Abstract
Cadmium has appeared as an important element for certain types of solar cells and rechargeable batteries. It is possible that there will be a large increase in demand for technical cadmium in the future. This is in conflict with environmental policies for phasing out cadmium from any technical use worldwide because of its great toxicity to humans. Cadmium toxicity is on par with that of mercury, and data suggests that cadmium exposure has no safe lower limit. There is no shortage of cadmium to extract, and no shortage from lack of cadmium available in the future zinc flow is to be expected. There is a global treaty to ban it from all use. The Integrated Assessment Model WORLD7 was used to assess different aspects of the supply of cadmium to society. It would be possible to produce at least 250,000 tons/year; in reality, the 2023 production is about 24,000 tons/year. The price is about 3500–4500 $/ton and is volatile. Because there is a United Nations agreed global policy to phase out cadmium from all use, demand for cadmium will soon not be met, and there will be an actual shortage of cadmium for any use, including photovoltaic technologies and semiconductors. This is good news for nature, but bad news for the CdTe and CIGS types of photovoltaic panels. It is estimated that only 25% of the planned future capacity may not be available unless good substitutes for cadmium can be found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cadmium, a very toxic element, is on the way to being phased out of all global use, according to the UN/ECE-LRTAP (UN/ECE, 1998). Cadmium use is banned by the UN globally at present. It can only be used as long as full recovery can be secured under special permission (Bakker et al., 1998; de Vries et al., 1998a, 1998b; Sverdrup, 2001; Sverdrup & Ashmore, 2001; van der Voet et al., 2013). It is considered to be of great importance for human health and environmental impacts that cadmium is phased out of all use in human society. The UN/ECE heavy metals protocol was long in preparation and took about 10 years to develop (1988–1998) and 25 years from first beginning to final implementation (1988–2013). A global ban was not agreed until 2015 and was not implemented until after 2020 (UN/ECE-LRTAP 1998, Balali-Mood et al., 2021).
Cadmium has appeared as an important element for certain types of solar cells and rechargeable batteries. New technologies for solar panels use cadmium in small amounts, such as the cadmium-tellurium technology (CdTe), and can also occur in the CIGS type of solar panel. Bleiwas (2010) explains the basics of this technology for cadmium, as well as gallium, germanium, indium, tellurium and selenium, which also are used in this technology (International Cadmium Association, 2019, Öhrlund, 2011; Halada et al., 2008; Marwede & Reller, 2014). In the future, a significant part of global energy production should come from renewable sources to avoid major climate change problems (Gordon et al., 2006; Grandell & Höök, 2015; Halada et al., 2008; Moss et al., 2011; Nassar et al., 2015; Elshkaki & Graedel, 2013; Fthenakis et al., 2009; Zuser & Rechenbeger, 2011). This is assumed to be achieved by using a large number of solar photovoltaic panels and wind energy, as well as much more efficient technologies hel** to reduce energy use. In the available policy plans, there is planned a large capacity coming from the use of photovoltaic technologies. Earlier research has indicated (Sverdrup & Ragnarsdottir, 2014; Sverdrup et al., 2024) that the amounts of some of the key materials may come in limited supply (silver, indium, germanium, gallium and tellurium), limiting the installed capacity for a certain technology (Sverdrup et al., 2024). Based on silver, gallium and indium, it appears that about 23% of the demand may be covered with the available material. Thus, careful optimization may be needed to have as much capacity as possible, looking at a system using all technologies exploiting that they have different material demands.
The implication is that there is an increasing demand for cadmium for use in some types of thin-film solar cells. This exposes a goal conflict between phasing cadmium out of society because of its great toxicity to humans and the use of cadmium to generate much-demanded electricity by harvesting solar power. In the past, cadmium was used for permanent colours like yellow and red colours (now forbidden), for electroplating (now forbidden), in dental amalgam (now forbidden), in low-meting point soldering alloys (now forbidden), as stabilisers and chemical additive in polymers and in some industrial processes (being phased out), in photocells, light sensors (looking for alternatives on-going) and nickel–cadmium rechargeable batteries (being phased out).
Figure 1 shows the cadmium production and market price in 1900–2022 and data from USGS 2019, pieced together from various other websites accessed by the authors. Cadmium production peaked in 2018 (Sverdrup & Ragnarsdottir, 2014; see Fig. 1) and is expected to decline to a very low level by 2030 according to the UN/ECE-LRTAP protocol (UN/ECE, 1998). If cadmium production has peaked remains to be verified, but that should be the result of respecting the UN/ECE-LRTAP Århus 1988 Heavy Metals Protocol, ratified in 2013 and implemented after 2020. The price has dropped in the later years, possibly because of the collapse of demand for cadmium and the policy of phasing cadmium out from all uses globally.
2 Objectives and Scope
The first goal was to develop an integrated dynamic model for the global market for cadmium as a part of building a long-term supply assessment tool for technology metals required for new energy transition technologies. The WORLD7 model was used to simulate mother metal production rates of copper, zinc and lead (Sverdrup et al., 2017b, 2021). The cadmium sub-model will be validated and embedded inside the WORLD7 model using data for extraction and market price. It will be ensured that it can reconstruct the past with respect to extraction, recycling, supply and market price. In a second step, the WORLD7 model with cadmium will be used to assess the long-term risk for soft or hard scarcity with respect to important new technologies such as thin-film photovoltaic technologies and rechargeable batteries. The effect of having conflicting goals for the fate of cadmium and its use will be investigated.
3 Methods and Theory Used
3.1 Modelling
The main tool employed here is system dynamics modelling. For the modelling, we use the standard methods of systems analysis. We analyse the system using stock-and-flow charts and causal loop diagrams. The mass balance expressed differential equations resulting from the flow charts and the causal loop diagrams were numerically solved using the STELLA® Architect modelling environment (Meadows et al., 1974; Senge, 1990; Sverdrup et al., 2022). We use causal loop diagrams for map** out where the causalities are, to find intervention points in the system and to propose policy interventions. This method gives more detail, demands more insight and can include more factors including recycling rates and pricing. The Integrated Assessment Model WORLD7 was used for this study (Sverdrup & Olafsdottir, 2019). The reserves and resource estimates for the source metals are based on geological estimates, the interpretation of geological data and the allocation of extractable amounts according to ore quality, stratified with extraction costs (Mudd et al., 2014; Sverdrup & Olafsdottir, 2019; Sverdrup & Ragnarsdottir, 2014; Sverdrup & Olafsdottir, 2019; Krautkraemer, 1988).
The WORLD7 model addresses a large number of metals, and they are all in some way linked in their extraction. The WORLD7 energy module supplies energy from fossil fuels, renewables and nuclear power, with a market price generated by supply and demand in the model. For cadmium, the price has an effect on demand, but not any significant impact on the supply, as this is dependent on the source metal extraction rate. All modules are interconnected. Figure 4 shows the flow chart for the sub-model inside the WORLD7 model dealing with cadmium, with the parent ores and the dependent secondary extraction of many technology metals. The price affects demand, but it does not have any significant impact on the supply, as this is dependent on the mother metal extraction rate. The model as a simple causal loop diagram for the system is shown in Fig. 5. The mining rate is driven by profit from operations. The price is determined by how much metal is available in the market in the same way as in earlier models (Sverdrup & Ragnarsdottir, 2014; Sverdrup et al., 2017b; Sverdrup & Olafsdottir, 2019). A high metal price will increase profits, promote a larger supply to the market and limit demand. More supply to the market will increase the amount available and lower the price.
