Abstract
Catechol is a substance that is commonly found in wastewaters from a variety of sectors including paper, paint, petroleum, dyes, antioxidants, pesticides, iron and steel, solvents, nylon, detergent, textile, plastic, rubber, cosmetics, and medicine. In this study, sequential electrochemical and chemical multi-polymerization of catechol was investigated for environmental pollution abatement. The effect of operating parameters like catechol concentration (2–10 g/L), ammonium persulphate (APS) concentration (2–10 g/L) and reaction temperature (20–60 °C) were evaluated using response surface methodology. Catechol concentration was determined using HPLC in a gradient mobile phase. The electrochemical behavior of the polymer was investigated by cyclic voltammetry (CV). The structural and morphological properties of polycatechol were characterized by Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy with energy dispersive X-ray (SEM–EDX) analysis. It was observed from the SEM images a polymeric structure developed from a crystalline and heterogeneous structure when the APS concentration increased. Similarly, it was seen in SEM images that the polymers transitioned from a bulk and heterogeneous structure to a homogeneous structure as the temperature increased, and back to a heterogeneous structure as the catechol concentration increased. It was also found that catechol removal increased and reaction selectivity decreased by increasing the reaction temperature. The optimum operating conditions were found as 4 g/L catechol concentration, 9.5 g/L APS concentration, 30 °C reaction temperature with 100 cycles at 50 mV/s of electrochemical polymerization and 72 h of chemical polymerization. The results of this study show the potential of challenging new routes not only facile polymerization of organic monomers but also to decrease the undesirable pollutant concentration in the wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Water pollution causes serious problems for both human health and aquatic lives. In particular, the organic molecules such as phenolic structures widely originated from the industrial disposal that results in water contamination. Catechol is a colorless, toxic, highly carcinogenic and water-soluble phenolic compound, which is widely existed in many industrial wastewaters including petroleum, dyes, antioxidants, pesticides, iron and steel, solvents, nylon, detergent, textile, plastic, rubber, medicine, cosmetics, paint, and paper (Caetano et al., 2009; Chen et al., 2010; Kazemi et al., 2014; Saha & Mukhopadhyay, 2022; Shakir et al., 2008). Several techniques have been investigated in removal of catechol from aqueous solutions using physical, chemical, and physicochemical methods. Catechol and its derivatives represent one of the most important classes of naturally abundant small molecules. It has the capacity to react with a wide variety of chemical groups such as amines or thiols when oxidized. It is not surprising that it has opened the door to the design of catechol-based multifunctional polymers because of this property of catechols. Due to their privilege properties, catechols are used in various fields including tissue engineering; as redox active polymers in the battery field; to maintain the adhesion of a particular material to a substrate. Catechol molecules have not only been proven to be potent antioxidants, anti-inflammatory, redox active or exhibit excellent adhesion to different substrates, but they have also been found to be easily oxidized. Catechol functionality can be incorporated into polymers using different techniques. The most studied method is the direct polymerization of functional catechols such as dopamine by oxidative or enzymatic methods. In recent studies, catechol functionality in catechol polymerization was investigated in applications of supercapacitors (Yao et al., 1999; Nemtollahi & Golabi, 1996; Ribeiro et al., 2015; Santos et al., 2019). Polymerization of catechol takes place by the formation of C–C bonds between benzene rings following the deprotonation of catechol. At pH 8, approximately 3% of the catechol will be deprotonated, which is likely sufficient to initiate polymerization (Jha & Halada, 2011). Catechol is susceptible to oxidation, called auto oxidation when exposed to air. During the catechol auto oxidation process, catechol loses two hydrogen atoms and undergoes the extraction of two electrons in turn. In the first step, catechol is oxidized to the o-semiquinone radical with the production of O2−. The resulting O2− then reacts with catechol to form α-semiquinone radicals and H2O2. The formation of free radicals in the whole process was confirmed by electron paramagnetic resonance (Yan et al., 2021).
Multiple polymerization can be used as a basic method to synthesize polymers with certain properties that different polymerization mechanisms are carried out together (Ge et al., 2023). The method consists of three types of mechanisms: (i) orthogonal polymerization, (ii) hybrid polymerization, and (iii) sequential polymerization. Orthogonal polymerization refers to the simultaneous progression of various mechanisms without mutual influence. The superiority of orthogonal polymerization is to allow different mechanisms to be performed together under similar conditions without the need of adding reagents or changing the conditions of the reaction (Ge et al., 2023). The difference between hybrid polymerization and orthogonal polymerization that is more flexible, as it allows the combination of mechanisms with mutual action. It achieves this broad range of compatibility by considering several mechanisms through its hybrid polymerization. In comparasion to orthogonal polymerization and sequential polymerization, hybrid polymerization has a wide range of compatibility given the different mechanisms. Sequential polymerization is the most advanced multi-polymerization technique and different polymerization methods occur independently at predetermined phases in sequential polymerization (Ge et al., 2023). The advantage of sequential polymerization is to combine different polymerization mechanisms. Sequential polymerization makes it possible to synthesize block copolymers with different chain segments synthesized from different polymerization mechanisms that encompasses many mechanisms, including radical polymerization, coordination polymerization, cationic/anionic polymerization, and ring-opening metathesis polymerization. The main strategy for sequential polymerization is using a bifunctional initiator, monomer, or catalyst capable of initiating two mechanisms under different conditions. A typical example of sequential polymerization is the sequential polymerization of difunctional molecules containing both hydroxyl group and alkyl bromide, ring-opening polymerization (ROP) and atom transfer radical polymerization (ATRP). The second polymerization mechanism requires the conversion of existing chain terminals or functional groups, the conversion is often accomplished elegantly and efficiently for sequential polymerization (Ge et al., 2023).
