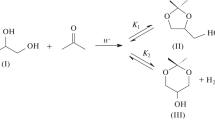
Abstract
The treatment of clay minerals with a preliminary acid wash and titration to pH 7 has proven to generate catalysts for the most interesting of oligomerization reactions in which activated RNA-nucleotides generate oligomers up to 40-mers. Significantly, not all clay minerals become catalytic following this treatment and none are catalytic in the absence of such treatment. The washing procedure has been modified and explored further using phosphoric acid and the outcomes are compared to those obtained when clay samples are prepared following a hydrochloric acid wash.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The RNA world hypothesis for the origins of life proposes that RNA stored the genetic code and catalyzed the first life on Earth. Evolution of the RNA world resulted in the formation of the DNA/protein world present on the Earth today. So far, there is no prebiotic route to RNA but it has been possible to generate RNA oligomers by the montmorillonite-catalyzed reaction of activated monomers. Reactions in the terrestrial environment forming oligonucleotides having 40 or more bases are generally one of the key requirements for the RNA world scenario for the origin of life on Earth (Ferris 2006). This proposal requires that RNA both store genetic information and catalyze chemical reactions. Exceptionally, short oligonucleotides can also have catalytic activity (Turk et al. 2010). The oligomerization of activated nucleic acids can be achieved by the use of a catalyst, montmorillonite, which occurs naturally on Earth. However, not all montmorillonites are catalytic.
We have reported the need to remove the interlayer metal cations and replace them with alkali metal and some alkaline earth cations before catalytic activity is observed (Joshi et al. 2009). Then, a variety of activated nucleotides can be successfully oligomerized on montmorillonite and analyzed (Huang and Ferris 2006; Joshi et al. 2007; Miyakawa et al. 2006; Zagorevskii et al. 2006). Analytical findings lead to the general conclusion that the mineral could have initiated the RNA world and metal ion catalyzed reactions on the early Earth. Our studies are merely a model since, currently, it is not known whether montmorillonites or nucleotides were present on the early Earth. The recent identification of phyllosilicates including montmorillonite on Mars (Bishop et al. 2008) raises the possibility that such processes may have taken place there too. Our recent findings (Joshi et al. 2009) emphasize the characteristics of the RNAs formed by montmorillonite catalysis. The mechanism by which montmorillonite catalyzes the formation of RNA remains a current focus of our investigations. The montmorillonite-catalyzed reactions of the 5′-phosphorimidazolide of adenosine following the use of a phosphoric acid wash were investigated to compound our study on the effect of acids on the preparation of the catalysts for this reaction.
Experimental Details
General
Adenosine-5′-monophosphate (AMP), anhydrous sodium perchlorate (NaClO4); 2, 2′-dithiodipyridine, imidazole, perchloric acid (HClO4), sodium phosphate monobasic (NaH2PO4), trifluoroacetic acid (TFA), triphenylphosphine, triethylamine (TEA) and Trizma base (Tris) were obtained from Sigma/Aldrich. N, N-Dimethyl-formamide (DMF), dimethylsulfoxide (DMSO), acetonitrile (CH3CN), ether and acetone were obtained from Mallinckrodt. Anion exchange resin (IONAC®NA-38, OH− Form, type 1, Beads 16–50 Mesh) and sodium chloride (NaCl) were procured from J.T. Baker. Sodium hydroxide (NaOH) was obtained from Macron Fine Chemicals, PA. Orthophosphoric acid (75%) and HPLC grade sodium perchlorate (NaClO4) were purchased from Fisher. Ultrapure molecular biology grad nuclease-free water was obtained from USB Corp., Cleveland, OH.
Analytical Methods
HPLC analysis was performed on a Hitachi L-6200A intelligent pump system equipped with a Hitachi L-4000 UV detector operating at 260 nm. The oligomers as anions were separated on a Dionex DNAPac®-100 (4.0 × 250 mm) analytical anion exchange column from Dionex Corporation, Sunnyvale, CA, using a gradient of 0–0.4 M NaClO4 with 2 mM Tris at pH 8. The analysis of activated nucleotides (ImpA and ImpU) for examination of their purity, was performed on a reverse phase Alltima C-18, 5 μ (4.6 mm × 250 mm) column (Grace) using a gradient of 0.02 M NaH2PO4 with 0.2% TFA (pH 2.5) and 30% CH3CN in H2O with 0.2% TFA (pH 2.5). For the collection of oligomers for mass spectral analysis, the desalting of the montmorillonite free oligomer mixture from sodium chloride was carried out by passing it through a C-18 column (24 mm × 100 mm), washing the material with water (50 mL) and extracting the oligomers with 30% CH3CN (100 mL). Electrospray HPLC mass spectra (ESI HPLC/MS) were obtained on an Agilent 1100 series LC/MSD in trap system. Samples were introduced into the ion source using a syringe pump at a flow rate of ~7 μL/min or using an auto sampler.
