Abstract
For industrial processes—like waste incineration—it is necessary to reduce solid components (like dust or fly ash) as well as gaseous components (like dioxins, CO and other harmful hydrocarbons) to fulfill legal requirements. Therefore, catalytically functionalized filters based on polymers already exist. However, it is known that such filters are always constructed in multiple layers to prevent the migration of catalyst particles. This study demonstrates that it is possible to prepare a stable catalytic functionalized single-layer filter based on polyester needle felt by using flame spray pyrolysis. The catalyst is a low temperature active Pt/TiO2 with a loading weight of 38 g/l on the filter. Via SEM images the uniform distribution of the catalytic particles even in the deeper regions of the single-layer filter was proven. The structure was confirmed after experiments under realistic conditions—migration could not be obtained. Likewise, it was obtained that the oxidative conversion of carbon monoxide (CO) to carbon dioxide (CO2) is completely even at temperatures below 100 °C. Furthermore, comparative studies with catalysts on a honeycomb and a ceramic foam have shown that the conversion on the polyester needle felt textile catalyst is comparable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Industries across the spectrum, from manufacturing to chemical processing and transportation, invariably generate airborne and waterborne particles as byproducts of their operations. These particles, often comprising hazardous substances, pose a dual threat: they contribute to air, degrading the quality of our natural resources and compromising the well-being of ecosystems and human populations. Moreover, these emissions frequently contain greenhouse gases and other pollutants like NOx, HC or SOx that exacerbate climate change and contribute to the broader spectrum of environmental challenges faced globally [1, 2]. Waste incineration exemplarily is one of the main sources of industrial dust and gas emissions. The exhaust gases contain high levels of dust (> 10 mg/m3) and harmful hydrocarbons (> 10 mg/m3). To remove the gaseous and solid emissions different setups are in use and the temperature range at the removal point varies between 100 and 300 °C [3]. These conditions allow to use of filters and separate catalysts. The filters used for this purpose often rely on polymer or ceramic materials and are characterizes by face velocities of 0.5 to 2 m3/m2·min. Catalysts, on the other hand, consist almost exclusively of metallic or ceramic substrates with varying cell density and coating thickness. Depending on the specific application, the thickness of the catalytic material layer ranges from 15 to 100 µm, which can result in loading weights of 30 to 200 g/liter, depending on the material. Cell densities typically ranges from 50 to 400 cpsi depending on the system requirements. With increasing cell density, activity and pressure drop usually also increase. As the pressure drop is a critical factor for economic reasons, ceramic honeycombs with a maximum of 233 cpsi are typically used in industrial plants. Depending on the conditions of use, these can be used as solid extrudates or as coated honeycombs, typically using cordierite or Al2O3 [4]. Besides honeycomb substrates, ceramic foams serve as another option for catalyst supports. In the field of emission reduction in small combustion plants, foams based on Al2O3 with a pore size in the range of 10–30 ppi are often the preferred choice. Due to their irregular structure and spacious pores, these foams can be utilized with low back pressure. Typical space velocities for ceramic foams and honeycombs fall within the range of 30,000 to 80,000 h−1. The type of oxidation catalyst in the field of emission reduction often depends on the operating temperature. While precious metal-free oxide materials such as MnO2, Fe2O3 or mixed oxides are commonly used at temperatures above 200 °C, precious metal-based catalysts with platinum or palladium are often used for applications below 200 °C [4].
When using separate catalysts and filters to remove dust and emissions, the respective pressure losses must be considered additively and may be detrimental to the operation of the plant. Furthermore, separate units require a considerable amount of space. Against this backdrop, the concept of simultaneous particle filtration and emissions reduction has become a focal point in efforts to achieve sustainable industrial practices. An example of this is the exhaust air purification of waste incineration plants, which already relies on this technology [5]. The type of filter medium depends on many factors, with the process parameters temperature and exhaust composition being particularly decisive. In addition, the amount and size of the materials to be filtered is, of course, crucial for the type of filter. Additionally, the necessity of periodic filter cleaning through short pressure shocks is pertinent. These cleaning cycles involve the injection of pressure shocks, causing a reverse flow to dislodge filtered dust particles from the filter. These pressure shocks impose a mechanical stress on the catalytic particles fixed on the filter, emphasizing the critical need for their secure attachment to the filter.
