the electrolyte or electrochemical oxidation–reduction reaction of desorption. Thus, the capacitance of the electrode is the sum of the pseudo and the double layer. The specific capacitance values can be calculated as high as 482.61 F g−1 with current density of 5 mA cm−2.
Figure 5 shows the electrochemical impedance spectroscopy (EIS) measurements, which are good to further understand the capacitive behavior of the electrodeposited cobalt oxide electrode. It can also be seen from the figure that the electrochemical impedance test was carried out at a capacitor operating potential of 0.4 V in a frequency range from 105 to10−2 Hz. In Fig. 5, the impedance spectra are composed of one semicircle in the high-frequency range corresponding to the double-layer capacitance and ionic diffusion process which a straight slo** line in the low-frequency range corresponding to the diffusive resistance resulted by Warburg behavior. At high frequencies, the impedance behaves like a resistance; as the frequency becomes lower, the slope of all the impedance plots increases and tends to become purely capacitive, which demonstrates that the electrochemical capacitance of this capacitor is higher.
To further confirm the influence of the nanostructure on the electrochemical performance, the equivalent circuit which is consistent with the above behaviors is shown in Fig. 6. The charge-transfer resistance of Co3O4 is called R ct, which has been described as a pseudocharge-transfer resistance and a straight slo** line in the low-frequency range corresponding to the diffusive resistance. R sol is the solution resistance in the circuit. Besides, the two constant-phase elements CPE1 and CPE2 are denoted as the double-layer capacitance and ionic diffusion process resulting in Warburg behavior in the electrode, respectively.
In order to evaluate the stability of the Co3O4 electrodes, the charge–discharge cycling tests were conducted for 250 cycles. Figure 7 shows the cyclic performance of Co3O4 electrode in 2 mol L−1 of KOH electrolytes at 5 mA cm−2. As shown in Fig. 6, the stability of the material is excellent except for an initial decrease of capacitance in the first 50 cycles. As the cycle number increases, the specific capacitance of the electrode gradually declines because of the long time of charge–discharge cycles, and the active materials composed of formless and loose structures expanded and shrank into some extent in order to induce a significant decrease. It may lead to the drop of activities of the nickel foam substrate, which results to the obvious decrease of capacitance. In the end, the initial capacitance of the Co3O4 electrode just decreased to 10.05%.
Conclusions
In summary, Co3O4 of amorphous structures which adopted sodium hexametaphosphate calgon as structure-directing agent were successfully synthesized in large scale via two step strategies: preparation of the precursor followed by calcination process. The results of XRD indicate that the prepared material is mainly composed of Co3O4. The SEM showed that the morphology of as-prepared Co3O4 was formless and loose in structure. Cyclic voltammetry, chronopotentiometry, and electrochemical impedance measurements are applied in a mild aqueous electrolyte to investigate the performance of the Co3O4, which show a high SC of 482.61 F g−1 at 5 mA cm−2. Besides, the SC degradation is only 10.05 % after 250 continuous charge–discharge cycles at 5 mA cm−2, which indicates excellent electrochemical stability.
References
Rolison DR, Long RW, Lytle JC, Fischer AE, Rhodes CP, McEvoy TM, Bourga ME, Lubers AM (2009) Chem Soc Rev 38:226
Simon P, Gogotsi Y (2008) Nat Mater 7:845
Miller JR, Simon P (2008) Science 321:651
Zhang H, Zhang WF, Cheng J, Cao GP, Yang YS (2008) Solid State Ionics 179:1946
Fang BZ, Binder L (2006) J Power Sources 163:616
Futaba DN, Hata K, Yamada T, Hiraoka T, Hayamizu Y, Kakudate Y, Tanaike O, Hatori H, Yumura M, Iijima S (2006) Nat Mater 5:987
Cormie A, Cross A, Hollenkamp AF, Donne SW (2010) Electrochem Acta 55:7470
Wu MS, Huang CY, Lin KH (2009) J Power Sources 186:557
Wang YG, **a YY (2006) Electrochem Acta 51:3223
Frackowiak E, Beguin F (2001) Carbon 39:937
**e K, Qin X, Wang X, Wang Y, Tao H, Wu Q, Yang L, Hu Z (2012) Adv Mater 24:347
Mai LQ, Yang F, Zhao YL, Xu X, Xu L, Luo YZ (2011) Nat Commun 2:381
Liu C, Li F, Ma LP, Cheng HM (2010) Adv Mater 22:E28
Arico AS, Bruce P, Scrosati B, Tarascon JM, Van Schalkwijk W (2005) Nat Mater 4:366
Brezesinski T, Wang J, Tolbert SH, Dunn B (2010) Nat Mater 9:146
Hu CC, Chang KH, Lin MC, Wu YT (2006) Nano Lett 6:2690
Wang GL, Cao DX, Yin CL, Gao YY, Yin JL, Cheng L (2009) Chem Mater 21:5112
Ding Y, Wang Y, Su LA, Bellagamba M, Zhang H, Lei Y (2010) Biosens Bioelectron 26:542
Yu T, Zhu YW, Xu XJ, Shen ZX, Chen P, Lim CT, Thong JTL, Sow CH (2005) Adv Mater 17:1595
Wang X, Wu XL, Guo YG, Zhong YT, Cao XQ, Ma Y, Yao JN (2010) Adv Funct Mater 20:1680
Wang GX, Shen XP, Horvat J, Wang B, Liu H, Wexler D, Yao J (2009) J Phys Chem C 113:4357
Srinivasan V, Weidner JW (2002) J Power Sources 108:15
Yuan CZ, Yang L, Hou LR, Shen LF, Zhang F, Li DK, Zhang XG (2011) J Mater Chem 21:18183
Wu JB, Lin Y, **a XH, Xu JY, Shi QY (2011) Electrochem Acta 56:7163
Hosono E, Fujihara S, Honma I, Zhou HS (2005) J Mater Chem 15:1938
Xu JA, Gao L, Cao JY, Wang WC, Chen ZD (2010) Electrochem Acta 56:732
He T, Chen DR, Jiao XL, Wang YL (2006) Adv Mater 18:1078
**ong S, Yuan C, Zhang X, ** B, Qian Y (2009) Chem Eur J 15:5320
Zhu LP, Zhang WD, **ao HM, Yang Y, Fu SY (2008) J Phys Chem C 27:112
Hu L, Peng Q, Li Y (2008) J Am Chem Soc 130:16136
Liu B, Zeng HC (2004) J Am Chem Soc 126:8124
Zhao ZW, Guo ZP, Liu HK (2005) J Power Sources 147:264–258
Zhang KC, Zhang LH (1981) Crystal Growth [M]. Science Press, Bei**g, pp 216–220
Jiao SH, Xu DS, Xu LF, Zhang XG (2012) Acta Phys Chem Sin 28(10):2436–2446
Barbero C, Planes GA, Miras MC (2001) Electrochem Commun 3(3):113–116
Li YH, Huang KL, Liu SQ, Yao ZF, Zhuang SX (2011) J Solid State Electrochem 15:587
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 51062011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Xu, H., Zhuang, JX., Li, JL. et al. Liquid precipitation synthesis of Co3O4 for high-performance electrochemical capacitors. Ionics 20, 489–494 (2014). https://doi.org/10.1007/s11581-013-0998-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0998-7