Preparation of Catalytic Montmorillonite
From the raw montmorillonite samples obtained from the Pembina Hill region, 2 km south of Treherne (southwestern Manitoba, Canada) (Aldersley et al. 2011a, b) and Belle Fourche, South Dakota, USA), their acidic forms (H+-montmorillonite) were prepared as follows: four separate 12 g samples of Treherne clay were treated with 50 mL of 0.125 M, 0.25 M, 0.50 M and 1.0 M H3PO4 respectively with constant stirring (30 min) at room temperature. At the end of each treatment, excess acid was removed by centrifugation (3500 rpm) and decanting of the supernatant. Fresh acid (50 mL) was added to the montmorillonite pellet and the treatment was repeated for one or two more times. The H+-montmorillonite samples were washed with 100 mL deionized water at room temperature for 30 min with constant stirring. At the end of the washing, excess water was separated, again by centrifugation (3500 rpm) and decanting of the supernatant. Washing with water was repeated three times. For each sample, the H+-montmorillonite slurry was added to water (1000 mL) and to this was added 45 mL of wet anion exchange resin to remove any residual acid. The mixture was stirred for 30 min, the pH is measured (3.00 ± 0.2), the anion exchange resin was removed by filtration using a stainless steel sieve (115 mesh), and the H+-montmorillonite slurry was removed and freeze dried. For each sample, one gram of H+-montmorillonite was dispersed in 100 mL of deionized water and titrated with 0.02 M aqueous sodium hydroxide to pH 7. The water was separated by centrifugation (3500 rpm) and the Na+-montmorillonite pellet was freeze-dried. Similarly, a 12 g sample of the Belle Fourche clay was prepared following washing three times with 0.5 M hydrochloric acid. Subsequent washing with water and resin treatment followed by titration to pH 7 were carried out in the same fashion as with the Treherne clay.
Preparation of the Activated Nucleotides
A mixture of AMP (1.76 mmol) and imidazole (22 mmol) was added to DMF (10 mL) in a 50-mL flask and the solvent evaporated to dryness at reduced pressure. The evaporation was repeated twice with DMF (2 × 10 mL) thereby removing residual water. The residue was dissolved in a mixture of DMF (10 mL) with DMSO (10 mL) and stirred with 2, 2´-dithiodipyridine (6.5 mmol), triphenylphosphine (6.36 mmol), and TEA (6.5 mmol) for 2 h. The resulting product was recovered from the clear yellow reaction mixture as a precipitate by adding the reaction mixture drop wise to a solution of anhydrous NaClO4 (3.5 g) in a mixture of ether (100 mL), acetone (100 mL), and TEA (20 mL) with stirring. The stirring was continued for 30 min (to ensure complete formation of the Na+ salt) and the colorless, flocculent solid, which precipitated, was allowed to settle (30 min). The supernatant was decanted and the remaining reaction mixture was centrifuged. The resulting colorless pellet was washed twice with a mixture of ether (100 mL) and acetone (100 mL) and then with ether (100 mL) and dried overnight in a vacuum desiccator. The purity of ImpA was determined by reverse phase HPLC (purity > 99.5%).
Montmorillonite-Catalyzed Oligomerization of Activated Nucleotides
The stock solution of ImpA (15 mM) was prepared in 1 M NaCl. To a 200-μL reaction mixture was added 10 mg Na+-montmorillonite, the suspension vortexed and then allowed to stand at 24 °C for 72 h. After the completion of reaction, the supernatant was collected from the reaction mixtures by centrifugation at 13,200 rpm/16,100 × g (6 min). The reaction products were further desorbed from the Na+-montmorillonite by extracting four times (2 × 1 h, 1 × overnight, and 1 × 1 h) with 200 μL of 30% CH3CN in 0.1 M NaCl elution reagent. Each extract was collected by centrifugation and combined with the supernatant to give 0.9 mL of combined extracts. The combined extract was diluted to 1.0 mL, filtered with an Alltima 0.45 μm nylon syringe filter, adjusted to pH 4 with 1% HClO4, and incubated at 40 °C for 2.5 h to cleave any unreacted imidazole groups from the activated nucleotides. The samples were diluted (0.1 mL/mL) and 50 μL aliquots are analyzed by HPLC using an ion exchange column, so using 15 nmoles of material per HPLC analysis. The percentage yields of the different products are obtained as an average from three independent determinations. Experimental outcomes were always within 5% of one another. The longest oligomer present was determined from the HPLC traces (Fig. 3) by a visual comparison of retention times.