The use of ceramic filter cartridges has become established in the exhaust air purification of many industrial processes with exhaust gas temperatures above 300 °C [6]. Generally, they are characterized by high filtration efficiency of above 99.5% at broad temperature ranges [7, 8]. However, it is necessary to distinguish between different types of ceramic filters. In addition to ceramic membrane filter cartridges which are characterized by low porosity and high filter performance, there are also ceramic fiber filters existing with a high porosity and lower filter performance. The latter is also suitable for catalytic coating, as the catalyst particle do not clog the huge channels and thus not increase the backpressure [9,10,11,12,13,14,15,16,17,18]. Those ceramic materials can be coated be several procedures like dip coating or incipient wetness coating, e.g. ceramic fibers coated with SCR catalysts are widely used for simultaneous particle filtration and DeNOx [19,20,21].
For applications at temperatures below 250 °C also PTFE filter candles can be used. They could also be catalytically coated however the coating process is more complicated as there are no attractive interactions between the PTFE surface and the inorganic catalyst particles. Thus, the addition of binders is necessary which makes the preparation more demanding. Typically, a bag over bag principle is used consisting of at least two layers of PTFE filter. This has the advantage that the dust particles are captured on the outer layer while the emissions pass the second catalytically functionalized filter on the inner filter bag [22]. However, multilayer systems naturally increase the pressure drop, which has an unfavorable effect on the operating conditions. Moreover, as the preparation contains binders, the catalytic particles are deposited not only on the fibers but also in the interstices, which also increases the pressure drop [23]. Finally, there is the question of whether polyfluorinated materials such as PTFE should be used for this purpose in the long term, as their impact on the environment is considered to be of concern [24].
For filtration tasks at temperatures below 150 °C, polyester needle felts are very popular. These materials are inexpensive and are characterized by high filtration performance. Depending on the filtration task, they can be used in a wide range of basis weights ranging between 250 and 600 g/m2 [25]. Catalytic functionalization of polyester needle felt textiles has not been considered so far, as polyester, similar to PTFE, has no functional groups to which the inorganic particles could be chemically bound. Consequently, a binder-based process would also have to be used, but this is not feasible due to the high cost and limited applicability in terms of temperature stability. A process for direct coating of textile fibers could overcome this problem and make the catalytic functionalization of polyester materials attractive. The flame spray pyrolysis (FSP) used in this paper addresses this point by baking the catalytic particles into the polyester needle felt textile fibers in a form closuring way. FSP is typically used to prepare nanoparticles by contacting a precursor solution with a flame that initiate the chemical transformation [26]. Within this paper the FSP is used the heat up the already prepared catalytic particles in order to contact them with the polyester textile. This novel and successful approach is described in detail below.
2 Experimental
2.1 Reagents
A solution of Bis(ethanolammonium)hexahydroxoplatinum(IV) (Precious metal content 9.88 wt%) in liquid form was acquired from Heraeus. The SiO2-doped TiO2, identified as DT-S10, was purchased from Tronox. Carl Roth supplied the solution of Cerium(III) nitrate hexahydrate. The polyester needle felt (with a basis weight of 550 g/m2) was obtained from Kayser Filtertech. Hoffman Ceramic provided the 20ppi Al2O3 foam. The 233 cells per square inch (cpsi) Al2O3 honeycomb was supplied by Porzellanfabrik Hermsdorf. Acetic acid was procured from Sigma Aldrich.
2.2 Catalyst Powders
2.2.1 Pt/TiO2
The catalyst was prepared via incipient wetness impregnation. For this purpose, 5 g of DT-S10 was placed in a 50 ml tumbler. Subsequently an aqueous solution of bis(ethanolammonium)hexa-hydroxoplatinum(IV) was slowly dropped onto the DT-S10 while stirring the solid. The mixture was dried overnight at 80 °C followed by a calcination step at 400 °C for 1 h. The adjusted mass fraction of platinum was set to 4 wt%, based on the DT-S10.
2.2.2 Pt/Ce/TiO2
Pt/Ce/TiO2 was synthesized following the same route as described for Pt/TiO2. However, prior to the addition of platinum, cerium oxide was impregnated by incipient wetness impregnation. Therefore, an aqueous solution of cerium(III) nitrate hexahydrate was used. The four resulting catalysts contain between 1 and 10 wt% of cerium by mass. The nomenclature of the catalysts is adjusted according to the mass content and is therefore Pt/Ce2/TiO2 for the catalyst loaded with 2 wt% cerium. The adjusted mass fraction of platinum is again 4 wt% based on DT-S10.