We have made one important assumption for the business-as-usual scenario for the assessment of future cadmium supply. It was assumed that the use of cadmium for photovoltaic solar panels will be permitted and exempt from the internationally agreed ban under the UN/ECE-LRTAP convention. We have assumed this will be politically justified by the great need for photovoltaic energy and made contingent on reaching a certain level of recycling. If this does not occur, then the assessment will show how fast cadmium will disappear from use in society.
3.2 Resource Estimations
Cadmium is found mostly in zinc ores, lead–zinc ores and multi-metal Ca-Zn-Pb ores, where it is mostly found in the sphalerite mineral, a zinc sulphide (Frenzel et al., 2016; Wellmer et al., 1990; Zhucheng et al., 2007). Cadmium is found in trace amounts in some iron ores and in some phosphate deposits (USGS, 2022). Phosphate is an important part of industrial fertilisers and, through this, an important pathway to human exposure. Further literature was consulted for numbers (Kelly & Matos, 2014; Brown et al., 2015; Idoine et al., 2022).
The estimation of cadmium resources is problematic (Mudd & Weng, 2012; Mudd et al., 2017; Papp et al., 2008). There are no cadmium mines and thus no dedicated cadmium mining industry. Cadmium is extracted as a by-product of zinc refining, a few copper ores and from recycling (Table 1). In this study, some key assumptions were made: (1) Cadmium is only extractable in mother metals from primary mining. (2) Recycled mother metals have very little content of cadmium. (3) Cadmium is only available if the ore is hydrometallurgical processed and very little technology metals come out with heap leaching methods. Only a few studies make detailed studies of the available resources cadmium; Plachy (2009) and the Mineral Commodities Summaries of the USGS (USGS 2015–2022). The supply security of cadmium depends on source ores for copper and zinc. There is no earlier process-oriented systems dynamics model for cadmium available (Busch et al., 2014; Elshkaki & Graedel, 2013, 2015; Goe & Gustard, 2014; USGS, 2022).
Extraction cut-off is dependent on technology and the degree of repetitiveness of the extraction method (Krautkraemer, 1988; Singer, 1993, 2007). It is composed of different elements: (1) access yield YA is the part of the deposits that will be available for this kind of extraction. Some deposits lack physical or legal access and have a composition that prevents extraction. (2) The secondary extraction yield is for when the primary extraction operation does not have the infrastructure to extract the technology metal when the operation is running (YS). The secondary yield is the fraction of the potential in the source metals that will be extracted. Some methods, such as heap leaching do not readily give such a secondary substrate. (3) The refining yield is the fraction of the metal recovered from the refining substrate (YR). The beneficiation yield is sometimes linked to the cut-off. The extraction cut-off is dependent on technology, extraction costs and the metal price at the time.
where XO is the ore content, XCO is the ore content that is not captured, the cut-off, and YB is the beneficiation yield. For example, if the ore grade is 1.44%, the cut-off is at 0.5%, then the beneficiation yield YB has a value of 0.67. If the cut-off is 0.3%, then YB = 0.79. The refining yield will be a function of the extractive efficiency when treating the ore shipped to the refinery. The material contained below the cut-off grade is lost with the waste. The extractable amount depends on the difference between the ore grade and the refining cut-off grade. It is necessary to account for limited access, the right kind of extraction method, if the infrastructure is available, if the ore allows for it to be extracted and the extraction yield. The total yield is thus
This formula is applied in Table 1 and 2. Table 1 shows the recoverable resources of source metals in million tons of metal, and this was used as input data to the WORLD7 model (Sverdrup & Olafsdottir, 2019; Sverdrup & Ragnarsdottir, 2014; Sverdrup et al., 2019). In addition, experiences learned from studying corporate reports and scientific literature discussed in the text were reworked into the resource data and the estimates of costs of extraction. Cadmium is not really scarce. Most of the high and low ore grades of zinc have already been mined, and at present, cadmium comes from the low-grade zinc ore with the highest cadmium content. Not very much globally generalizable information is available. For YA and YS, there is no data; for YB, there are a few hints. Thus, the overall yield is a very approximate estimate, most based on the generic mining experiences of the authors; 80% or 90% yield in a single step may sound very good, but since many steps are involved in the pathway from geological deposits to metal in the market, the total yield is 40–50% as can be seen in Table 1. In summary, Table 1 shows that the available zinc resources contain about 20 million tons of cadmium; we can only expect to be able to extract a maximum of about 8 million tons. This is because of limitations in access to substrate, lack of technological installations and limitations in yields that are realistic. The overall yield for zinc in itself is maybe 80%; thus, from the first geological occurrence of metal in the market, the total yield for cadmium is perhaps no more than 32%.
Table 2 shows a simple cadmium production estimate using source metal resources and contents. The global production was about 22,000 tons in 2013 and about 24,000 tons/year in 2022. There is more cadmium in high-grade ores and less in low-grade ores, as cadmium closely follows zinc. The cadmium extraction rate peaked in 2021 and is now in steady decline. The extraction potential is far above the actual extraction, and demand is in decline in all traditional areas of application. The mother metal ore data has been stratified with respect to ore metal content and relative extraction cost (Phillips & Edwards, 1976). Cadmium comes almost exclusively from zinc ores refining residuals, but historically with small contributions from copper and lead refining.
Table 3 shows the cadmium content for how much cadmium is needed for different photovoltaic harvest technologies in the construction phase, as tons of technology metals are used per installed MW photovoltaic panel capacity (Cesaro et al., 2018; Cucchiella et al., 2015). Table 3 is an important input for any photovoltaic panel sustainability assessment. Only the photovoltaic panel types using cadmium have been included in the table. Another important technical use for cadmium is in rechargeable batteries. However, these are being slowly phased out, as there are good substitutes.
3.3 Simulation Model Description
The cadmium model is based on differential equations derived from the mass and energy balances of the cadmium system. Figure 2 shows a causal loop diagram for the extraction process. The mining of zinc is driven by its own economic merit, but supplies a waste, the raw material for cadmium extraction. Provided the substrate is available and is suitable, it will be used for extraction as long as it is profitable. Figure 3 shows the market amount to the price diagram used in the simulations. This parameterizes the market amount to price relationship used in the model (Papp et al., 2008; Sverdrup & Olafsdottir, 2019, 2020a, Olafsdottir & Sverdrup 2021, Sverdrup 2019, Sverdrup et al., 2017a, b). Figure 4 shows the flow chart for cadmium in society, and that is represented in the WORLD7 model. Red lines are cadmium transaction losses. This is a graphical representation of the mass balance for cadmium in the metal supply system. In the model, we have worked with five different uses in society. The use of cadmium in plating and colour has traditionally been the two pathways that contributed most to human cadmium exposure in the past. These have also been the first to be phased out because of health concerns.