Reaction and Analysis in the Absence of Minerals
Control solutions of activated nucleotides (15 mM) were prepared in 1 M NaCl (200 μL) without Na+-montmorillonite. The reaction mixtures were allowed to stand at room temperature for 3 days. At the end of reaction time, the mixtures were diluted to 1.0 mL with the 30% CH3CN in 0.1 M NaCl reagent, adjusted to pH 4 with 1 M HClO4, and incubated at 37 °C for 2.5 h to cleave any unreacted imidazole groups from the reaction products and the residual activated nucleotides.
Discussion
There has been a gradual evolution of the methodology in the study of Montmorillonite and its ability to catalyze the formation of long RNA oligomers from activated RNA nucleotides. This has involved the systematic study of the role of the activated nucleotide, the role of the ions, both anions and cations, present both within the clay and in solution, as well as the varying efficiency of the oligomerization process with the pH of the system. This, in turn, generated a standard methodology of using three acid washes and subsequent titration to a desired pH with sodium hydroxide so forming Na+-montmorillonite; finally, the use of pH 7 in the presence of 1 M sodium chloride solution became the standard protocol for the testing of the catalysts since oligomer yields become lower at both higher and lower pH values. (Aldersley et al. 2011a, b)
To date, we have analyzed over 300 samples of montmorillonite, obtained from sources all over the world, and not all Na+-Montmorillonite samples are catalytic when the raw clays have been subjected to the acid-washing protocol of the day followed by titration to the optimum pH of 7. None were found to be catalytic in the absence of this pre-treatment. All catalytic clays, approximately 10% of the total studied, behave similarly with respect to their catalytic behavior and pH, with optimal oligomer yields of oligomers at pH 7 in the presence of 1 M sodium chloride solution, which led to a much more detailed appreciation of the type of mechanism involved in this reaction. (Aldersley et al. 2011a, b).
We have here explored the washing process using phosphoric acid, employing here both different concentrations and different numbers of wash cycles, always finishing with a titration to pH 7 with sodium hydroxide (Table 1) (and see Experimental). In this way, a comparison of the wash with phosphoric acid is compared with that with hydrochloric acid. Of the three acids studied in detail to date, phosphoric acid and hydrochloric acid are contenders as a prebiotic material, although the use of sulfuric acid in a prebiotic Martian context has been reported (Joshi et al. 2015).
Two representative HPLC traces, obtained from the two-oligomer mixtures from ImpA (Fig. 1) and separate Treherne clay samples washed with either hydrochloric acid or phosphoric acid, are shown (Fig. 3). The oligomer length is determinable by the retention time (e.g. by comparison with Joshi et al. 2015). The results were always within 5% of one another and reproducible to the extent that the retention times can be used to identify the oligomer length. For example, using our standard HPLC conditions for these analyses, linear dimers always have a retention time of 11–12 min and hexamers have a retention time of 21–22 min respectively.
Clearly, the analyses (Table 1) show that the distribution of products (Fig. 2) from the hydrolysis product, AMP, through dimers and trimers (both linear and cyclic) to oligomers of 8–10 nucleotides in length, are very similar. We are here comparing the outcomes of the washing procedures with the two acids (Fig. 3).
The longest oligomer identified in each case does not relate simply to either the concentration of the phosphoric acid nor the number of washes as we had hoped. There are several possible reasons for the somewhat anomalous behavior:
-
As phosphoric acid is tri-protic, it is worth reflecting that only the ionization of the first proton is germane to the washing process (pKa values =2, 7, 12 depending upon concentration) (Martell et al. 2001)
-
Phosphoric acid also forms polyphosphoric acids (PPAs) with which it is in equilibrium in aqueous solution. As the phosphoric acid is diluted, there is a lesser tendency to form these PPAs which of course demonstrate a range of pKa values similar to those of phosphoric acid, e.g. for triphosphoric acid pKa values of 1.0; 2.2; 2.3; 3.7; 8.5 (Holleman and Wiberg 2001).