The purpose of this study is to investigate the separation of catechol from aqueous solutions by sequential electrochemical and chemical multi-polymerization for the abatement of organic pollutants. Ammonium persulfate (APS) was used as both polymerization initiator and supporting electrolyte. The electrochemical behavior of the polymer was investigated by cyclic voltammetry (CV). FTIR and SEM analysis supported the evidence of polycatechol structure. This study provides a new route not only facile polymerization of organic monomers but also decreasing the concentrations of undesirable organic pollutants that can be found in wastewater.
2 Materials and Methods
2.1 Chemicals and Materials
Catechol (Merck), ammonium persulfate (APS) (Sigma), methanol (Merck) and acetonitrile (Merck) were received in extra pure grade and used as received. Double distilled water was produced using GFL-2008 water still and Millipore Simplicity® UV ultrapure water system at 18.2 MΩ.cm resistivity.
2.2 Experimental Set-up and Procedure
Sequential one-pot electrochemical and chemical multi-polymerization were carried out in a three-electrode cell with heating/cooling jacket in APS electrolyte solution. The reaction volume was 100 mL. Electrochemical cell was connected to VoltaLab 40 PGZ 301 potentiostat running VoltaMaster 4 software. The working electrode was graphite carbon (Meteor, Germany), the reference electrode was Ag/AgCl (3 M KCl), and the auxiliary electrode was Pt in a three-electrode cell. The surface of the carbon electrode was grinded by sandpaper and the electrodes were cleaned with deionized water before each experiment. Cyclic voltammograms (CV) were recorded at open circuit potential in the potential range from -0.3 V to + 1.5 V for 100 cycles at scan rate of 50 mV/s in APS electrolyte solution. Magnetic stirrer (Heidolph MR3001K) was used for the homogenization of reaction medium at 300 rpm. Reaction temperature was controlled with circulating water recycled in a temperature-controlled water bath (Memmert WB 22) using peristaltic pump (Heidolph PD 5206). The samples were withdrawn from the reaction medium at predetermined time intervals to determine the catechol concentration. After 100 cycles of electrochemical polymerization, the solution in the reactor was kept at room temperature for 72 h to carry out the chemical polymerization. Catechol polymer was obtained after filtering the solution through a slow grade quantitative filter paper (Isotherm) at the end of 72 h and washed with deionized water several times to remove the impurities. The solid product was dried on the filter paper in drying and heating chamber with natural convection (BINDER ED 115) at 60 °C for 24 h. The filter paper was then washed on a funnel with 50 mL of methanol to remove impurities and the polymer solution was obtained in a glass petri dish. The methanol from the filtered solution in the petri dish was evaporated in BINDER ED 115 at 60 °C for 24 h and the solid polymer was obtained. Experimental design and optimization were accomplished by response surface methodology (RSM) using Design-Expert® 12 software (Stat-Ease).
2.3 Analysis
Catechol concentration was determined using Shimadzu Prominence LC-20AD HPLC system. The system was equipped with SIL 20-A auto sampler, CBM-20Alite system control, LC-20AD gradient pump, DGU-20A5 degasser, CTO-20A column oven, SPD-20A UV/Vis detector. Gradient mobile phase was acetonitrile and water (50:50%) with 1 mL/min flow rate. The injection volume was 30 µL, the column temperature was 30 °C and the UV wavelength was set at 254 nm. The samples were taken from the reaction medium and catechol concentration was determined by using a calibration curve with the regression coefficient R2 = 0.993. The structural and morphological properties of polycatechol were characterized by FTIR, SEM and EDX analysis. Before SEM analysis polycatechol samples were coated with platinum by Quorum Q150R to increase electrical conductivity. Then the coated samples were placed in Zeiss SUPRA 55 for SEM analysis. An electron beam was sent to polycatechol samples to obtain SEM images at different magnifications. EDX analysis was used to identify the elemental composition of polycatechols. FTIR spectra used to determine the functional groups of polycatechols in the range of 400–4000 cm−1. 0.1 g polycatechol samples were placed in a Perkin Elmer FTIR/FIR/NIR Spectrometer Frontier ATR to obtain the spectrums.
3 Results and Discussions
Cyclic voltammograms (CV) were obtained at open circuit potential in the potential range from -0.3 V to + 1.5 V for 100 cycles at scan rate of 50 mV/s in APS electrolyte solution in electrochemical polymerization of catechol. APS was used as both polymerization initiator (oxidizing reagent) and supporting electrolyte. Petran et al. (2020) was also investigated the oxidative polymerization of catechol with APS as oxidizing reagent in the presence of Tris-buffer. The electrochemical polymerization of catechol proceeds in one step at low pH values, and in two steps at high pH values. These two steps involve the oxidation of the catechol followed by its polymerization (Cortés et al., 2001; Kong & Mu, 2003; Pourghobadi et al., 2020). Kong and Mu (2003) observed a peak at 0.94 V that caused polymerization of catechol in a three-electrode system using a rotating disc electrode from pH 1–10 at potential of 0–1.2 V. The authors also stated that the electrode type can affect the oxidation and polymerization potential of catechol.