2.3 Coated Catalyst Materials
2.3.1 Flame Spray-Based Synthesis of Polyester Needle Felt Textile Catalyst
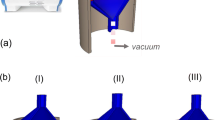
To prepare the catalytic polyester needle felt textile, a 120 mm round piece of polyester needle felt was used in a self-designed FSP as shown in Fig. 1 (left). The FSP consists of a two-substance nozzle (Lechler, Model 136.316.16.A) with a spray angle of 25° and two commercial gas burners. Furthermore, it consists of a black steel tube with 120 mm diameter and variable length between 400 and 500 mm, in which the polyester needle felt was fixed to the bottom. To suck the air containing particles into the black tube, a compressor was connected underneath (SKV-NS-95-1-101) with an 80 mm flexible pipe. During preparation, the pressure above and below the polyester needle felt was measured with a differential pressure system. For synthesis, the polyester needle felt textile was attached to the bottom of the tube and the compressor was adjusted to maintain a constant differential pressure of 30 mbar. After starting the burners, a temperature of approx. 130 °C was reached on the polyester needle felt textile, measured with two K-Type thermocouples placed directly above the textile and in the middle of the black tube. The Pt/TiO2 catalyst particles (solids content approx. 20 wt%) suspended in a solution of water and acetic acid (2:1) are metered into the two- fluid nozzle by a peristaltic pump (ProMinent Type Dulcoflex DF4a). The dosing rate of the stable suspension was 0.00143 l/min while the air flow rate was 10 l/min. When the atomized suspension passes the burner the catalytic particles immediately heat up before they impact the polyester needle felt textile. As the particle temperature as well as the polyester needle felt textile temperature was above the glass transition temperature of the polyester needle felt (at around 75 °C) the particle can easily penetrate the textile fiber. As shown in Fig. 1 (right) the particle deposition exclusively takes place in the area where the compressor sucks air through the polyester needle felt. The dosing time of the suspension was adjusted so that the loading weight with catalyst reaches almost 38 g catalyst per liter of substrate (textile), what was also confirmed gravimetrically and is within the typical loading range for oxidation catalysts, which is often between 30 and 80 g/l. It has also been observed that this coating method is highly reproducible at a loading weight of approx. 38 g/l.
2.3.2 Coated Ceramic Catalysts
The Pt/TiO2 catalyst was coated on both honeycomb (233 cpsi) and foam (20 ppi). The Pt/Ce/TiO2 was exclusively coated on a 20 ppi ceramic foam.
2.3.2.1 Preparation of Catalyst Solution
For coating the ceramic carriers, the powdered Pt/TiO2 catalyst was transferred to an aqueous suspension (Solid content between 30 und 40 wt%). First, pH was adjusted to 4 by using diluted HNO3 before adding 1 wt% of a powdered boehmite (Pural, Fa. Sasol) while stirring. In addition, a small amount of methyl cellulose was added to enhance viscosity. The solids content was adjusted so that the target amount of solid could be achieved with a single dip coating process. A catalyst loading of 38 g dry mass per liter substrate volume was the targeted value.
2.3.2.2 Foam Catalyst
A cylindrical Al2O3 foam ceramic (20 ppi) with 25.4 mm diameter and a length of 12 mm is coated by a dip coating process. First, the substrate was dipped into the liquid catalyst solution for several seconds. Subsequently it is carefully blown out with air. The so prepared ceramic was then dried at 80 °C overnight before calcination at 400 °C for two hours in air atmosphere. As previously mentioned, the Pt/Ce(2 to 10)/TiO2 materials are also prepared on foams. For preparation the same synthesis route was used.
2.3.2.3 Honeycomb Catalyst
A cylindrical Al2O3 honeycomb (233 cpsi) with dimensions 25.4 mm (Diameter) and a length of 12 mm was prepared by dip coating. The procedure used was the same as described for the ceramic foam.
3 Characterization
3.1 Powder Catalysts
3.1.1 Nitrogen Sorption Experiments
Nitrogen sorption experiments (N2) were conducted on a Surfer (Thermo Fisher Scientific, Waltham, MA, USA) at a temperature of − 196 °C. Data evaluation was done using the Thermo Fisher Scientific Software Surfer Version 1.7.2). BET-plots were acquired a relative pressure range from 0.05 to 0.3.
3.1.2 X ray Diffraction
The X ray diffraction analysis was performed on a D2 PHASER 2nd Gen from Bruker. The X-ray source is a Cu anode with a wavelength of λ(Kα) = 1.52 Å and an accelerating voltage of 30 kV at 10 mA. The primary optic was a 4° Soller aperture with a 2 mm divergence slit. The secondary optic was a 4° Soller aperture with a nickel filter. The detector was a LEE-T sensor with 192 channels and a resolution below 380 eV (Cu).