The market amount to price diagram used in the simulations; see also Sverdrup and Olafsdottir (2019)
Figure 5 shows the causal loop diagram for the cadmium module made for WORLD7. There are three reinforcing loops kee** the system running. They all involve profit and sales and are run by commercial suppliers. The loops marked R1 and R2 run over recapture, recycling and supplies society, and this is shown in blue. Cadmium recapture and recycling have been driven by profit in the past and, more recently, by environmental legislation. The loop marked R3 runs over production from extraction from zinc refining residuals, sales and profits and is marked in red. The loop is driven by commercial profits of the sales of cadmium in the markets. For cadmium, it is necessary to distinguish between recapture and recycling. A steadily growing fraction of the recaptured cadmium will not be recycled, but put into permanent safe storage as a part of removing it from society.
The causal loop diagram for the cadmium module made for WORLD7. There are three reinforcing loops kee** the system running: R1 runs over recycling and supplies society and is shown in blue; R2 runs over production, sales and profits and is marked in red; and R2 runs over recycling and is marked in red
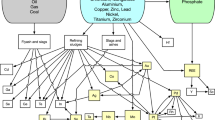
Figure 6 shows the cadmium module inside WORLD7 as it was developed for this study. It has three parts: one for handling cadmium in society, one for the market and one for extraction. Figure 7 shows an overview of the whole WORLD7 system, version 7.358 of 1 August 2023. Every red line is one or more causal links. Every box contains one or more system dynamics modules, as specified in the box.
Table 4 shows some aspects of the parameterization of the cadmium sub-model rate coefficients used in the WORLD7 model simulations. The WORLD7 has gone through a number of development stages since 2011; the first version of WORLD7 appeared in 2019 after a major reorganisation of WORLD6 by Olafsdottir and Sverdrup.
4 Results
4.1 The Mother Metal Simulations as Input to the Cadmium Assessment
Figure 8 shows the simulated copper and zinc production using the WORLD7 model. The primary extraction of zinc and copper is the basis for cadmium extraction. The rates do not include recycling nor secondary sources.
The primary extraction rate of the source metals (a) zinc and (b) copper in the WORLD7 model (see Sverdrup et al., 2019, for a full description)
4.1.1 The Business-as-usual Simulations for Cadmium
The simulated and observed cadmium production is shown in Fig. 9. In the calculation for cadmium, we have assumed cadmium to be extractable from high-grade and low-grade zinc ores, but it does not occur in extractable amounts and concentrations in ultralow-grade zinc ore. This has to do with the ore genesis process.
Results from the WORLD7 model simulations. (a) The extraction, loss, supply, recycling and removed from circularity. (b) The demand to different sectors. (c) The flow to cadmium uses. (d) The cadmium stocks-in-use and amount of waste in scrap. (e) The price simulation for cadmium. (f) The fraction of supply as recycled and how much is captured and sent to final deposit. (g) The simulation of the cadmium price. (h) The cumulative amount of cadmium demand, modified demand, supply and extraction
Figure 9a shows the simulated demand, modified demand after price feedback, supply, extraction, removed and recycled cadmium. With time, removal replaces recycling as cadmium is phased out. Cadmium is fairly abundant and is produced well below what is possible as measured by occurrence. Cadmium is expected to peak soon, but demand is decreasing for its use as pigment (now 16%) in soldering alloys and in other metal alloys because of health and environmental concerns. Demand is larger than price-modified demand after 1985; this is called soft scarcity. Price-modified demand separates from supply in 2080, and after that, cadmium will be in hard scarcity.
Figure 9b shows the simulated stocks-in-use in different sectors. It can be seen how batteries containing cadmium will be phased out, even if this takes time. This is caused by a long lifetime for such batteries and a systemic delay. It can be seen that after 2020, to use of cadmium will be only for photovoltaic technologies. It is estimated that at full planned capacity, some 240,000 tons of cadmium would be required for solar panels alone, which is a substantial amount.
Figure 9c shows the model simulated flow to different cadmium uses. Cadmium use, in general, is at present in decline. Note how the full demand for cadmium to photovoltaic technologies cannot be delivered in the future. This is caused by a limitation to future production capacity and environmental legislation according to international agreements (UN/ECE-LRTAP, 1998).
Figure 9d shows the simulated cadmium flow to colours, plating and alloys as compared to the demand for each of them. All show peak behaviour due to the intended international UN/ECE environmental policy to phase cadmium totally out of use in society.
Figure 9e shows the simulated degree of recycling to society and the flow to the permanent final deposit of cadmium. The captured fraction of supply exceeds the value one when the stocks-in-use declines and as cadmium is phased out. This is a sign of cadmium being long term removed from the system.
Figure 9f shows the simulated amount lost to the environment. First, the cadmium ban creates a collapse of the demand and the price and, later, an increase when there is a lack of production capacity.
The amount of cadmium lost to the environment from 1900 to 2200 is about 700,000 tons. During the same period, about 1 million tons will have been captured and sent to the final safe repository. Figure 9g shows the price simulation for cadmium. Figure 7h shows the cumulative amount of cadmium demand, modified demand, supply and extraction. Note how soft scarcity sets in 2020, when demand and modified demand separate. Modified demand and supply will separate in 2100, signalling hard scarcity.
4.2 Testing the Model
The model has been tested against the recorded mining data derived from the USGS (2022) databases. Figure 10a shows a comparison of simulated extraction as compared to the data. Figure 10b shows the cumulative extraction as compared to the cumulative simulated amount. The fit is excellent. There is no systematic error building up in the model with time, suggesting the model gets the mass balances right. Figure 10c shows the simulated price as compared to the observed data. Figure 9d shows the stocks-in-use in kg per capita and supply in kg per capita and year. It shows a typical peak behaviour caused by the phasing out of cadmium rather than because of significant scarcity. Take note that the cadmium module inside the WORLD7 model is not calibrated to any production data, but does this up from basic principles of trade, economics of supply and demand and mining dynamics. This can be seen throughout the results section and in the comparisons with data. The model does reproduce the observed mining rates satisfactorily when the model is driven by market demand and price dynamics. All metals, minerals and commodities are modelled simultaneously this way in WORLD7 without any time-series calibration.