Figure 1 shows the effect of catechol concentration on electrochemical polymerization of catechol where the current density increased with increasing catechol concentration. Aktaş and Tanyolaç (2003) synthesized polycatechol using oxidative polymerization by laccase enzyme and they found that increasing catechol concentration enhanced the initial reaction rate. Sayyah et al. (2010) also reported that the current density increased up to 0.06 M p-phenylenediamine concentration in their electropolymerization study of p-phenylenediamine between concentration of 0.02–0.07 M. Yalçinkaya et al. (2008) synthesized poly (pyrole-co–o-toluidine) using Pt electrode. It was found that the current density increased with the increase of scan rate and increase of o-toluidine concentration at 0.3 M oxalic acid. Kiss et al. (2020) investigated the electrochemical polymerization of phenol in mesityl oxide using platinum and glassy carbon electrode. It was determined that the current density increased with increasing the number of cycles. Similarly, Bao et al. (2010) reported that the current density increased with increasing scan rate at 0.1 M phenol concentration in the study of electrochemical polymerization of phenol using 304 SS electrode. The reason for this situation may be related to the increasing monomer concentration and the diffusion process on the electrode surface (Liu & Oliveira, 2007). Figure 2 shows increasing current density with increasing APS concentration. Tiwari (2007) investigated gum arabic‐graft‐polyaniline for sensor applications and used APS as an initiator in experimental studies. It was stated that the grafting ability of PANI increased with increasing APS concentration. As it can be seen in Fig. 3 that the current density decreased with increasing reaction temperature. Khezri et al. (2021) studied cyclic voltammetry and potentiodynamic polarization of chalcopyrite. The researchers found that raising the reaction temperature from 60 °C to 90 °C led to a drop in anodic current density. Kalu et al. (2001) determined that the anodic current density decreased with increasing reaction temperature in the cyclic voltammetry study of nickel oxide. Qu et al. (2007) investigated the effect of ethylene diamine tetra acetic acid (EDTA) on corrosion of cold rolled steel in the presence of benzotriazole in hydrochloric acid. EDTA was used as anodic type inhibitor, EDTA and benzotriazole (BTA) were used as mixed type inhibitors in the study. The researchers reported that the inhibition activities increased with increasing reaction temperature.
Electrochemical properties of the polycatechol synthesized by electrochemical polymerization are given Table 1. In Table 1, E(i=o), Rp, and icorr are referring to corrosion potential (mV), polarization resistance (ohm.cm2), and corrosion current (mA/cm2), respectively. Polarization measurements are an important research tool in the study of different electrochemical phenomena. These measurements fit well with studies of the reaction mechanism and kinetics of corrosion and metal deposition (Stern & Geary, 1957). The polarization resistance of polycatechol decreased with increasing APS concentration. The corrosion current of polycatechol increased with increasing catechol concentration. The increase in the reaction temperature led to an increase in the corrosion current of the polycatechol. Salasi et al. (2007) investigated the electrochemical behavior of 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP) and sodium silicate on carbon steel. The researchers found that the polarization resistance for HEDP and sodium silicate increased over time. Öncül et al. (2011) investigated the inhibition of corrosion of stainless steel (SS) by poly-N-vinylimidazole (NVI) and N-vinylimidazole (PVNI). It was stated that the corrosion current density decreases as the thickness increases on the SS electrode.
Response surface methodology with central composite design (CCD) was used to determine the effect of operating parameters on catechol removal and to identify the optimum conditions. The effect of catechol concentration (2–10 g/L), APS concentration (2–10 g/L) and reaction temperature (20–60 °C) was investigated on separation of catechol by sequential electrochemical and chemical multi-polymerization. The effect of parameters on catechol removal are given in Table 2 and in Figs. 4, 5, 6. The quadratic response surface models of CCD in Eqs. 1–3 were found to be acceptable for the experimental design according to R2adj, F-values, and P-values. In Eqs. 1–3, x1, x2 and x3 were the factors of catechol concentration, APS concentration, and reaction temperature, respectively; y1, y2 and y3 are the responses of catechol removal, polymer conversion, and reaction selectivity respectively.
Polymer conversion (P) was defined as the conversion of monomer to polymer. The polymer conversion percent was calculated by Eq. 4. In Eq. 4, Ct and Co refer to the final catechol concentration after the completion of chemical polymerization and the initial catechol concentration, respectively.
It is well known that the oxidative polymerization of catechol involves the steps of the oxidation of the catechol followed by its polymerization (Cortés et al., 2001; Kong & Mu, 2003; Pourghobadi et al., 2020). Therefore, reaction selectivity could be defined in Eq. 5 as the ratio of the concentration of polycatechol obtained in electrochemical polymerization, to the concentration of reaction intermediates and by-products (redox products, dimer, trimer, etc.).
In Eq. 5, A and B refer to the polycatechol concentration, and the concentration of reaction intermediates and by-products, respectively. The amount of reaction intermediates and by-products was evaluated by component mass balance from HPLC chromatograms. The application of the method was reported elsewhere (Körbahti & Alaca, 2021; Körbahti & Taşyürek, 2015, 2016).
The effect of catechol concentration, APS concentration and reaction temperature on catechol removal is shown in Fig. 4A and B. At constant catechol concentration increasing APS concentration increased catechol removal. The highest catechol removal was achieved 81% at an APS concentration of 8–10 g/L and a catechol concentration of 2–4 g/L. At 6–8 g/L APS concentration and 30–40 °C reaction temperature, the maximum removal of catechol was obtained as 70%. Aktaş (2005) synthesized polycatechol by biopolymerization at various reaction temperatures and pH levels. The optimum reaction temperature and pH were found using RSM as 31 °C and 4.87, respectively. Yahiaoui et al. (2011) studied electrochemical degradation of phenol using Pb/PbO2 electrode. The researchers investigated the effects of reaction temperature, current density, and stirring speed on electrochemical degradation of phenol. The optimum result was found at reaction temperature of 60 °C, current density of 19.66–25 mA/cm2, and stirring speed of 600 rpm. The phenol degradation was obtained 71% under optimal conditions.
Figure 5A and B shows the effect of catechol concentration, APS concentration, and reaction temperature on polymer conversion. The highest polymer conversion was obtained 22% at a concentration of 4–6 g/L catechol and 6–8 g/L APS, respectively. At an APS concentration of 8–10 g/L and a reaction temperature of 50–60 °C, the maximum polymer conversion was achieved as 23%. Kumar et al. (2000) synthesized phenol/formaldehyde resin by chemical methods. It was specified that increasing the reaction temperature increases the reaction rate. Tahar and Savall (2009b) investigated electropolymerization of phenol using vitreous carbon electrode at reaction temperature of 25–85 °C. They reported that the current density increased as the reaction temperature increased. In another study of Tahar et al. (2009), the electrochemical polymerization of phenol in aqueous solutions using Ta/PbO2 electrode was investigated. The polymer conversion was obtained as 3%, 4%, 13%, and 15% at 25 °C, 35 °C, 50 °C, and 65 °C reaction temperature, respectively. The conversion of phenol monomer to polymer increased with increasing anodic current density, reaction temperature, and phenol concentration.