3.1.3 Transmission Electron Microscopy
The Transmission electron microscopic measurements were performed on a Jeol JEM-2100 Plus transmission electron microscope at an applied accelerating voltage of 200 kV. For the preparation, the sample was first mortared in ethanol for one minute. Then the suspension was sedimented for another 10 min and a small amount was taken up from the top layer using a pipette. A small drop of the absorbed volume was consumed on the holey carbon layer of the copper TEM grid. After a short time, the ethanol was volatilized and the sample carrier including the sample can be placed in the measuring instrument.
3.2 Substrate Based Catalysts
3.2.1 Scanning Electron Microscopy
SEM images were taken with a Zeiss Gemini DSM 982 equipped with an Inlens detector. Prior to the measurements the samples were sputtered with a 20 nm carbon film in order to enhance the electric conductivity.
3.2.2 Pressure Drop Analysis
The pressure drop was assessed within a custom-built test bench comprising a 40 mm inner diameter tube. A sample could be attached to the end of the tube. For pressure measurement, a differential pressure sensor was connected to the tube upstream of the sample, while a second sensor operated against ambient pressure. The pressure drop was recorded across various flow rates ranging from 0 to 30 l/min. The sample dimensions were fixed at a 20 mm diameter. The effects of wall friction can be neglected under the specified parameters.
3.2.3 Migration Tests Procedure
The same setup (as in 3.2.2.) is used to analyze the particle adhesion on the polyester needle felt textile. The polyester needle felt textile is clamped into the apparatus, and pressure pulses (1 s duration) are generated using an automated valve, resulting in a differential pressure of 50 mbar upstream and downstream of the filter. The textile subjected to 1000 pressure pulses during the test. The proportion of migrated particles is determined by gravimetric measurements before and after the experiment.
3.2.4 Catalytic Activity in CO Oxidation
The catalytic activity of the polyester needle felt textile samples was tested in a different test cell than the other samples, as described below. The results are shown as temperature-dependent conversion.
Conversion was given as: \({X}_{CO}=1-\frac{{c}_{CO}}{{C}_{CO,0}}\). The initial CO concentration CO, 0 is given to 100 ppm.
The coated polyester needle felt was measured in a self-constructed cell consisting of two parts (Fig. 2). The 20 mm round piece of polyester needle felt textile was clamped between the two halves. It was fixed with O-rings (NBR). A type K thermocouple was mounted under the polyester needle felt textile to measure the inlet gas temperature. The gas enters horizontally at the bottom of the cell in order to ensure a uniform flow through the polyester needle felt textile. The cell was electrically heated. Prior to the measurement the sample was pre-treated for 30 min at 150 °C in air. Subsequently the sample was cooled to 100 °C and the gas mixture was initiated. Data points were collected from the cooling curve. The space velocity was set to 25,000 h−1, 50,000 h−1 and 75,000 h−1, with the gas matrix consisting of 100 ppm CO, 16 vol% O2, 2 vol% H2O by volume, and N2 as balance. The flow rate of each gas was adjusted via independent mass flow controllers (Bronkhorst® Germany North,). Gas concentrations were measured at the reactor outlet using an electrochemical sensor (Testo 340, Fa. Testo).
3.2.5 Foam and Honeycomb
For the CO oxidation activity measurements, the respective samples were placed in a tubular quartz reactor with an inner diameter of 30 mm and sealed with quartz wool. The reactor was placed in a horizontal tube furnace, connected to the exhaust manifold, and heated up to 150 °C in air for 30 min. After the short pre-treatment the sample were cooled to 100 °C and gas mixture was initiated. Data points were collected from the cooling curve. The space velocity was set to 25,000 h−1, 50,000 h−1 and 75,000 h−1, with the gas matrix consisting of 100 ppm CO, 16 vol% O2, 2 vol% H2O by volume, and N2 as balance. The flow rate of each gas was adjusted via mass flow controllers (Bronkhorst® Germany North,). Gas concentrations were measured at the reactor outlet using an NDIR spectrometer (Saxon Junkalor Type Infralyt 80).
4 Results
4.1 Powder Catalysts
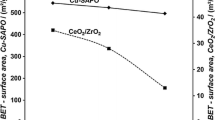
The specific surface area (SSA) of the powdered materials, determined by N2 physisorption, provides values of 110 m2/g for the pure DT-S10 (TiO2) and 101 m2/g for the Pt/TiO2 catalyst. The decrease in SSA is more pronounced for the ceria containing catalysts. Samples with 1 and 2 wt% cerium have an SSA of 85 m2/g, while samples with 5 and 10 wt% have a SSA of 82 m2/g and 68 m2/g respectively. The reduction in specific surface area (SSA) could be attributed to blockage of pores caused by ceria particles.