Results from the WORLD7 model simulations. (a) A test of the model simulation against data for the cadmium extraction (b) and the cumulative cadmium extraction. (c) The cadmium price as simulated with the model versus data. (d) Supply per person and year in kg per person and year and stocks-in-use in kg per capita for Cd. The cadmium model captures the cadmium history with great accuracy
5 Discussion
5.1 Environmental Concerns versus Technical Usefulness
The cadmium supply has peaked and is declining according to the international plan to phase it out of all uses. Cadmium is a technically useful metal, but its very significant toxicity is a problem that cannot be ignored. Cadmium is one of the metals that will be phased out in Europe in accordance with the LRTAP1996 Heavy Metals Protocol (UN/ECE-LRTAP, 1998). The use of cadmium is restricted in Europe because of human health risks. Cadmium is very toxic, and a serious poisoning has virtually no cure. It is still used in electric accumulators, but these may eventually be phased out. If cadmium can be used for CdTe solar cells on a large scale, it appears uncertain; it will be dependent on whether a significant cadmium recovery after use can be done or not. Unless near recapture of the used cadmium in the new technical applications, it would probably not be permitted. For cadmium, the underproduction is caused by the environmental policy in Europe on cadmium due to its great toxicity (UN/ECE-LRTAP 1998, Genchi et al., 2020, Nriagu & Pacyna 1988). Cadmium supply per person per year is shown in Fig. 9d. In the long run, the success of the UN/ECE-LRTAP 1998 protocol will be determined by the degree of global enforcement as well as unintentional and intentional policy leakage. It must be counted on that lobbying will be brought on to make exceptions for cadmium use in alternative energy technologies such as photovoltaic panels of the CIGS type and some types of batteries. This will be argued on the great need for energy and pointing to the smaller hazard of using cadmium in photovoltaic panels as compared to the damage from large scale use of coal for power generation.
5.2 Environmental Impacts of Cadmium Use, Pollution and Human Exposure
Figure 9f shows the predicted loss rate to the environment per year from the technical sphere of the model simulations. In total, 970,000 tons of cadmium is lost to the environment that originated from mined cadmium. Much of the technically lost cadmium ends up in the environment. In addition to this comes a significant cadmium flow to the environment and humans from the use of agricultural fertilisers which have cadmium contamination in the phosphate deposits (Nriagu & Pacyna 1988, Pacyna & Pacyna 2001). This is an issue when sedimentary cadmium is used. The main global phosphate resources are of the sedimentary type, such as those from Morocco.
Figure 11 shows the flow chart for the interaction between society and the environment for cadmium. This system is also contained in the WORLD7 model where it interacts with public health, hospitalizations and mortality, and with the population model. These aspects do not come into this narrative in any detail and will be the subject of a later study. Cadmium reaches humans through several pathways, the two most important being through contaminated food and secondarily through environmental pollution. In earlier times, the use of cadmium in consumer products caused significant exposure.
Cadmium is a volatile element with low melting and boiling points and thus can also escape from technical environments easily. However, cadmium is not long lived the environment. Under anaerobic conditions, cadmium encounters sulphide ions and is very strongly bound as cadmium sulphide that will not readily redissolve. Recycling of cadmium from technical uses has challenges, but work is being done to improve recycling (Feddersen & Lee, 1954; Marwede & Reller, 2012, 2014; Reuter et al., 2013a, 2013b).
Figure 12 shows the dose–response diagram for mercury, cadmium and lead as derived many years ago from literature data by some of the authors (Bakker et al., 1998; Friberg et al., 2019; Pfitzer & Vouk, 1979; Sverdrup, 2001; Sverdrup & Ashmore, 2001). The authors did a more recent review of some literature (Balali-Mood et al., 2021, Bernhoft, 2012; Buchet et al., 1980; Chen et al., 2006; Chen et al., 2019; Choong et al., 2014; Li et al., 2019; Lin et al., 2018; Lv et al., 2017; Djordjevic et al., 2019; Järup et al., 1998; Nishijo et al., 2017; Perry & Erlanger, 1974; Proshad et al., 2020; Rani et al., 2014; Satarug, 2018; Satarug et al., 2000; Yang & Shu, 2015; Kjellström & Nordberg, 1978; Mezynska & Brzóska, 2018; VKM, 2015; Food Safety News, 2012; ANSES, 2019; Shar et al., 2012), as confirmed the picture shown in Fig. 12. The picture was radical in 2001, and in 2023, it appears as very appropriate and in line with the latest research on their topic. Cadmium is very toxic, and there is no valid lower limit. Take careful note that mercury and cadmium show the same dose–response system behaviour, despite the fact that the physiological mechanisms for effect are different. This stands in contrast to lead, where the medical effect disappears below 300 µg per day (Fig. 12). This difference arises from the fact that the body can get rid of lead in small amounts through some mechanisms that move calcium in the body. For cadmium and mercury, there is no such mechanism. The diagram strongly suggests that there is no safe exposure level and that both mercury and cadmium are damaging from the first molecule of exposure.
Dose–response diagram for mercury, cadmium and lead as derived many years ago from literature data by some of the authors (Bakker et al., 1998; Sverdrup & Ashmore, 2001; Sverdrup & Olafsdottir, 2020b; Genchi et al., 2020) for use in the UN/ECE-LRTAP convention and the 1998 Århus Heavy Metal Protocol. Median consumption in the USA was 0.16 µg per kg body weight per day
Due to the volatility of cadmium and mercury and their methylated compounds, cadmium and mercury also move freely across any cell barrier, including the blood–brain barrier. The half-life time for cadmium in the body is 37 years, implying that there is no way for a normal grown-up person to recover from significant exposure (Kjellström & Nordberg, 1978, Chunhabundit 2016). Thus, long-term exposure and acute exposure of any kind must be avoided. Cadmium is as poisonous to animals as to humans, and the effects on the natural environment are very serious. The response function shown in Fig. 12 shows why it is urgent and necessary to have a total ban on all use of both cadmium and mercury (Balali-Mood et al., 2021; Sverdrup & Olafsdottir, 2020b) and why any exceptions to that rule must be so few that any cadmium leakage to nature is irrelevant. This dictates that the photovoltaic technologies listed in Table 3, as using cadmium, cannot be allowed until a recovery after use of at least 90% elimination of any leakage to nature can be guaranteed (EFSA, 2009). At present, the industry is very far from any such goal.
It would be technically possible to produce at least 250,000 tons of cadmium per year. In reality, the 2023 production is about 24,000 tons of cadmium per year in 2022. This much cadmium never reaches humans as the environment is in between, filtering out more than 99% of the cadmium pollution as an environmental service. Without this environmental service, humanity would have been in deep trouble. The background cadmium exposure is at about 3 µg per day per person and is declining.
There is also a consequence for climate change mitigation and adaptation. Several metals set limits for how much photovoltaic capacity can be installed in the future as a compensating effort when fossil fuels are phased out from 2025 to 2050. Preliminary calculations (Sverdrup & Ragnarsdottir, 2014; Sverdrup & Olafsdottir, 2020b; Sverdrup et al., 2024) suggest that the amounts extractable for indium, gallium, germanium, cadmium, and silver are sufficient for about 20–25% of the projected demand for solar photovoltaic power. This emphasises renewable energy for solar photovoltaics that will not be able to replace the energy not made when fossil fuels are phased out. For climate mitigation and adaptation measures to be successful, a strategy with different new technologies and significant reductions in total energy use will be required. This is much talked about in preambles and prefaces to different strategy documents, but is absent from most action plans.