The effect of reaction temperature, APS concentration, and catechol concentration on reaction selectivity can be seen in Fig. 6A and B. The highest reaction selectivity was obtained 43% at in 2–4 g/L catechol and 8–10 g/L APS concentration, respectively. At APS concentration 8–10 g/L and reaction temperature 50–60 °C, the maximum reaction selectivity was achieved as 47%. By increasing catechol concentration from 2 to 10 g/L catechol removal decreased from 78.01% to 66.15%, both polymer conversion and reaction selectivity increased up to 6 g/L catechol concentration, and then both decreased. It was reported that catechol can be rapidly oxidized by ambient oxygen to the corresponding semiquinone or quinone (Cortés et al., 2001). The increase in catechol concentration and the decrease in the reaction selectivity indicate the increase in the amount of reaction intermediates and by-products. Pourghobadi et al. (2020) also determined that the peak current ratio decreased and the dimerization rate increased with increasing pH value in electropolymerization of catechol on graphite electrode at different pH values. These results clearly indicate that the complete polymer conversion could not be achieved and somehow oxidation products also obtain depending on the operating conditions. Considering that the operating conditions have a substantial influence on the reaction, although higher catechol concentrations favor the catechol oxidation it was concluded that the catechol concentrations below 6 g/L was more suitable for the polymer conversion due to formation of reaction intermediates and by-products. Reaction selectivity increased with increasing APS concentration when the reaction temperature is constant. The increase in the reaction selectivity reflects that the amount of reaction intermediates and by-products decreases as APS concentration increases. Increasing the APS concentration from 2 g/L to 10 g/L catechol removal increased from 65.16% to 77.63%, and the reaction selectivity increased from 1.30% to 37.57%. Higher APS concentrations promoted polymer conversion rather than the formation of reaction intermediates and by-products. At constant catechol concentration and APS concentration, increasing temperature from 20 °C to 60 °C increased the catechol removal from 61.62% to 68.95%, however both polymer conversion and reaction selectivity were decreased. This result indicates that the low temperatures such as below 40 °C favor the polymer conversion rather than the formation of reaction intermediates and by-products.
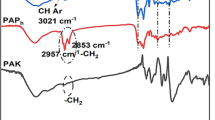
The functional groups of polycatechol were identified using the FTIR spectra shown in Figs. 7, 8, 9. The broad peaks between 3600–3200 cm−1 indicates O–H stretching. The peaks around 3000–2840 cm−1, 1650–1566 cm−1, 1450–1375 cm−1, 1420–1330 cm−1, and 1275–1200 cm−1 vibration corresponding to C-H stretching, C–C stretching, C-H bending, O–H bending, and C-O stretching, respectively. The peaks at 860 cm−1 and 780 cm−1 could be defined as C-H bending. These findings are very similar to the FTIR spectra of polycatechol and polyphenol in aqueous media (Aktaş et al., 2003; Zhang et al., 2012a). The peak at 1187 cm−1 between 1250–1020 cm−1 could be attributed to C-N stretching due to the use of APS as both polymerization initiator and supporting electrolyte. New peak formations were observed and FTIR spectra became more complicated with increasing catechol concentration. Since there was no evidence of the formation of ether bonds according to FTIR spectra, the polymerization mechanism of catechol can be suggested by the formation of C–C bonds between catechol molecules. Similar result was also reported by Jha and Halada (2011). Increasing the catechol concentration from 2 g/L to 10 g/L, it was observed that the peak at 2981 cm−1 formed a very sharp downward, and the vibrations at 3700 cm−1 and 2300 cm−1 increased. The vibrations at 2980 cm−1 increased when the catechol concentration was increased from 2 g/L to 10 g/L. It was observed that increasing the catechol concentration from 6 g/L to 10 g/L increased the vibrations between 2300–2400 cm−1. The vibrations around 1500 cm−1 increased by increasing the catechol concentration from 2 g/L to 10 g/L. The peak at 2889 cm−1 appeared at 10 g/L catechol concentration when increasing catechol concentration from 2 g/L to 10 g/L. It was observed that as the APS concentration increased from 2 g/L to 10 g/L the intensity of the vibrations at 3700 cm−1 increased. The intensity of the peak observed at 1689 cm−1 decreased with increasing APS concentration from 2 g/L to 10 g/L and disappeared at 10 g/L concentration. The peak at 2982 cm−1 formed at 6 g/L APS concentration and sharpened with increasing the APS concentration from 6 g/L to 10 g/L. Increasing the reaction temperature from 40 °C to 60 °C a new peak was formed at 2923 cm−1. The peak at 2982 cm−1 can be seen at 20 °C and 40 °C reaction temperatures that was disappeared at 60 °C when the reaction temperature increased from 40 °C to 60 °C. According to EDX analysis, C/O atomic ratio decreased with increasing catechol concentration from 2 g/L to 6 g/L; and it was increased with the increase of APS concentration from 2 g/L to 6 g/L. As the reaction temperature increased from 20 °C to 40 °C the C/O ratio decreased as well. Sun et al. (2013) synthesized polycatechol by enzymatic polymerization. FTIR peaks at 3451–3326 cm−1, 1487 cm−1, and 1260–1050 cm−1 were corresponding to phenolic O–H vibration, ortho-substitute benzene ring, and C–O–C stretching, respectively. Long et al. (2008) synthesized m-cresol/phenol and FTIR peaks at 3460 cm−1, 2970 cm−1, and 2780 cm−1 were corresponding to O–H stretching, C-H stretching, and CH3 respectively. The peaks at 1180 cm−1, 1020 cm−1 and 1700 cm−1 belong to C–O–C stretching. Li et al. (2010) synthesized polyphenol by enzymatic polymerization. FTIR peaks at 3220 cm−1, 1370–1205 cm−1, and 1488–1450 cm−1 were corresponding to -OH group, C-O group, and C–C aromatic bond, respectively. Zhang et al. (2012b) synthesized polyphenol by electrochemical method and FTIR peaks located at 3424 cm−1, 1646 cm−1, 1608 cm−1, 1409 cm−1, and 1211 cm−1 were corresponding to -OH stretching, aromatic C–C stretching, and C-O stretching. Garces et al. (2000) synthesized polyphenol by electrochemical polymerization and FTIR peaks at 700–850 cm−1, 900 cm−1, and 1150 cm−1 were reported as C-H deformation, C-O bond, and = C–O–C = vibration respectively.
