Abstract
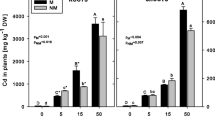
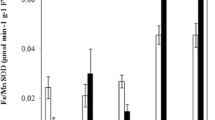
In this paper, we examined Cd accumulation and PC synthesis in two clones of Dittrichia viscosa, one with a metallicolous (DV-A) and the other with a non-metallicolous origin (DV-W). The clones were cultured in vitro with 0 and 10 mg Cd L−1 in both short-term treatments (up to 72 h) and over 10 days. We also examined the influence of the culture medium dilution and the PC-synthesis inhibitor, l-buthionine-sulfoximine (BSO), on these parameters. Similar Cd accumulation values were found in the two clones. No synthesis of new thiolic compounds was observed in Cd-treated plants cultured in vitro in Murashige and Skoog medium up to 72 h when compared to controls. Dilution of the culture medium affected PC production, increasing it in 1/2 MS and especially in 1/4 MS. Cd uptake did not increase in the same way, but still hyperaccumulation levels were exceeded in all Cd treatments. BSO addition increased the sensitivity of D. viscosa to Cd and diminished Cd accumulation. Nevertheless, a poor correlation between PCs and Cd accumulation capacity was observed since the highest Cd content did not correspond to the highest PC levels. All these results obtained suggest that PCs are important in Cd accumulation and detoxification in D. viscosa and also that other mechanisms might be involved in these traits.





Similar content being viewed by others
References
Akhter F, McGarvey B, Macfie SM (2012) Reduced translocation of cadmium from roots is associated with increased production of phytochelatins and their precursors. J Plant Physiol. doi:10.1016/j.jplph.2012.07.011
Arnetoli M, Vooijs R, ten Bookum W, Galardi F, Gonnelli C, Gabbrielli R, Schat H, Verkleij JAC (2008) Arsenate tolerance in Silene paradoxa does not rely on phytochelatin-dependent sequestration. Environ Pollut 152:585–591. doi:10.1016/j.envpol.2007.07.002
Barbafieri M, Dadea C, Tassi E, Bretzel F, Fanfani L (2011) Uptake of heavy metals by native species growing in a mining area in Sardinia, Italy: discovering native flora for phytoremediation. Int J Phytoremed 13:985–997. doi:10.1080/15226514.2010.549858
Bert V, Bonnin I, Saumitou-Laprade P, de Laguerie P, Petit D (2002) Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol 155:47–57. doi:10.1046/j.1469-8137.2002.00432.x
Bräutigam A, Schaumlöffel D, Krauss G-J, Wesenberg D (2009) Analytical approach for characterization of cadmium-induced thiol peptides—a case study using Chlamydomonas reinhardtii. Anal Bioanal Chem 395:1737–1747. doi:10.1007/s00216-009-2921-7
Bräutigam A, Wesenberg D, Preud’homme H, Schaumlöffel D (2010) Rapid and simple UPLC–MS/MS method for precise phytochelatin quantification in alga extracts. Anal Bioanal Chem 398:877–883. doi:10.1007/s00216-010-3970-7
Chen A, Komives EA, Schroeder JI (2006) An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis. Plant Physiol 141:108–120. doi:10.1104/pp.105.072637
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719. doi:10.1016/j.biochi.2006.07.003
Clemens S, Peršoh D (2009) Multi-tasking phytochelatin synthases. Plant Sci 177:266–271. doi:10.1016/j.plantsci.2009.06.008
Delhaize E, Jackson PJ, Lujan LD, Robinson NJ (1989) Poly(γ-glutamylcysteinyl)glycine synthesis in Datura innoxia and binding with cadmium. Plant Physiol 89:700–706. doi:10.1104/pp.89.2.700
Doran PM (2009) Application of plant tissue cultures in phytoremediation research: incentives and limitations. Biotechnol Bioeng 103:60–76. doi:10.1002/bit.22280
Duan G-L, Hu Y, Liu W-J, Kneer R, Zhao F-J, Zhu Y-G (2011) Evidence for a role of phytochelatins in regulating arsenic accumulation in rice grain. Environ Exp Bot 71:416–421. doi:10.1016/j.envexpbot.2011.02.016
Ellman G (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. doi:10.1016/0003-9861(59)90090-6
Farzadfar S, Zarinkamar F, Modarres-Sanavy SAM, Hojati M (2012) Exogenously applied calcium alleviates cadmium toxicity in Matricaria chamomilla L. plants. Environ Sci Pollut Res. doi: 10.1007/s11356-012-1181-9