5.3 Sustainability of Supply
In the model, the demand is made on the market, and when the market amount goes low, then the prices go higher. Figure 10d shows the supply per person and year in kg per person and year and stocks-in-use in kg per capita for Cd. The supply per person and year must cover up for wear and losses, use for maintenance, and any extra over that can allow growth. If less is supplied than the losses, then the stock will decline. Higher prices push the mining rate by increasing profits, causing the price to increase, which in turn makes the demand decrease. The model becomes self-regulating. The market dynamics are fully expressed in the present cadmium model and that gives a significantly smoother production curve and better dynamics. This is the case for all minerals, metals and commodities represented in the WORLD7 model. In the discussion of sustainability, we should note that recycling can delay symptoms of scarcity for a significant time, even after the primary production from mines has stopped. Supply per person and year reflects the amount available to compensate for continuous losses and any surplus available for growth in the stock-in-use. Stock-in-use per person is an indicator of the utility gained from the resource, and a decline in stock-in-use suggests a decline in service provision from that resource. The production of the dependent metals is limited to some extent by the fact that only a fraction of the metal refineries is technically equipped for efficient recovery of these metals.
6 Conclusions
The case of cadmium represents a classical conflict of goals. Present and future energy needs stand in opposition to the protection of the natural environment and human health. All types of available photovoltaic technologies will be required for reaching sufficient amounts of renewable energy in order to replace fossil fuels before 2050 (Sverdrup et al., 2022). At the same time, it is of great importance to phase out cadmium, potentially taking away 25% of the future photovoltaic capacity that will be available (Sverdrup et al., 2024). From earlier studies (Sverdrup & Ragnarsdottir, 2014, Sverdrup et al., 2022), we do know that indium, gallium and silver will limit the photovoltaic technologies using indium, gallium and silver by 75% as compared to the projected activities. This is a very serious warning that there is something fundamentally wrong with the projections of future capacity for solar electric power on a large scale. All of this urges towards doing research to substitute cadmium with something less toxic in solar photovoltaic panels and battery technologies.
There is no shortage of cadmium to extract, and no shortage from lack of cadmium available in the future zinc flow is to be expected. Thus, from a technical perspective, all cadmium demanded can be supplied. It would be possible to produce at least 250,000 tons/year; in reality, the 2023 production is about 24,000 tons/year and declining. This is so low in 2022 because there is a United Nations agreed global policy to phase out cadmium from all use. Technical demand for cadmium will soon not be met, and there will be an actual shortage of cadmium for any use, including photovoltaic technologies and semiconductors. This is very good news for the environment and human health, but bad news for those that want the CdTe and CIGS types of photovoltaic panels to mitigate future energy shortages. There are strong arguments against allowing the use of cadmium in applications where recycling is problematic. For photovoltaic panels using cadmium as an ingredient, the recycling degree is very poor, far from what must be demanded for cadmium.
The environmental policy of the UN/ECE-LRTAP Convention has been successful, and it seems like cadmium will probably be phased out of all use, including photovoltaics. Thus, pursuing research towards any technology that uses cadmium does not seem to be a good business strategy. A departure from the heavy metals policy should only be permitted if a better than 95% recapture efficiency can be secured for the cadmium used.
Data Availability
There are no databases associated with this study that can be shared. All data used were taken from open public sources or scientifically published sources. The model is available in STELLA Architect format upon request from the corresponding author.
References
ANSES. (2019). ANSES makes recommendations to limit cadmium exposure from consumption of edible seaweed. https://www.anses.fr/en/content/anses-makes-recommendations-limit-cadmium-exposure-consumption-edible-seaweed. Accessed during 2023.
Bakker, D., de Vries, W., & Sverdrup, H. (1998). Effects-based approaches to assess the risk of heavy metal inputs to surface waters–Overview methods and models. In: Workshop on critical limits and effect based approaches for heavy metals and persistent organic pollutants, Gregor, H., Spranger, T., Hörnerbach, F. (eds). Umweltbundesamt Texte 5:98:225–278 ISSN 0722–186X.
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M. R., & Sadeghi, M. (2021). Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Frontiers in Pharmacology, 12, 643972. https://doi.org/10.3389/fphar.2021.643972
Bernhoft, R. A. (2012). Mercury toxicity and treatment: A review of the literature. Journal of Environmental Public Health, 2012, 460508. https://doi.org/10.1155/2012/460508
Bleiwas, D. I. (2010). Byproduct mineral commodities used for the production of photovoltaic cells: United States of America Geological Survey, USGS Circular 1365, 10 pages, available at http://pubs.usgs.gov/circ/1365/. Downloaded January 2023.
Boyd, R., & Gautneb, H. (2016). Mineral resources of Norway: Potential and strategic importance, 2016 update. Norges Geologiske Undersøkelse, Report no. 2016.034, ISSN 2387–3515. Trondhjem, Norway.
Brown, T. J., Wrighton, C. E., Raycraft, E. R., Shaw, R. A., Deady, E. A., Rip**ale, J., Bide T., & Idoine, N. (2015). World mineral production 2009–2013, 88pp. Keyworth, Nottingham British Geological Survey. ISBN 978–0–85272–848–2
Buchet, J. P., Roels, H., Bernard, A., & Lauwerys, R. (1980). Assessment of renal function of workers exposed to inorganic lead, cadmium or mercury vapor. Journal of Occupational Medicine, 22, 741–750.
Busch, J., Steinberger, J. K., Dawson, D. A., Purnell, P., & Roelich, K. (2014). Managing critical materials with a technology-specific stocks and flows model. Environmental Science and Technology, 48, 1298–1305. ISSN 0013-936X.
Cesaro, A., Marra, A., Kuchta, K., Belgiorno, V., & van Hullebusch, E. D. (2018). WEEE management in a circular economy perspective: an overview. Global NEST Journal, 20, 743–750.
Chen, L., **, T., Huang, B., Chang, X., Lei, L., Nordberg, G. F., et al. (2006). Plasma metallothionein antibody and cadmium-induced renal dysfunction in an occupational population in China. Toxicological Science, 91, 104–112. https://doi.org/10.1093/toxsci/kfj053
Chen, X., Wang, Z., Zhu, G., Nordberg, G. F., **, T., & Ding, X. (2019). The association between cumulative cadmium intake and osteoporosis and risk of fracture in a Chinese population. Journal of Exposure Science & Environmental Epidemiology, 29, 435–443. https://doi.org/10.1038/s41370-018-0057-6
Choong, G., Liu, Y., & Templeton, D. M. (2014). Interplay of calcium and cadmium in mediating cadmium toxicity. Chemico-Biological Interactions, 211, 54–65. https://doi.org/10.1016/j.cbi.2014.01.007
Chunhabundit, R. (2016). Cadmium exposure and potential health risk from foods in contaminated area. Thailand. Toxicol. Res., 32, 65–72. https://doi.org/10.5487/tr.2016.32.1.065
Cucchiella, F., D’Adamo, I., Lenny Koh, S. C., & Rosa, P. (2015). Recycling of WEEEs: an economic assessment of present and future e-waste streams. Renewable and Sustainable Energy Reviews, 51, 263–272.
de Vries, W., Bakker, D. J., & Sverdrup, H. U. (1998). Manual for calculating critical loads of heavy metals for aquatic ecosystems; guidelines for critical limits, calculation methods and input data. Alterra Institute, Wageningen, SC-DLO, RIVM. Report 165, 92 pp.
de Vries, W., Bakker, D., Sverdrup, H., Paces, T., & Tip**, T. (1998) Effects-based approaches to assess the risk of heavy metal inputs to soils–Overview methods and models. In: Workshop on critical limits and effect based approaches for heavy metals and persistent organic pollutants, H. Gregor, T. Spranger, F. Hörnerbach (Eds). Umweltbundesamt Texte 5:98:125–224 issn 0722–186X.