X-ray analysis shows reflexes (2θ) at angles of 25.3°, 37.60°, 40.5°, 49.8°, 54.36° and 62.70° for all samples attributed to anatase phase of TiO2 according to JCPDS with reference number 23-0278. Anatase phase is marked as empty dots in Fig. 3. In addition, a broad reflection for the platinum (dotted grey) is found at 46.2° (2θ). Using this signal from the Pt/TiO2 sample, the estimated average crystallite size for the platinum is 5.2 nm. The reflex attributed to platinum is also found in the ceria loaded samples wherefore similar crystallite sizes between 3.5 and 5.2 nm can be estimated. Reflexes at 28.43° and 32.98° (2θ) are attributed to CeO2 according to JCPDS card 34-0394 marked as black dots. These reflexes can exclusively be found for the samples with 5 and 10 wt% ceria content. Calculation of the CeO2 crystallite size for the Pt/Ce10/TiO2 sample is 4.3 nm. This suggests that the estimated size of the cerium oxide and platinum crystallites is evidently in the same range. The found crystallite sizes are in good agreement with literature data for Pt/CeO and Pt/TiO2 catalysts [27, 28]
TEM analysis is presented in Fig. 4 for the sample containing 10 wt% cerium. Small entities, measuring between 3 and 14 nm in size, are observable, although precise attribution to either platinum or ceria is not possible. To estimate an average particle size, several hundred particles were counted and analyzed. Despite the broad particle size distribution, an average particle size of 5.9 nm can be computed, which aligns well with the findings obtained from X-ray diffraction. As previously mentioned, these findings align with data found in literature on Pt/TiO2 or Pt/CeO2 catalysts, known for their low-temperature oxidation activity.
4.2 Coated Catalysts
To assess the catalytic activity of the coated polyester needle felt textile in comparison to conventional catalysts, substrates comprising a honeycomb and a ceramic foam were also coated. A coating weight of 38 g/l was targeted for all samples. The effective loadings are shown in Table 1.
The as-prepared polyester needle felt textile was analyzed using scanning electron microscopy, revealing catalytic particles uniformly deposited on the polyester fibers. (Fig. 5a, b). Moreover, the coating does not form a closed catalytic layer, but is deposited evenly and sporadically on the fiber, without large agglomerates. This is consistent with theoretical calculations of the layer thickness, which showed that due to the enormously high geometric surface of the textile sample (62.3 m2 per liter of substrate), a layer in the sub-micrometer range would result. As can be seen in Fig. 5a, no large particle agglomerates are observed in the areas between the fibers. It can therefore be assumed that the air permeability of the coated polyester needle felt textile is not significantly influenced by the coating. The coating can even be observed in the deepest layers of the textile (downstream side), but with slightly decreasing intensity. This result is surprising considering that the process is based on a one-sided coating on the upstream side of the polyester needle-felt. Fortunately, however, this means that coating from both sides is not necessary, which is advantageous from an economic point of view.
As shown in Fig. 5c, the coating of the ceramic foam results in a thin layer with a variable thickness between 20 and 120 µm. The uneven coating thickness is a consequence of the process in which the aqueous solution is absorbed into the substrate by dip coating and subsequently dried. With these highly curved porous materials, capillary effects occur during the drying process, resulting in an uneven deposition. The coating on the honeycomb yields a continuous layer, albeit with varying thicknesses as shown in Fig. 5d. Here, too, the corners are significantly more heavily coated than the flat surfaces due to capillary effects. Considering the relatively small geometric surface of the ceramic honeycomb and ceramic foam (2.2 m2/l and 3.6 m2/l, respectively), the determined coating thicknesses agree with the theoretically calculated average values of 18 and 11 µm, respectively.
4.3 Migration Tests
In addition to the uniform distribution of the catalytic particles on the polyester needle felt, it is crucial for the technical application that the particles adhere to the textile fibers even in the case of process-related pressure surges and do not begin to migrate. The aim of the migration tests was therefore to evaluate the adhesion properties of the catalytic particles on the polyester needle felt under realistic conditions. For this purpose, the polyester needle felt is subjected to a treatment involving 1000 pressure shocks, which resulted in a mass loss of less than 1% relative to the coating mass. An additional 1000 pressure shocks did not result in a further reduction of particle mass. Evidently, the adhesion of the particles to the polyester needle felt is robust enough to withstand the applied pressure shocks.