Fernández R, Bertrand A, Casares A, García R, González A, Tamés RS (2008) Cadmium accumulation and its effect on the in vitro growth of woody fleabane and mycorrhized white birch. Environ Pollut 152:522–529. doi:10.1016/j.envpol.2007.07.011
Fernández R, Bertrand A, García JI, Tamés RS, González A (2012) Lead accumulation and synthesis of non-protein thiolic peptides in selected clones of Melilotus alba and Melilotus officinalis. Environ Exp Bot 78:18–24. doi:10.1016/j.envexpbot.2011.12.016
Fernández R, Carballo I, Nava H, Sánchez-Tamés R, Bertrand A, González A (2011) Looking for native hyperaccumulator species useful in phytoremediation. In: Golubev I (ed) Handbook of phytoremediation, 1st edn. Nova Science, New York, pp 297–330
Friederich M, Kneer R, Zenk MH (1998) Enzymic synthesis of phytochelatins in gram quantities. Phytochemistry 49:2323–2329. doi:10.1016/S0031-9422(98)00353-7
Gadapati WR, Macfie SM (2006) Phytochelatins are only partially correlated with Cd-stress in two species of Brassica. Plant Sci 170:471–480. doi:10.1016/j.plantsci.2005.09.017
Gisbert C, Almela C, Vélez D, López-Moya JR, de Haro A, Serrano R, Montoro R, Navarro-Aviñó J (2008) Identification of As accumulation plant species growing on highly contaminated soils. Int J Phytoremed 10:185–196. doi:10.1080/15226510801997457
Grill E, Thuman J, Winnacker EL, Zenk MH (1988) Induction of heavy-metal binding phytochelatins by inoculation of cell cultures in standard media. Plant Cell Rep 7:375–378. doi:10.1007/BF00269516
Haag-Kerwer A, Schafer HJ, Heiss S, Walter C, Rausch T (1999) Cadmium exposure in Brassica juncea causes a decline in transpiration rate and leaf expansion without effect on photosynthesis. J Exp Bot 50:1827–1835. doi:10.1093/jxb/50.341.1827
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11. doi:10.1093/jexbot/53.366.1
Hernández-Allica J, Garbisu C, Becerril JM, Barrutia O, García-Plazaola JI, Zhao FJ, McGrath SP (2006) Synthesis of low molecular weight thiols in response to Cd exposure in Thlaspi caerulescens. Plant Cell Environ 29:1422–1429. doi:10.1111/j.1365-3040.2006.01525.x
Jemal F, Didierjean L, Ghrir R, Ghorbal MH, Burkard G (1998) Characterization of cadmium binding peptides from pepper (Capsicum annuum). Plant Sci 137:143–154. doi:10.1016/S0168-9452(98)00120-4
Kinraide TB (1998) Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol 118:513–520. doi:10.1104/pp.118.2.513
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61:517–534. doi:10.1146/annurev-arplant-042809-112156
Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2000) Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol 145:11–20. doi:10.1046/j.1469-8137.2000.00560.x
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68:1–13. doi:10.1016/j.envexpbot.2009.10.011
Maier EA, Matthews RD, McDowell JA, Walden RR, Ahner BA (2003) Environmental cadmium levels increase phytochelatin and glutathione in lettuce grown in a chelator-buffered nutrient solution. J Environ Qual 32:1356–1364. doi:10.2134/jeq2003.1356
Martínez M, Bernal P, Almela C, Vélez D, García-Agustín P, Serrano R, Navarro-Aviñó J (2006) An engineered plant that accumulates higher levels of heavy metals than Thlaspi caerulescens, with yields of 100 times more biomass in mine soils. Chemosphere 64:478–485. doi:10.1016/j.chemosphere.2005.10.044
Milner MJ, Kochian LV (2008) Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann Bot-London 102:3–13. doi:10.1093/aob/mcn063
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Pal R, Rai JPN (2010) Phytochelatins: peptides involved in heavy metal detoxification. Appl Biochem Biotechnol 160:945–963. doi:10.1007/s12010-009-8565-4
Pérez-Sirvent C, Martínez-Sánchez MJ, García-Lorenzo ML, Bech J (2008) Uptake of Cd and Pb by natural vegetation in soils polluted by mining activities. Fresen Environ Bull 17:1666–1671
Rauser W (1995) Phytochelatins and related peptides. Structure, biosynthesis, and function. Plant Physiol 109:1141–1149. doi:10.1104/pp.109.4.1141
Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, del Río LA, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243. doi:10.1104/pp.108.131524