Djordjevic, V. R., Wallace, D. R., Schweitzer, A., Boricic, N., Knezevic, D., Matic, S., et al. (2019). Environmental cadmium exposure and pancreatic cancer: evidence from case control, animal and in vitro studies. Environment International, 128, 353–361. https://doi.org/10.1016/j.envint.2019.04.048
EFSA. (2009). "EFSA sets lower tolerable intake level for cadmium in food." https://www.efsa.europa.eu/en/news/efsa-sets-lower-tolerable-intake-level-cadmium-food. Downloaded Jan 2023.
Elshkaki, A., & Graedel, T. E. (2013). Dynamic analysis of the global metals flows and stocks in electricity generation technologies. Journal of Cleaner Production, 59, 260–273.
Elshkaki, A., & Graedel, T. E. (2015). Solar cell metals and their hosts: a tale of oversupply and undersupply. Applied Energy, 158, 167–177.
Feddersen, P. C. & Lee, H. E. (1954). Cadmium recovery practice in lead smelting. https://www.911metallurgist.com/cadmium-recovery-lead-smelting/ accessed 2023.
Food Safety News. (2012). EU lowers lead and cadmium limits for food products. https://www.foodsafetynews.com/2021/08/eu-lowers-lead-and-cadmium-limits-for-food-products. Downloaded during 2023.
Frenzel, M., Hirsch, T., & Gutzmer, J. (2016). Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type – a meta-analysis. Ore Geology Reviews, 76, 52–78.
Friberg, L. T., Elinder, G. G., Kjellstrom, T., & Nordberg, G. F. (Eds.) (2019). Cadmium and health: A toxicological and epidemiological appraisal: Volume 2: effects and response (Vol. 1); CRC Press: Boca Raton, FL, USA.
Fthenakis, V., Wang, W., & Kim, H. C. (2009). Life cycle inventory analysis of the production of metals used in photovoltaics. Renew. Sustainable Energy Review, 13, 493–517.
Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A., & Catalano, A. (2020). The effects of cadmium toxicity. International Journal of Environmental Research and Public Health, 17, 3782. https://doi.org/10.3390/ijerph17113782
Goe, M., & Gaustad, G. (2014). Identifying critical materials for photovoltaics in the US: A multi-metric approach. Applied Energy, 123, 387–396.
Gordon, R., Bertram, B., & Grädel, M. (2006). Metal stocks and sustainability. Proceedings of the National Academy of Science, 103, 1209–1214.
Grandell, L., & Höök, M. (2015). Assessing rare metal availability challenges for solar energy technologies. Sustainability, 7, 11818–11837.
Halada, K., Shimada, M., & Ijima, K. (2008). Forecasting of the consumption of metals up to 2050. Materials Transactions., 49, 402–410.
Idoine, N. E., Raycraft, E. R., Shaw, R. A., Hobbs, S. F., Deady, E. A., Everett, P., Evans, E. J., & Mills, A. J. (2022). World mineral production 2016–2020. British Geological Survey, Keyworth, Nottingham.
International Cadmium association. (2019). Downloaded november 2022 from https://www.cadmium.org/cadmium-applications
Järup, L., Berglund, M., Elinder, C. G., Nordberg, G., & Vahter, M. (1998). Health effects of cadmium exposure - a review of the literature and a risk estimate. Scandinavian Journal of Work, Environment & Health, 24(supl1), 52p.
Kelly, T.D., & Matos, G.R. (2014). With major contributions provided by Buckingham, D.A., DiFrancesco, C.A., Porter, K.E., and USGS mineral commodity specialists. Historical Statistics for Mineral and Material Commodities in the United States. Downloaded november 2022: https://www.usgs.gov/centers/nmic/historical-statistics-mineral-and-material-commodities-united-states
Kjellström, T., & Nordberg, G. F. (1978). A kinetic model of cadmium metabolism in the human being. Environmental Research, 16, 248–269.
Krautkraemer, J. A. (1988). The cut-off grade and the theory of extraction. Canadian Journal of Economics., 21, 146–160.
Li, H., Fagerberg, B., Sallsten, G., Borné, Y., Hedblad, B., Engström, G., et al. (2019). Smoking-induced risk of future cardiovascular disease is partly mediated by cadmium in tobacco: Malmö Diet and Cancer Cohort Study. Environmental Health, 18, 56. https://doi.org/10.1186/s12940-019-0495-1
Lin, X., Peng, L., Xu, X., Chen, Y., Zhang, Y., & Huo, X. (2018). Connecting gastrointestinal cancer risk to cadmium and lead exposure in the Chaoshan population of Southeast China. Environmental Science and Pollution Research International, 25, 17611–17619. https://doi.org/10.1007/s11356-018-1914-5
Lv, Y., Wang, P., Huang, R., Liang, X., Wang, P., Tan, J., et al. (2017). Cadmium exposure and osteoporosis: a population-based study and benchmark dose estimation in southern China. Journal of Bone Minerals Research, 32, 1990–2000. https://doi.org/10.1002/jbmr.3151
Marwede, M., & Reller, A. (2012). Future recycling flows of tellurium from cadmium telluride photovoltaic waste. Resources, Conservation and Recycling, 69, 35–49.
Marwede, M., & Reller, A. (2014). Estimation of life cycle material costs of cadmium telluride – and copper indium gallium diselenide – photovoltaic absorber materials based on life cycle material flows. Journal of Industrial Ecology, 18, 254–267.
Meadows, D. L., Behrens, W. W., III., Meadows, D. H., Naill, R. F., Randers, J., & Zahn, E. K. O. (1974). Dynamics of growth in a finite world. Wright-Allen Press Inc.
Mezynska, M., & Brzóska, M. M. (2018). Environmental exposure to cadmium—a risk for health of the general population in industrialized countries and preventive strategies. Environmental Science and Pollution Research, 25, 3211–3232.
Moss, R. L., Tzimas, E., Kara, H., Willis, P., & Kooroshy, J. (2011). Critical metals in strategic energy technologies: Assessing rare metals as supply-chain bottlenecks in low-carbon energy technologies; Luxembourg, European Commission Joint Research Centre – Institute for Energy and Transport. Downloaded march 2023: https://setis.ec.europa.eu/system/files/CriticalMetalsinStrategicEnergyTechnologies-def.pdf.
Mudd, G. M., & Weng, Z. (2012). Base metals. In T. Letcher & J. L. Scott (Eds.), Materials for a sustainable future: United Kingdom (pp. 11–59). Royal Society of Chemistry.