4.4 Pressure Drop
As previously stated, due to the coating methods, it can be assumed that the catalytic particles are deposited exclusively on the textile fibers and have only a minor impact on the air permeability. To investigate this, pressure drop experiments were carried out with both the uncoated and the coated polyester needle felt. In addition, the pressure drop of the ceramic catalysts was also tested. Due to the different thickness of the test samples, the pressure drop is presented as a normalized pressure drop related to the same substrate length.
The choice of air volume in these experiments is based on the operating conditions for polyester-based textile filters. In this context, the face velocity is used instead of the space velocity as defined below. The face velocity is calculated by dividing the volumetric flow \(V\) per minute by the filter area A, as shown here:
Typically, this value ranges from 0.5 to 2 m/min. In the experiments shown here, the face velocity is investigated in the range between 0.32 and 0.95, which corresponds to a volume flow of 10 to 30 l/min as shown in Table 1.
As depicted in Fig. 6, the pressure drop for the ceramic honeycomb and ceramic foam is notably low under the conditions investigated, as expected. The investigation of the uncoated and coated textile reveals an almost linear relationship between pressure drop and face velocity in the observed range. At a flow rate of 30 l/min, the normalized backpressure of the coated polyester needle felt textile is 12.1 mbar, while that of the uncoated is only 8.4 mbar. However, the effective difference in pressure drop between the uncoated and coated polyester needle felt textiles also decreases with decreasing face velocity. Since a structural change of the textile due to the coating process could not be observed with any analytical method, it can be assumed that the pressure difference is due to the catalytic coating.
4.5 Catalytic Activity Tests
The catalytic activity of the polyester needle felt filter is examined on the basis of CO oxidation to CO2. The oxidation of carbon monoxide is an important reaction in almost all technical combustion processes that generate particulate matter. Three different space velocities were chosen for the experiments. Converting these space velocities to face velocities shows that this range covers the typical face velocity for filters, roughly from 0.5 to 2 m3/m2 min.
Regarding Fig. 7 it can be stated that all catalysts exhibit high activity at the space velocities and temperatures selected, confirming the low-temperature activity of the Pt/TiO2 catalyst. High CO conversions in oxygen-rich environments at temperatures below 100 °C are already documented in the literature for Pt/TiO2 catalysts [29]. When comparing the catalysts investigated here, it can be observed that the catalytically functionalized polyester needle felt provides the highest activity (Fig. 7a). This material initiates CO conversion at 40 °C. At the lowest space velocity, complete CO conversion is already evident at 70 °C. Even at higher space velocities, complete conversion is observed from 90 °C onwards. Thus, the temperature range is well-suited for the mentioned applications regarding simultaneous filtration and emission reduction. Looking at the CO conversion on the honeycomb catalyst (Fig. 7b), it is evident that although the conversion starts at lower temperatures, it follows a different trend with increasing temperature compared to the textile, and complete CO conversion cannot be achieved within the temperature range independent of the space velocity. A very similar activity trend can be observed for the ceramic foam (Fig. 7c). Here, a noticeable CO conversion is evident at 30 °C, irrespective of the space velocity. However, complete conversion cannot be achieved for this catalyst at any space velocity. Yet, with the lowest space velocity of 25,000 h−1, an almost complete (98%) CO conversion is attained at 100 °C. It is worth noting that the superior activity of the polyester needle felt catalyst is not necessarily attributable to the substrate structure and the resulting low coating thickness. Instead, it is presumed that the catalytic Pt/TiO2 particles undergo a chemical reduction during the flame spray coating process, a phenomenon well described in the literature. [30]. Often, pre-reduction of a catalyst increases its catalytic activity. For this reason, a pre-conditioning at 150 °C in air for 30 min was conducted, which, however, might not have been sufficient to establish a comparable state of the catalyst particles. It should be noted that higher temperatures are not feasible due to the limited temperature resistance of the polyester textile. In conclusion, it can be stated that all catalysts exhibit excellent CO conversion at the observed temperatures. However, compared to the honeycomb and foam ceramics, the polyester needle felt textile, in addition to emission reduction, has the ability to filter particles from the exhaust gas with high efficiency.