Sarma H (2011) Metal hyperaccumulation in plants: a review focusing on phytoremediation technology. J Environ Sci Technol 4:118–138. doi:10.3923/jest.2011.118.138
Schat H, Llugany M, Vooijs R, Hartley-Whitaker J, Bleeker PM (2002) The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J Exp Bot 53:2381–2392. doi:10.1093/jxb/erf107
Srivastava S, Tripathi RD, Dwivedi UN (2004) Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa—an angiospermic parasite. J Plant Physiol 161:665–674. doi:10.1078/0176-1617-01274
Steffens JC (1990) The heavy metal-binding peptides of plants. Annu Rev Plant Physiol 4:553–575. doi:10.1146/annurev.pp.41.060190.003005
Sun Q, Ye ZH, Wang XR, Wong MH (2007) Cadmium hyperaccumulation leads to an increase of glutathione rather than phytochelatins in the cadmium hyperaccumulator Sedum alfredii. J Plant Physiol 164:1489–1498. doi:10.1016/j.jplph.2006.10.001
Thangavel P, Long S, Minocha R (2007) Changes in phytochelatins and their biosynthetic intermediates in red spruce (Picea rubens Sarg.) cell suspension cultures under cadmium and zinc stress. Plant Cell Tiss Org 88:201–216. doi:10.1007/s11240-006-9192-1
Tian S, Lu L, Zhang J, Wang K, Brown P, He Z, Liang J, Yang X (2011) Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere 48(1):63–69. doi:10.1016/j.chemosphere.2011.02.05
Verbruggen N, Hermans C, Schat H (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181:759–776. doi:10.1111/j.1469-8137.2008.02748.x
Vernoux T, Wilson R, Seeley KA, Reichheld J-P, Muroy S, Brown S, Maughan S, Cobbett C, Van Montagu M, Inzé D, May MK, Sung ZR (2000) The root meristemless1/ cadmium sensitive2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12:97–110. doi:10.1105/tpc.12.1.97
Vurro E, Ruotolo R, Ottonello S, Elviri L, Maffini M, Falasca G, Zanella L, Altamura MM, Sanità di Toppi L (2011) Phytochelatins govern zinc/copper homeostasis and cadmium detoxification in Cuscuta campestris parasitizing Daucus carota. Environ Exp Bot 72:26–33. doi:10.1016/j.envexpbot.2010.04.017
Wan G, Najeeb U, Jilani G, Naeem MS, Zhou W (2011) Calcium invigorates the cadmium-stressed Brassica napus L. plants by strengthening their photosynthetic system. Environ Sci Pollut Res 18:1478–1486. doi:10.1007/s11356-011-0509-1
Wójcik M, Tukiendorf A (2004) Phytochelatin synthesis and cadmium localization in wild type of Arabidopsis thaliana. Plant Growth Regul 44:71–80. doi:10.1007/s10725-004-1592-9
Wójcik M, Tukiendorf A (2011) Glutathione in adaptation of Arabidopsis thaliana to cadmium stress. Biol Plantarum 55:125–132. doi:10.1007/s10535-011-0017-7
Wójcik M, Vangronsveld J, Tukiendorf A (2005) Cadmium tolerance in Thlaspi caerulescens: I. Growth parameters, metal accumulation and phytochelatin synthesis in response to cadmium. Environ Exp Bot 53:151–161. doi:10.1016/j.envexpbot.2004.03.010
Wood B, Feldmann J (2012) Quantification of phytochelatins and their metal(loid) complexes: critical assessment of current analytical methodology. Anal Bioanal Chem 402:3299–3309. doi:10.1007/s00216-011-5649-0
Zeng X, Ma LQ, Qiu R, Tang Y (2009) Responses of non-protein thiols to Cd exposure in Cd hyperaccumulator Arabis paniculata Franch. Environ Exp Bot 66:242–248. doi:10.1016/j.envexpbot.2009.03.003
Acknowledgments
This work was supported by FICYT (PEST 08-07) and by Ministerio de Ciencia y Tecnología (CTM2008-04272). AB and DF were funded by fellowship FICYT. We also thank Royal Botanic Gardens, Kew (UK) for the supply of D. viscosa seeds, Prof. Meinhart H. Zenk for kindly providing PC standards, and the anonymous referees whose comments substantially improved this manuscript. The provision of FEDER funds for the purchase of the LC–MS/MS instrument is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 3383 kb)
Rights and permissions
About this article
Cite this article
Fernández, R., Fernández-Fuego, D., Rodríguez-González, P. et al. Cd-induced phytochelatin synthesis in Dittrichia viscosa (L.) Greuter is determined by the dilution of the culture medium. Environ Sci Pollut Res 21, 1133–1145 (2014). https://doi.org/10.1007/s11356-013-1954-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1954-9