Mudd, G. M., Yellishetty, M., Reck, B. K., & Graedel, T. E. (2014). Quantifying the recoverable resources of companion metals: a preliminary study of Australian mineral resources. Resources, 3, 657–671. https://doi.org/10.3390/resources3040657
Mudd, G. M., Jowitt, S. M., & Werner, T. T. (2017). The World’s by-product and critical metal resources, part I: uncertainties, current reporting practices, implications and grounds for optimism. Ore Geology Reviews, 86, 924–938.
Nassar, N. T., Graedel, T. E., & Harper, E. M. (2015). By-product metals are technologically essential but have problematic supply. Science Advances, 1: https://doi.org/10.1126/sciadv.1400180
Nishijo, M., Nakagawa, H., Suwazono, Y., Nogawa, K., & Kido, T. (2017). Causes of death in patients with Itai-itai disease suffering from severe chronic cadmium poisoning: A nested case–control analysis of a follow-up study in Japan. British Medical Journal Open, 7(7), e015694. https://doi.org/10.1136/bmjopen-2016-015694
https://unece.org/environment-policy/air/protocol-heavy-metals
Nriagu, J., & Pacyna, J. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, 333, 134–139.
Öhrlund I. (2011). Future metal demand from photovoltaic cells and wind turbines - Investigating the potential risk of disabling a shift to renewable energy systems. Science and Technology Options Assessment (STOA), European Parliament.
Olafsdottir, A. H., & Sverdrup, H. (2021). System dynamics modelling of mining, supply, recycling, stocks-in-use and market price for nickel. Mining, Metallurgy & Exploration, 38, 819–840. https://doi.org/10.1007/s42461-020-00370-
Pacyna, J. M., & Pacyna, E. G. (2001). An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environmental Reviews, 9, 269–298.
Papp, J. F., Bray, E. L., Edelstein, D. L., Fenton, M. D., Guberman, D. E., Hedrick, J. B., Jorgenson, J. D., Kuck, P. H., Shedd, K. B., & Tolcin, A. C. (2008). Factors that influence the price of Al, Cd, Co, Cu, Fe, Ni, Pb, rare earth elements, and Zn. U.S. Geological Survey Open-File Report, 1356, 61. Downloaded in 2014. http://pubs.usgs.gov/of/2008/1356/pdf/ofr2008-1356.pdf
Perry, H. M., & Erlanger, M. W. (1974). Metal-induced hypertension following chronic feeding off low doses of cadmium and mercury. Journal of Laboratory and Clinical Medicine, 83, 541–547.
Pfitzer, E. & Vouk, V. (1979). Mathematical and statistical aspects of dose-effect and dose response relationships, Elsevier/North-Holland Biomedical Press.
Phillips, W.G.B., & Edwards, D.P. (1976). Metal prices as a function of ore grade. Resources Policy 167—179
Plachy, J. (2009). Cadmium recycling in the United States in 2000, chap. O of Sibley, S.R., Flow studies for recycling metal commodities in the United States: U.S. Geological Survey Circular 1196–T, pagesT1–T7, Downloaded in 2014. http://pubs.usgs.gov/circ/circ1196t/.14pp
Proshad, R., Zhang, D., Uddin, M., & Wu, Y. (2020). Presence of cadmium and lead in tobacco and soil with ecological and human health risks in Sichuan province, China. Environmental Science and Pollution Research., 27, 18355–18370. https://doi.org/10.1007/s11356-020-08160-1
Rani, A., Kumar, A., Lal, A., & Pant, M. (2014). Cellular mechanisms of cadmium-induced toxicity: a review. International Journal of Environmental Health Research, 24, 378–399. https://doi.org/10.1080/09603123.2013.835032
Reuter, M. A.; Hudson, C.; van Schaik, A., Heiskanen, K., Meskers, C., & Hagelüken, C. (2013a) A Report of the working group on the global metal flows to the international resource panel. UNEP. p 320.
Reuter, M., Hudson, C., Schalk, A., Heiskanen, K., Meskers, C., & Hagelüken, C. (2013b). The international resource panel. UNEP. Metal recycling; Opportunities, Limits, Infrastructure. p 317.
Satarug, S. (2018). Dietary cadmium intake and its effects on kidneys. Toxics, 6, 15.
Satarug, S., Haswell-Elkins, P., & Moore, M. R. (2000). Safe levels of cadmium intake to prevent renal toxicity in human subjects. British Journal of Nutrition., 84, 791–802.
Senge, P. (1990). The fifth discipline. The art and practice of the learning organisation. Century Business, New York.
Shar, Z. H., Pirhot, O. P., Shar, H. H., & Channa, M. K. (2012). Assessing the risk of heavy metals contamination in milk from Pakistan. International Journal of Science and Research Archive, 03, 107–113. https://doi.org/10.30574/ijsra.2021.3.1.0114
Singer, D. A. (1993). Basic concepts in three-part quantitative assessments of undiscovered mineral resources Nonrenewable. Resources, 2, 69–81.
Singer, D. A. (2007) Short course introduction to quantitative mineral resource assessments: U.S. Geological Survey Open-File Report 2007–1434. Downloaded 2014. http://pubs.usgs.gov/of/2007/1434/.
Sverdrup, H. (2001). Setting critical limits for mercury, cadmium and lead to be used in calculation of critical loads for different receptors. In J. Curlik, P. Sefcik, & Z. Viechova (Eds.), Report from the Ad Hoc Expert Meeting in Bratislava, Slovakia, 93–100. Bratislava: Peer reviewed through a series of official UN/ECE workshops held in Schwerin, Berlin and Bratislava. Published by the Slovak Ministry of Environment.
Sverdrup, H. U. (2019). The global sustainability challenges in the future: The energy and materials supply, pollution, climate change and inequality nexus. In E. Holden, J. Meadowcraft, O. Langhelle, D. Banister, & K. Linnerud (Eds.), Our common future, what next for sustainable development? 30 years after the Brundtland report. Chapter 4:49–72. Frankfurt: Springer Verlag.