Considering the use of catalysts that achieve high pollutant conversion even at lower temperatures, the scope of application could expand from industry to households in the future. Current indoor air purification systems tend to focus on carbon capture and storage techniques [31]. The use of catalytic pollutant reduction at ambient temperature and slightly elevated levels, however, would be quite interesting for health reasons. To assess the extent to which this is achievable with conventional catalytic materials based on Platinum, cerium oxide-modified catalysts were developed, showing promising results, as depicted in Fig. 8. In comparison to the Pt/TiO2 foam catalyst (Fig. 7c) at a space velocity of 25,000 h−1, all modified materials exhibit better conversion rates. Complete conversion can be achieved as early as 65 °C, despite the CO concentration in this experiment being five times higher. With the exception of Pt/Ce2/TiO2, a continuous increase in activity can be observed with cerium loading. The previously described nonlinear correlation between cerium loading and increased activity is well-documented in the literature. It is presumed that the interfacial area between cerium oxide and platinum is particularly responsible for increased activity at low temperatures [32]. The most active catalyst, Pt/Ce10/TiO2, already shows low conversions at 25 °C and reaches 50% conversion at 30 °C. Complete conversion is observed at 55 °C. Hence, it is assumed that polyester needle felt filters equipped with such low-temperature catalysts could also be used in indoor settings (e.g., air purification of odors in the kitchen). Future work may also investigate whether catalytic functionalization by FSP can be extended to other types of textiles. Especially in the field of nitrogen oxide reduction on PTFE-based textiles, the new coating method could present an interesting alternative to previous preparation methods.
5 Conclusion
This study demonstrates that a flame spray pyrolysis setup is suitable for coating an air-permeable polyester textile. At a catalyst loading of 38 g/l, the deposition of the particles on the polyester needle felt textile substrate does not form a continuous layer on the fibers, but rather a scattered and uniform deposition. This is consistent with the fact that the specific surface area of the textile is up to 25 times larger than that of the ceramic substrates. Migration tests under realistic conditions have shown that the adhesion of the catalytic particles to the polyester textile is high and the catalytic particles do not migrate. The results from the carbon monoxide conversion clearly indicate that the activity of the coated textile and the ceramic substrates is similar. The conversion rate for the coated textile is slightly higher, suggesting a potential influence of the coating process on the catalytic behavior. It has been demonstrated that complete conversion of carbon monoxide can be achieved below 100 °C under the flow conditions typical of textile filter materials. Additional experiments with ceria-modified Pt/TiO2 samples show that high CO conversion can already be achieved at 40 °C and below. Low-temperature catalysis on a textile filter material can be useful for various applications, including indoor pollutant reduction.
Data availability
The data that support the findings of this study are available from the corresponding author, Andreas Roppertz, upon reasonable request.
Change history
06 March 2024
Copyright year has been changed as 2024 in XML.
References
Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions, https://eur-lex.europa.eu. Accessed 23 Aug 2010
Dincer I (2018) Comprehensive energy systems, vol 1. Part A, Energy fundamentals. Elsevier
Thabit Q, Nassour A, Nelles M (2022) Flue gas composition and treatment potential of a waste incineration plant. Appl Sci 12:5236
Ertl G, Knözinger H, Weitkamp J (eds) (1997) Handbook of heterogeneous catalysis, Vol 2. Weinheim: VCH, pp 427–440. https://doi.org/10.1002/9783527619474
Hsu WH, Hung PC, Chang MB (2015) Catalytic destruction vs. adsorption in controlling dioxin emission. Waste Manag 46:257–264
Li H, Huangfu L, Li J, Gao S, Xu G, Yu J (2022) Recent advances in catalytic filters for integrated removal of dust and NOx from flue gas: fundamentals and applications. Resour Chem Mater 1:275–289
Sharma SD, Dolan M, Ilyushechkin AY, McLennan KG, Nguyen T, Chase D (2010) Recentdevelopments in dry hot syngas cleaning processes. Fuel 89:817–826