In Figs. 10, 11, 12, the morphology of polycatechol was determined by SEM images at 25,000 × magnification. In Fig. 10, polycatechol showed a bulky and heterogeneous structure at 6 g/L APS concentration and 2 g/L catechol concentration at 40 °C reaction temperature. The polymer chain structure has started at 6 g/L APS concentration and 6 g/L catechol concentration at reaction temperature of 40 °C. The chain structure of the polymer was distorted and a heterogeneous structure was obtained with a catechol concentration of 10 g/L. Polycatechol has crystalline and bulky structure with an APS concentration of 2 g/L as can be seen in Fig. 11. Polycatechol begins to form in a chain structure with APS concentration of 6 g/L and it has homogeneous structure with APS concentration of 10 g/L. Increasing APS concentration showed positive effect on the formation of the polymer chain. Chen et al. (2019) studied electrochemical polymerization of 4,4′-thiobis-phenol (TDP) in alkaline solution and it was observed in SEM analysis that the polyTDP film has a thick homogeneous structure. Figure 12 shows that polycatechol has heterogeneous and bulk structure at a reaction temperature of 20 °C. Polycatechol has chain structure with the reaction temperature of 40 °C whereas the chain structure of the polycatechol was destroyed and a heterogeneous structure obtained with the reaction temperature of 60 °C. Li et al. (2010) also reported the fractured surfaces in SEM images of the synthesized polyphenol. Santos et al. (2019), investigated electropolymerization of phenol and aniline derivatives in the presence of 0.5 M sulfuric acid. It was determined from the SEM analysis that phenol has specific morphological character on each polymeric film in the potential range of 0.3–0.8 V on the graphite electrode.
In Fig. 13, the shaded region shows the process efficiency for the highest polymer conversion by multi-polymerization of catechol with catechol removal higher than 75% and reaction selectivity higher than 25%. According to the findings of this study, the optimum values of the parameters were determined as catechol concentration, 4 g/L; APS concentration, 9.5 g/L; and reaction temperature, 30 °C with 100 cycles at 50 mV/s of electrochemical polymerization and 72 h of chemical polymerization. An experiment carried out at these optimum conditions and the electrochemical properties of polycatechol were found as E(i=o), Rp, icorr, and corrosion rate as 181.6 mV, 112.95 Ω.cm2, 0.7682 mA/cm2, and 8.985 mm/year, respectively. At the end of multi-polymerization; catechol removal, polymer conversion, and reaction selectivity were obtained under optimum conditions as 72.89%, 25.80%, and 54.80%, respectively.
4 Conclusions
The separation of catechol from aqueous solutions by sequential electrochemical and chemical multi-polymerization was successfully accomplished for the abatement of an organic pollutant. The polymerization mechanism of catechol can be suggested by the formation of C–C bonds between catechol molecules since there was no evidence of the formation of ether bonds according to FTIR spectra. The operating conditions had a substantial influence on the reaction. New peak formations were observed and FTIR spectra became more complicated with increasing catechol concentration. Although higher catechol concentrations favor the catechol oxidation, catechol concentrations below 6 g/L was more suitable for the polymer conversion due to formation of reaction intermediates and by-products. On the other hand, increasing APS concentration showed positive effect on polymer chain formation. Catechol removal, polymer conversion, and reaction selectivity increased with increasing APS concentration. High APS concentrations promoted polymer conversion rather than the formation of reaction intermediates and by-products. Increasing reaction temperature increased the catechol removal, however both polymer conversion and reaction selectivity were decreased. This result indicated that the low temperatures such as below 40 °C favor the polymer conversion rather than the formation of reaction intermediates and by-products. It was determined from the SEM images that a polymeric structure was obtained as a crystalline and heterogeneous structure with the increasing APS concentration. The polymers changed from a bulk and heterogeneous structure to a homogeneous structure with increasing reaction temperature. The optimum operating conditions were determined as 4 g/L catechol concentration, 9.5 g/L APS concentration, 30 °C reaction temperature with 100 cycles at 50 mV/s of electrochemical polymerization and 72 h of chemical polymerization. Under optimum conditions, catechol removal, polymer conversion, and reaction selectivity were obtained as 72.89%, 25.80%, and 54.80%, respectively. The results of this study indicates that electrochemical polymerization coupling with chemical polymerization as a multi-polimerization process designed to remove catechol in high concentrations from aqueous solutions and to prevent environmental pollution will be promising.
Data availability
The datasets supporting the conclusions of this article are included within the article.