Sverdrup, H., & Olafsdottir, A. H. (2020a). System dynamics modelling of the global extraction, supply, price, reserves, resources and environmental losses of mercury. Water, Air and Soil Pollution, 231, article 8-14. https://doi.org/10.1007/s11270-020-04757-x
Sverdrup, H. U., & Olafsdottir. (2020b). Assessing the sustainability of extraction, supply, resources and price for Sb, Bi, Cd, Ge, Ga, In, Se and Te with WORLD7. 12 pages. Presented at the World Resources Forum, Geneva 22–24. October, 2019. World Resources Forum Conference Proceedings. Open access publication. https://www.wrforum.org/world-resources-forum-2019/poster-sessions/assessing-future-supply-of-technology-metals-of-bi-cd-ge-ga-in-sb-se-and-te-for-new-technologies-using-the-world6-model/
Sverdrup, H. U., Ragnarsdottir, K. V., & Koca, D. (2017a). An assessment of global metal supply sustainability: global recoverable reserves, mining rates, stocks-in-use, recycling rates, reserve sizes and time to production peak leading to subsequent metal scarcity. Journal of Cleaner Production, 140, 359–372. https://doi.org/10.1016/j.jclepro.2015.06.085
Sverdrup, H., Ragnarsdottir, K. V., & Koca, D. (2017b). Modelling the copper, zinc and lead mining rates and co-extraction of dependent metals, supply, price and extractable amounts using the BRONZE model. Proceedings of the 2015 World Resources Forum, 11–15 September. Davos, Switzerland. In: (Eds) Ludwig, C., Matasci, C., Boosting resource productivity by adopting the circular economy. Pages 100–108. Paul Scherrer Institute, Villingen, Switzerland. ISBN 978–3–9521409–7–0
Sverdrup, H., Olafsdottir, A. H., & Ragnarsdottir, K. V. (2019). Assessing global copper, zinc and lead extraction rates, supply, price and resources using the WORLD6 integrated assessment model. Resources, Conservation and Recycling, 1–26(X4), 100007. https://doi.org/10.1016/j.rcrx.2019.100007.Openaccesspublication
Sverdrup, H. and M. Ashmore 2001. Conclusion from the ad hoc expert meeting on setting critical limits for heavy metals. 11–13 October 2000 in Bratislava, Slovakia, under the auspices of the UN-ECE and the ICP on modelling and map**. In: Report from the Ad Hoc Expert Meeting in Bratislava, Slovakia, 109–125, J. Curlik, P. Sefcik, Z. Viechova (Eds.). Peer reviewed through a series of official UN/ECE workshops held in Schwerin, Berlin and Bratislava. Published by the Slovak Ministry of Environment, Bratislava.
Sverdrup, H., & Olafsdottir, A. H. (2019). Conceptualization and parameterization of the market price mechanism in the WORLD7 model for metals, materials and fossil fuels. 31 pages. Mineral Economics. https://doi.org/10.1007/s13563-019-00182-7
Sverdrup, H. U., & Ragnarsdottir, K. V. (2014). Natural resources in a planetary perspective. Geochemical Perspectives 2:1–156. European Geochemical Society.
Sverdrup, H, Olafsdottir, A. H., & Ragnarsdottir, K. V. (2021). Development of a biophysical economics module for the global integrated assessment WORLD7 model. In: Cavana, R., Pavlov, O., Dangerfield, B., Wheat, D., (Eds) Modelling Feedback Economics. Chapter 10, 247–283. Springer Verlag, Frankfurt. https://www.springer.com/gp/book/9783030671891
Sverdrup H. U. (Ed.), Haraldsson, H. V., Olafsdottir, A. H., Belyazid, S., Svensson, M., & Nordby, A. (2022). System thinking, system analysis and system dynamics: Find out how the world works and then simulate what would happen. 316 pages. 7th revised and rewritten edition. ISBN Oplandske Bokforlag, Hamar, Norway. ISBN 978–82–7518–280–5.
Sverdrup, H. U., Nordby, A., van Allen, O., Schlyter, P., Stjernquist, I, Nordby, A, Olafsdottir, A. H., & van Dieren, W. (2024). The final count-down: Estimating the total value of the world’s natural resources. World Resources Forum 2023. Geneva, Switzerland. Oral Presentation. 4. September 2023. WRF 2023 Conference proceedings. Accepted.
UN/ECE. (1998). The 1998 Århus Heavy Metal Protocol. United Nations Economic Commission for Europe, Long Range Transboundary Air Pollution Convention. Downloaded 2014https://www.unece.org/env/lrtap/hm_h1.html
USGS. (2022). Mineral Commodity Summaries. Commodity statistics for a large number of metals. United States Geological Survey. <http://minerals.usgs.gov/minerals/pubs/commodity/. The websites of the USGS have been used systematically every year in the period 1993–2023 by the authors.
van der Voet, E., Salminen, R., Eckelman, M., Mudd, G., Norgate, T., & Hischier, R. (2013) Environmental risks and challenges of anthropogenic metals flows and cycles, a report of the working group on the global metal flows to the international resource panel. UNEP ISBN: 978–92–807–3266–5. 234pp
VKM. (2015) Risk assessment of dietary cadmium exposure in the Norwegian population. Opinion of the Panel on Contaminants of the Norwegian Scientific Committee for Food Safety. VKM Report 2015:12, ISBN: 978–82–8259–167–6, Oslo, Norway.
Wellmer, F. W., Hannak, W., Krauss, U., & Thormann, A. (1990). Deposits of rare metals. In: Kürsten, M. (Ed.), Raw Materials for New Technologies, Proceedings of the Fifth International Symposium, held in Hannover, Fed. Rep. of Germany, at the Federal Institute for Geosciences and Natural Resources, October 19–21 1988, Schweizerbart, Stuttgart, 71–122.
Yang, H., & Shu, Y. (2015). Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. International Journal of Molecular Sciences, 16, 1484–1494. https://doi.org/10.3390/ijms16011484
Zhucheng H., Yuanbo Z., Tao J., Guanghui L., Yongbin, Y, & Yufeng G. (2007). Investigation on comprehensive utilization of silver, gallium and other valuable elements in zinc leaching residue. Metal Mine, 2007–03, http://en.cnki.com.cn/Article_en/CJFDTOTAL-JSKS200703021.htm.
Zuser, A., & Rechberger, H. (2011). Considerations of resource availability in technology development strategies: the case study of photovoltaics. Resources, Conservation and Recycling, 56, 56–65. https://doi.org/10.1016/j.resconrec.2011.09.004
Acknowledgements
We acknowledge that Dr. Anna Hulda Olafsdottir, now at the Meteorological Institute in Reykjavik, Iceland, worked with the development of earlier versions of the WORLD6 and WORLD7 models, including an earlier very simplified cadmium model.
Funding
Open access funding provided by Inland Norway University Of Applied Sciences
Author information
Authors and Affiliations
Contributions
Dr. H. Sverdrup developed the cadmium module for the WORLD7 model, as well as the cadmium toxicity module in the public health assessment module. The human dose–response data and the response function were developed by Sverdrup and colleagues about 25 years ago in projects for the LRTAP convention (Sverdrup, 2001; Sverdrup & Ashmore, 2001). Sverdrup wrote up the first manuscript draft and did the simulation runs. Ole van Allen worked on the further general development of the WORLD7 model into European resource-integrated assessment platforms. Dr. Hördur Valdimar Haraldsson participated in the environmental assessment and developed the relevance to the European Environmental Policy, cross-cutting to public health and heavy metal regulations.
Corresponding author
Ethics declarations
Ethics Approval
This work is original research done by the authors. All text herein comes from the hands of the authors and nowhere else. No artificial intelligence language models were used in creating this work.
Conflict of Interest
We declare that we have no conflict of interest within the field of cadmium nor any vested interests in the cadmium sector. H. Sverdrup has worked in the past with assessments concerning setting limits for heavy metals for UN/ECE-LRTAP 1990–2002.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sverdrup, H.U., van Allen, O. & Haraldsson, H.V. Assessing Aspects of Cadmium Supply, Recycling and Environmental Pollution with Respect to Future Photovoltaic Technology Demands and Envionmental Policy Goals. Water Air Soil Pollut 235, 247 (2024). https://doi.org/10.1007/s11270-024-07027-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07027-2