Heidenreich J (2013) Hot gas filtration—a review. Fuel 104:83–94
Alonso-Farinas B, Lupion M, Rodriguez-Galan M, Martinez-Fernandez J (2013) Newcandle prototype for hot gas filtration industrial applications. Fuel 114:120–127
Choi HJ, Kim JU, Kim HS, Kim SH, Lee MH (2015) Effect of sintering temperaturein preparation of granular ceramic filter. Ceram Int 41:10030–10037
Krasnyi BL, Tarasovskii VP, Val’dberg AY, Kaznacheeva TO (2005) Porous permeable ceramics for filter elements cleaning hot gases from dust. Glass Ceram 62:134–138
Lin JCT, Hsiao TC, Hsiau SS, Chen DR, Chen YK, Huang SH, Chen CC, Chang MB (2018) Effects of temperature, dust concentration, and filtration superficial velocity on the loading behavior and dust cakes of ceramic candle filters during hot gas filtration. Sep Purif Technol 198:146–154
Park JK, Lee JS, Lee SI (2002) Preparation of porous cordierite using gelcasting method and its feasibility as a filter. J Porous Mat 9:203–210
Ewais EMM, Ahmed YMZ, Ameen AMM (2009) Preparation of porous cordierite ceramic using a silica secondary resource (silica fumes) for dust filtration purposes. J Ceram Process Res 10:721–728
Eggerstedt PM (1995) Lightweight ceramic filter components: Evaluation and application. Fuel Energy Abstracts 38:181
Ahluwalia RK, Novick VJ, Zhang L, Sutaria MP, Singh JP (2001) Performance of avacuum formed chopped ceramic fiber filter in a reducing environment. J Eng Gas Turbines Power-Trans ASME 123:293–302
Cuo ZX, Liu HD, Zhao F, Li WM, Peng SP, Chen YF (2018) Highly porous fibrousmullite ceramic membrane with interconnected pores for high performance dust removal. Ceram Int 44:11778–11782
Wang WJ, Hu XX, Li LY, **g W, Guo AR, Du HY (2019) Silica/mullite fiber compositemembrane with double-layer structure for efficient sub-micrometer dust removal. Ceram Int 45:6723–6729
Cho EH, Suh KS, Shin MC, Shin HG, Lee HS, Niihara K (2009) Thermal and chemicaldegradation behavior of a catalytic ceramic filter for dust/NO x removal. J Ceram Process Res 10:73–76
Saracco G, Specchia S, Specchia V (1996) Catalytically modified fly-ash filters for NO reduction with NH3. Chem Eng Sci 51:5289–5297
An Z, Niu GP, Tan ZQ, Shang T, Yang XG (2017) Preparation of ceramic catalytic filterand its deNOx performance. Therm Power Gen 46:36–41
Ebert J (2013) Innovative new air pollution control technologies to capture NO x, PM and Hg. American Society of Mechanical Engineers, New York
Gore Remedia: https://www.gore.com/products/dioxin-furan-filters-for-crematoriums-incinerations-metals-processing. Accessed 24 Aug 2023
Umwelt Bundesamt https://www.umweltbundesamt.de/sites/default/files/medien/2546/publikationen/-uba_sp_pfas_web_0.pdf. Accessed 24 Aug 2023
Innocentini MDM, Tanabe EH, Aguiar ML, Coury JR (2012) Filtration of gases at high pressures: permeation behavior of fiber-based media used for natural gas cleaning. Chem Eng Sci 74:38–48
Teoh W, Amala R, Mädler L (2010) Nanoparticles design and fabrication. Nanoscale 2:1324–1347
Boronin AI, Slavinskaya EM, Figueroba A, Stadnichenko AI, Kardash TYu, Stonkus OA, Fedorova EA, Muravev VV (2021) Appl Catal B 286:119931
Shi K, Wang L, Li L, Zhao X, Chen Y, Hua Z, Li X, Gu X, Li L (2019) Mild Preoxidation treatment of Pt/TiO2 catalyst and its enhanced low temperature formaldehyde decomposition. Catalysts 9:694
Zhangfeng HZ, Wenjuan Q, Wenjuan S, Jianguo S, Wang WJ (2005) Low-temperature oxidation of CO over Pd/CeO2–TiO2 catalysts with different pretreatments. J Catal 233:41–50
Trommer RM, Bergmann CP (2015) Flame spray technology. Springer, Berlin
Baus L, Nehr S (2022) Potentials and limitations of direct air capturing in the built environment. Build Environ 208:108629
Shi Y, Wang J, Zhou R (2021) Pt-support interaction and nanoparticle size effect in Pt/CeO2–TiO2 catalysts for low temperature VOCs removal. Chemosphere 265:129127
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bissinger, D., Honerkamp, J.H., Roldan, J. et al. Development of Catalytically Functionalized Polyester-Based Filters Produced by Flame Spray Pyrolysis. Top Catal 67, 539–550 (2024). https://doi.org/10.1007/s11244-023-01892-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-023-01892-7





 )
)



 ) and 75.000 h−1 (○). Conditions: 100 vppm CO, 16 vol% O2, 2 vol% H2O with N2 balance
) and 75.000 h−1 (○). Conditions: 100 vppm CO, 16 vol% O2, 2 vol% H2O with N2 balance