References
Abdelwahab, O., Amin, N. K., & Ashtoukhy, E. S. Z. E. (2009). Electrochemical removal of phenol from oil refinery wastewater. Journal of Hazardous Materials, 163, 711–716. https://doi.org/10.1016/j.jhazmat.2008.07.016
Aktaş, N. (2005). Optimization of biopolymerization rate by response surface methodology (RSM). Enzyme Microb Tech, 37, 441–447. https://doi.org/10.1016/j.enzmictec.2005.03.010
Aktaş, N., & Tanyolaç, A. (2003). Kinetics of laccase-catalyzed oxidative polymerization of catechol. Journal of Molecular Catalysis. B, Enzymatic, 22, 61–69. https://doi.org/10.1016/S1381-1177(03)00007-9
Aktaş, N., Şahiner, N., Kantoğlu, Ö., Salih, B., & Tanyolaç, A. (2003). A biosynthesis and characterization of laccase catalyzed poly (Catechol). J Polym Environ, 11, 123–128. https://doi.org/10.1023/A:1024639231900
Baby, M., Devapal, D., & Maniyeri, S. C. (2022). Mussel-inspired design of a catechol-polydimethyl siloxane covalent hybrid polymer for atomic oxygen resistant coating application. Surface & Coatings Technology, 448, 128886. https://doi.org/10.1016/j.surfcoat.2022.128886
Bao, L., **ong, R., & Wei, G. (2010). Electrochemical polymerization of phenol on 304 stainless steel anodes and subsequent coating structure analysis. Electrochimica Acta, 55, 4030–4038. https://doi.org/10.1016/j.electacta.2010.02.052
Caetano, M., Valderrama, C., Farran, A., & Cortina, J. L. (2009). Phenol removal from aqueous solution by adsorption and ion exchange mechanisms onto polymeric resins. Journal of Colloid and Interface Science, 338, 402–409. https://doi.org/10.1016/j.jcis.2009.06.062
Chen, H., Yao, J., Wang, F., Zhou, Y., Chen, K., Zhuang, R., Choi, M. M. F., & Zaray, G. (2010). Toxicity of three phenolic compounds and their mixtures on the gram-positive bacteria Bacillus subtilis in the aquatic environment. Science of the Total Environment, 408, 1043–1049. https://doi.org/10.1016/j.scitotenv.2009.11.051
Chen, L., **e, L., Jiang, Y., Meng, Q., & Huang, X. (2019). Electrochemical polymerization of 4,4′-thiobis-phenol in alkaline solution and properties of polymer. Ionics, 25, 4493–4498. https://doi.org/10.1007/s11581-019-02998-3
Cortés, S., Francioso, O., Ramos, J. V. G., Ciavatta, C., & Gessa, C. (2001). Catechol polymerization in the presence of silver surface. Colloid Surface A, 176, 177–184. https://doi.org/10.1016/S0927-7757(00)00630-0
Davis, J., Vaughan, D. H., & Cardosi, M. F. (1998). Modification of catechol polymer redox properties during electropolymerization in the presence of aliphatic amines. Electrochimica Acta, 43, 291–300. https://doi.org/10.1016/S0013-4686(97)00086-8
Faure, E., Daudre, C. F., Jerome, C., Lyskawa, J., Fournier, D., Woisel, P., & Detrembleur, C. (2013). Catechols as versatile platforms in polymer chemistry. Progress in Polymer Science, 38, 236–270. https://doi.org/10.1016/j.progpolymsci.2012.06.004
Fomo, G., Waryo, T., Feleni, U., Baker, P., & Iwuoha, E. (2019). Electrochemical Polymerization. Functional Polymers. Polymers and Polymeric Composites: A Reference Series., Springer 1–28. https://doi.org/10.1007/978-3-319-92067-2_3-1.
Garces, P., Lapuente, R., Andion, L. G., Cases, F., Morallon, E., & Vazquez, J. L. (2000). Electropolymerization of phenol on carbon steel and stainless steel electrodes in carbonate aqueous medium. Polymer Journal, 32, 623–628. https://doi.org/10.1295/polymj.32.623
Ge, M. Q., Wang, X. Y., Ren, N., Tong, G. S., & Zhu, X. Y. (2023). Multi-polymerization: from simple to complex. Chinese Journal of Polymer Science, 41, 179–186. https://doi.org/10.1007/s10118-022-2836-8
Jha, P. K., & Halada, G. P. (2011). The catalytic role of uranyl in formation of polycatechol complexes. Chemistry Central Journal, 5, 1–7. https://doi.org/10.1186/1752-153x-5-12
Kalu, E. E., Nwoga, T. T., Srinivasan, V., & Weidner, J. W. (2001). Cyclic voltammetric studies of the effects of time and temperature on the capacitance of electrochemically deposited nickel hydroxide. Journal of Power Sources, 92, 163–167. https://doi.org/10.1016/S0378-7753(00)00520-6
Kazemi, P., Peydayesh, M., Bandegi, A., Mohammadi, T., & Bakhtiari, O. (2014). Stability and extraction study of phenolic wastewater treatment by supported liquid membrane using tributyl phosphate and sesame oil as liquid membrane. Chemical Engineering Research and Design, 92, 375–383. https://doi.org/10.1016/j.cherd.2013.07.023
Khezri, M., Rezai, B., Abdollahzadeh, A. A., Wilson, B. P., Molaeinasab, M., & Lundström, M. (2021). Cyclic voltammetry and potentiodynamic polarization studies of chalcopyrite concentrate in glycine medium. Transactions of Nonferrous Metals Society of China, 31, 545–554. https://doi.org/10.1016/S1003-6326(21)65516-4
Khoo, S. B., & Zhu, J. (1999). Poly(catechol) film modifed glassy carbon electrode for ultratrace determination of cerium (III) by differential pulse anodic strip**. Voltammetry Electroanalysis, 11, 546–552. https://doi.org/10.1002/(SICI)1521-4109(199906)11:8%3c546::AID-ELAN546%3e3.0.CO;2-Z
Kiss, L., Kovacs, F., Li, H., Kiss, A., & Mate, S. K. (2020). Electrochemical polymerization of phenol on platinum and glassy carbon electrodes in mesityl oxide. Chemical Physics Letters, 754–137642. https://doi.org/10.1016/j.cplett.2020.137642
Kong, Y., & Mu, S. L. (2003). Investigation on the electrochemical polymerization of catechol by means of rotating ring-disk electrode. Chinese Journal of Chemistry, 21, 630–637. https://doi.org/10.1002/cjoc.20030210611
Kong, Y., Mu, S. L., & Mao, B. W. (2002). Synthesis of polycatechol with electrochemical activity and its properties. Chinese Journal of Polymer Science, 20, 517–524.