-
The amount of the various PPAs in a sample can be determined by titration methods or colorimetric methods, with the results providing comparable outcomes (Innophos Technical Bulletin 1991)
-
The amount of the various PPAs in a sample can also be determined by NMR (Guffy and Miller 1959) or a numerical method (Iordache et al. 1975)
-
Moreover, the formation of insoluble phosphates (representative solubility products shown in Table 2) from the initial interlayer cations which the acid washing process strives to remove, may in these cases confound the process and from detailed elemental analyses of the raw clays, we are aware that the interlayer cation material does differ from one clay sample to another. Evaluating the contribution of such precipitates to the catalytic behaviors is a subject of ongoing research using standard Mg2+- and Ca2+-Montmorillonites that we have prepared.
Nevertheless, the use of phosphoric acid in the preparation of the clay catalysts to be used in the synthesis of long RNA oligomers was successful (Table 1) providing results comparable and only slightly inferior to those obtained with hydrochloric acid.
References
Aldersley MF, Bamburak JD, Joshi PC, Thompson J, Delano JW, Ferris JP (2011a) Evaluation of Manitoba bentonites in the catalysis of RNA synthesis by montmorillonite (parts of NTS 62G1, 8, 10, K3, N1). Manitoba Geological Survey, Report of Activities 150–157
Aldersley MF, Joshi PC, Price JD, Ferris JP (2011b) The role of montmorillonite in its catalysis of RNA synthesis. Appl Clay Sci 54:1–14
Bishop JL, Dobrea EZN, McKeown NK, Parente M, Ehlmann BL, Michalski JR, Milliken RE, Poulet F, Swayze GA, Mustard JF, Murchie SL, Bibring JP (2008) Phyllosilicate diversity and past aqueous activity revealed at Mawrth Vallis, Mars. Science 321:830–833
Ferris JP (2006) Montmorillonite-catalyzed formation of RNA oligomers: the possible role of catalysis in the origin of life. Philos Trans R Soc B Biol Sci 361:1777–1786
Guffy JC, Miller GR (1959) Nuclear magnetic resonance method for analysis of polyphosphoric acids. Anal Chem 31(11):1895–1897
Holleman AF, Wiberg E (2001) Inorganic chemistry. Academic Press, San Diego, p 729
Huang W, Ferris JP (2006) One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis. J Am Chem Soc 128:8914–8919
Innophos Technical Bulletin (1991) https://www.innophos.com/__sitedocs/polyphosphoric-acid-assay.pdf. Accessed 29 Nov 2016
Iordache O, Zaharia A, Goergescu I (1975) Method for determining the composition of polyphosphoric acid mixtures. Rev Chim 26:8
Joshi PC, Pitsch S, Ferris JP (2007) Selectivity of montmorillonite catalyzed prebiotic reactions of D, L-Nucleotides. Orig Life Evol Biosph 37:3–26
Joshi PC, Aldersley MF, Delano JW, Ferris JP (2009) Mechanism of montmorillonite catalysis in the formation of RNA oligomers. J Am Chem Soc 131:13369–13374
Joshi PC, Dubey K, Aldersley MF, Sausville M (2015) Clay catalyzed RNA synthesis under Martian conditions: application for Mars return samples. Biochem Biophys Res Commun 462:99–104
Martell AE, Smith RM (1976) Critical stability constants, volume 4. Plenum Press, New York
Martell AE, Smith RM, Motekaitis RJ (2001) NIST Database 46. Gaithersburg, National Institute of standards and Technology
Miyakawa S, Joshi PC, Gaffey MJ, Gonzalez-Toril E, Hyland C, Ross T, Rybij K, Ferris JP (2006) Studies in the mineral and salt-catalyzed formation of RNA oligomers. Orig Life Evol Biosph 36:343–361
Turk RM, Chumachenko NV, Yarus M (2010) Multiple translational products from a five-nucleotide ribozyme. Proc Natl Acad Sci U S A 107:4585–4589
Zagorevskii DV, Aldersley MF, Ferris JP (2006) MALDI analysis of oligonucleotides directly from montmorillonite. J Am Soc Mass Spectrom 17:1265–1270
Acknowledgements
This paper is dedicated to the memory of Professor James P. Ferris, mentor, colleague and friend. We are grateful for support from the Undergraduate Research Program of the Department of Chemistry and Chemical Biology, Rensselaer Polytechnic Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Jim Ferris
Rights and permissions
About this article
Cite this article
Aldersley, M.F., Joshi, P.C. & Huang, Y. The Comparison of Hydrochloric Acid and Phosphoric Acid Treatments in the Preparation of Montmorillonite Catalysts for RNA Synthesis. Orig Life Evol Biosph 47, 297–304 (2017). https://doi.org/10.1007/s11084-017-9533-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-017-9533-6