Körbahti, B. K., & Alaca, S. (2021). Electrochemical degradation of tetracycline antibiotic with boron-doped diamond electrodes and effect of parameters on removal of reaction intermediates. Desalination and Water Treatment, 236, 285–299. https://doi.org/10.5004/dwt.2021.27716
Körbahti, B. K., & Taşyürek, S. (2015). Electrochemical oxidation of ampicillin antibiotic at boron-doped diamond electrodes and process optimization using response surface methodology. Environmental Science and Pollution Research, 22, 3265–3278. https://doi.org/10.1007/s11356-014-3101-7
Körbahti, B. K., & Taşyürek, S. (2016). Electrochemical oxidation of sulfadiazine antibiotic using boron-doped diamond anode: application of response surface methodology for process optimization. Desalination and Water Treatment, 57, 2522–2533. https://doi.org/10.1080/19443994.2015.1043590
Kumar, R. N., Nagarajan, R., Fun, F. C., & Seng, P. L. (2000). Effect of process variables on the exothermicity during the production of phenol-formaldehyde resins-modeling by response surface methodology. European Polymer Journal, 36, 2491–2497. https://doi.org/10.1016/S0014-3057(00)00016-1
Li, Z., Renneckar, S., & Barone, J. R. (2010). Nanocomposites prepared by in situ enzymatic polymerization of phenol with TEMPO-oxidized nanocellulose. Cellulose, 17, 57–68. https://doi.org/10.1007/s10570-009-9363-4
Li, J., Li, T., Zeng, Y., Chen, C., Guo, H., Lei, B., Zhang, P., & Feng, M. G. (2023). A novel sol-gel coating via catechol/lysine polymerization for long-lasting corrosion protection of Mg alloy AZ31. Colloid Surface A, 656, 130361. https://doi.org/10.1016/j.colsurfa.2022.130361
Liu, A. S., & Oliveira, M. A. S. (2007). Electrodeposition of polypyrrole films on aluminum from tartrate aqueous solution. Journal of the Brazilian Chemical Society, 18, 143–152. https://doi.org/10.1590/S0103-50532007000100016
Long, D. H., Zhang, J., Yang, J. H., Hu, Z. J., Li, T. Q., Cheng, G., Zhang, R., & Ling, L. C. (2008). Preparation and microstructure control of carbon aerogels produced using m-cresol mediated sol-gel polymerization of phenol and furfural. New Carbon Materials, 23, 165–170. https://doi.org/10.1016/S1872-5805(08)60020-7
Nemtollahi, D., & Golabi, S. M. (1996). Electrochemical study of catechol and 4-methylcatechol in methanol. Application to the electro-organic synthesis of 4,5-dimethoxy-and 4-methoxy-5-methyl-o-benzoquinone. Journal of Electroanalytical Chemistry, 405, 133–140. https://doi.org/10.1016/0022-0728(95)04402-7
Öncül, A., Çoban, K., Sezer, E., & Şenkal, B. F. (2011). Inhibition of the corrosion of stainless steel by poly-N-vinylimidazole and N-vinylimidazole. Progress in Organic Coatings, 71, 167–172. https://doi.org/10.1016/j.porgcoat.2011.02.001
Petran, A., Popa, A., Hadade, N. D., & Liebscher, J. (2020). New Insights into catechol oxidation-application of ammonium peroxydisulfate in the presence of arylhydrazines. Chemistry Select., 5, 9523–9530. https://doi.org/10.1002/slct.202002370
Pourghobadi, R., Nematollahi, D., Baezzat, R., Alizadeh, S., & Goljani, H. (2020). Electropolymerization of catechol on wireless graphite electrode. Unusual cathodic polycatechol formation. Journal of Electroanalytical Chemistry, 866, 114180. https://doi.org/10.1016/j.jelechem.2020.114180
Qian, G., Yang, C., Pu, W., Huang, J., & Zhang, J. (2007). A novel polycatechol/platinum composite film prepared by electrochemical synthesis. Synthetic Met, 157, 448–453. https://doi.org/10.1016/j.synthmet.2007.05.008
Qiu, B., Wang, J., Li, Z., Wang, X., & Li, X. (2020). Influence of acidity and oxidant concentration on the nanostructures and electrochemical performance of polyaniline during fast microwave-assisted chemical polymerization. Polymers, 12(310), 1–14. https://doi.org/10.3390/polym12020310
Qu, Q., Jiang, S., Bai, W., & Li, L. (2007). Effect of ethylenediamine tetraacetic acid disodium on the corrosion of cold rolled steel in the presence of benzotriazole in hydrochloric acid. Electrochimica Acta, 52, 6811–6820. https://doi.org/10.1016/j.electacta.2007.04.114
Raj, P., Patel, M., & Karamalidis, A. K. (2023). Chemically modified polymeric resins with catechol derivatives for adsorption, separation and recovery of gallium from acidic solutions. Journal of Environmental Chemical Engineering, 11, 110790. https://doi.org/10.1016/j.jece.2023.110790
Ribeiro, G. H., Vilarinho, L. M., Ramos, T. S., Bogado, A. L., & Dinelli, L. R. (2015). Electrochemical behavior of hydroquinone and catechol at glassy carbon electrode modified by electropolymerization of tetraruthenated oxovanadium porphyrin. Electrochimica Acta, 176, 394–401. https://doi.org/10.1016/j.electacta.2015.06.139
Sadaba, N., Salsamendi, M., Casado, N., Zuza, E., Munoz, J., Sarasua, J. R., Mecerreyes, D., Mantione, D., Detrembleur, C., & Sardon, H. (2018). Catechol end-functionalized polylactide by organocatalyzed ring-opening polymerization. Polymers, 10(2), 155. https://doi.org/10.3390/polym10020155
Saha, R., & Mukhopadhyay, M. (2022). Electrochemical analysis of catechol polymerization in presence of Trametes versicolor laccase and the mediator ABTS. Enzyme and Microbial Technology, 152, 109934. https://doi.org/10.1016/j.enzmictec.2021.109934
Salasi, M., Shahrabi, T., Roayaei, E., & Aliofkhazraei, M. (2007). The electrochemical behaviour of environment-friendly inhibitors of silicate and phosphonate in corrosion control of carbon steel in soft water media. Materials Chemistry and Physics, 104, 183–190. https://doi.org/10.1016/j.matchemphys.2007.03.008
Santos, C. C., Pimenta, T. C., Thomasini, R. L., Verly, R. M., Franco, D. L., & Ferreira, L. F. (2019). Electropolymerization of phenol and aniline derivatives: synthesis, characterization and application as electrochemical transducers. Journal of Electroanalytical Chemistry, 846, 113163. https://doi.org/10.1016/j.jelechem.2019.05.045
Sayyah, S. M., El-Rehim, S. S. A., El-Deeb, M. M., Kamal, S. M., & Azooz, R. E. (2010). Electropolymerization of p-Phenylenediamine on Pt-Electrode from Aqueous Acidic Solution: Kinetics, Mechanism, Electrochemical Studies, and Characterization of the Polymer Obtained. Journal of Applied Polymer Science, 117, 943–952. https://doi.org/10.1002/app.31476
Shakir, K., Ghoneimy, H. F., Elkafrawy, A. F., Beheir, S. G., & Refaat, M. (2008). Removal of catechol from aqueous solutions by adsorption onto organophilic-bentonite. Journal of Hazardous Materials, 150, 765–773. https://doi.org/10.1016/j.jhazmat.2007.05.037
Stern, M., & Geary, A. L. (1957). Electrochemical polarization: I. a theoretical analysis of the shape of polarization curves. Journal of the Electrochemical Society, 104, 56–63. https://doi.org/10.1149/1.2428496
Sun, X., Bai, R., Zhang, Y., Wang, Q., Fan, X., Yuan, J., Cui, L., & Wang, P. (2013). Laccase-catalyzed oxidative polymerization of phenolic compounds. Applied Biochemistry and Biotechnology, 171, 1673–1680. https://doi.org/10.1007/s12010-013-0463-0
Tahar, N. B., & Savall, A. (2009a). Electrochemical removal of phenol in alkaline solution. Contribution of the anodic polymerization on different electrode materials. Electrochimica Acta, 54, 4809–4816. https://doi.org/10.1016/j.electacta.2009.03.086
Tahar, N. B., & Savall, A. (2009b). Electropolymerization of phenol on a vitreous carbon electrode in alkaline aqueous solution at different temperatures. Electrochimica Acta, 55, 465–469. https://doi.org/10.1016/j.electacta.2009.08.040
Tahar, N. B., Abdelhedi, R., & Savall, A. (2009). Electrochemical polymerisation of phenol in aqueous solution on a Ta/PbO2 anode. Journal of Appled Electrochemistry, 39, 663–669. https://doi.org/10.1007/s10800-008-9706-0
Tiwari, A. (2007). Gum arabic-graft-polyaniline: an electrically active redox biomaterial for sensor applications. J Macromol Sci A, 44, 735–745. https://doi.org/10.1080/10601320701353116
Yahiaoui, I., Benissad, A., Fourcade, F., & Amrane, A. (2011). Response surface methodology for the optimization of the electrochemical degradation of phenol on Pb/PbO2 electrode. Environmental Progress & Sustainable Energy, 31, 515–523. https://doi.org/10.1002/ep.10572
Yalçinkaya, S., Tüken, T., Yazici, B., & Erbil, M. (2008). Electrochemical synthesis and characterization of poly(pyrrole-co-o-toluidine). Progress in Organic Coatings, 63, 424–433. https://doi.org/10.1016/j.porgcoat.2008.07.002
Yan, G., Chen, G., Peng, Z., Shen, Z., Tang, X., Sun, Y., Zeng, X., & Lin, L. (2021). The cross-linking mechanism and applications of catechol-metal polymer materials. Advanced Materials Interfaces, 8, 2100239. https://doi.org/10.1002/admi.202100239
Yao, Y., Zhou, R., Yu, Y., Chen, J., Du, C., Zhang, Y., Long, T., Wan, L., Wang, Q., & **e, M. (2023). Solvent-freely polymerizing catechol and paraformaldehyde to nitrogen-rich carbon for high-volumetric-performance supercapacitor. Chemical Engineering Journal, 472, 144905. https://doi.org/10.1016/j.cej.2023.144905
Zhang, L., Zhao, W., Ma, Z., Nie, G., & Cui, Y. (2012a). Enzymatic polymerization of phenol catalyzed by horseradish peroxidase in aqueous micelle system. European Polymer Journal, 48, 580–585. https://doi.org/10.1016/j.eurpolymj.2011.12.011
Zhang, W., Bao, L., Zhang, X., He, J., & Wei, G. (2012b). Electropolymerization treatment of phenol wastewater and the reclamation of phenol. Water Environment Research, 84, 2028–2036. https://doi.org/10.2175/106143012X13415215906771
Acknowledgements
The authors gratefully acknowledge Mersin University Scientific Research Projects Center (MEÜ BAP) for the financial support.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by Mersin University Scientific Research Projects Center (MEÜ BAP) with Grant No. 2015-TP2-1039.
Author information
Authors and Affiliations
Contributions
OCA: conceptualization, methodology, formal analysis, investigation, writing original draft. BKK: conceptualization, methodology, writing reviewing and editing, visualization, supervision, project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Altıncı, O.C., Körbahti, B.K. Sequential Electrochemical and Chemical Multi-Polymerization of Catechol for Abatement of Environmental Pollutants. Water Air Soil Pollut 235, 488 (2024). https://doi.org/10.1007/s11270-024-07306-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07306-y