Abstract
There is some evidence that the serotonin receptor subtype 7 (5-HT7) could be new therapeutic target for neuroprotection. The aim of this study was to compare the neuroprotective and neurite outgrowth potential of new 5-HT7 receptor agonists (AH-494, AGH-238, AGH-194) with 5-CT (5-carboxyamidotryptamine) in human neuroblastoma SH-SY5Y cells. The results revealed that 5-HT7 mRNA expression was significantly higher in retinoic acid (RA)-differentiated cells when compared to undifferentiated ones and it was higher in cell cultured in neuroblastoma experimental medium (DMEM) compared to those placed in neuronal (NB) medium. Furthermore, the safety profile of compounds was favorable for all tested compounds at concentration used for neuroprotection evaluation (up to 1 μM), whereas at higher concentrations (above 10 μM) the one of the tested compounds, AGH-194 appeared to be cytotoxic. While we observed relatively modest protective effects of 5-CT and AH-494 in UN-SH-SY5Y cells cultured in DMEM, in UN-SH-SY5Y cells cultured in NB medium we found a significant reduction of H2O2-evoked cell damage by all tested 5-HT7 agonists. However, 5-HT7-mediated neuroprotection was not associated with inhibition of caspase-3 activity and was not observed in RA-SH-SY5Y cells exposed to H2O2. Furthermore, none of the tested 5-HT7 agonists altered the damage induced by 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenylpyridinium ion (MPP +) and doxorubicin (Dox) in UN- and RA-SH-SY5Y cells cultured in NB. Finally we showed a stimulating effect of AH-494 and AGH-194 on neurite outgrowth. The obtained results provide insight into neuroprotective and neurite outgrowth potential of new 5-HT7 agonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases (ND) are becoming an increasingly serious medical and economical issue [1]. Lack of available clinical treatments and high prevalence of ND like Alzheimer’s disease, Parkinson’s disease (PD), Huntington’s disease, multiple sclerosis and amyotrophic lateral sclerosis creates an urgent need for therapies which could attenuate or stop the disease progression [2, 3]. Although the etiology of ND differs, pathomechanisms like mitochondrial dysfunction, neuroinflammation, excitotoxicity, excessive oxidative stress and dysregulation of cell death pathways are common among them [4,5,6,7]. Despite years of research, the scientist are still trying to discover potential therapeutic targets. In this context 5-HT7 (serotonin type 7 receptor) seems to be an interesting target for novel therapies. 5-HT7 belongs to rhodopsin-like transmembrane G-protein coupled receptors (GPCRs) and was discovered as the last one from the family of serotonin receptors in 1993 [8]. It is widely expressed throughout the whole brain with the highest expression found in thalamus, hypothalamus, hippocampus and amygdala [9, 10]. The mRNA for this receptor was present also in raphe nucleus, caudate nucleus, putamen as well as in substantia nigra. Moreover, neurons, as well as astrocytes and microglia express this receptor [11]. All the splice variants of this serotonin receptor, coupled with Gs protein connected with stimulation of cAMP production, show high level of constitutive activity [12, 13]. By acting on Gs protein, adenyl cyclase (AC) is activated which results in rapid increase of intercellular cAMP level. This secondary messenger acts on protein kinase A (PKA) and causes phosphorylation of downstream effector proteins like extracellular signal-regulated kinases (ERK) and protein kinase B (Akt) [14, 15]. The signaling pathways activated by stimulation of 5-HT7 like protein kinase A (PKA), extracellular signal-regulated kinases (ERK) and protein kinase B (Akt) play a crucial role in maintaining proper neuron function, support the establishment of neural networks and mediate neuroprotection [12, 16]. In addition, 5-HT7 receptors are coupled to Gα12 (G protein subunit alpha 12) activating the Rho-GEFs-Ras homolog family-Guanine Nucleotide Exchange Factor signaling and Cdc42 (Cell division control protein 42 homolog), which could significantly influence the cytoskeletal architecture [14, 17]. There are reports showing that Cdc42 activation by 5-HT7 receptor is associated with stimulation of neurites elongation in cortical, striatal and hippocampal primary cell cultures [18, 19]. Additionally, both of G-protein mediated pathways could have an impact on tyrosine receptor kinase B (TrkB) receptor expression, which has been associated with 5-HT7-mediated neuroprotective effects [20]. Serotonin (5-HT), through activation of 5-HT7, can evoke anti-inflammatory and anti-apoptotic effects. That effect has been captured as a decrease in caspase–3 and caspase–9 gene expression and was reduced after the addition of 5-HT7 antagonist, SB269970 [21]. Despite extensive studies, the full biological role of this receptor still remains unclear. The effects of 5-HT7 action has been studied using few, non-selective tool compounds, e.g.: LP-211, LP-44, LP-12, AS-19, E-55888, RA-7, 5-CT, 8-OH-DPAT [17, 22, 23]. 5-HT7 has been shown to exert a modulatory role in depression, anxiety, schizophrenia, sleep disruptions and obsessive–compulsive disorder [9, 11, 24]. It has been suggested, that 5-HT7 activation plays a role in neuroprotection and could increase the viability of neurons as well as have a beneficial role on neurite elongation and synaptogenesis [11, 21, 25,26,27]. Nevertheless, because of low selectivity, low metabolic stability and weak blood–brain barrier permeability, the search for applicable compounds is still ongoing [22, 24]. A recently emerged series of indole-imidazoles, weakly basic 5-HT7 agonists have been shown to exhibit high affinity and selectivity for 5-HT7, as well as high water solubility. Chosen compounds, such as AH-494 or AGH-192 exhibited favorable ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) properties [22, 28, 29].
In this study we compared the neuroprotective and neurite outgrowth potential of known (AH-494—compound 15, AGH-238—compound 37) [29], and previously unpublished (AGH-194) 5-HT7 agonists with 5-carboxyamidotryptamine (5-CT) (Table 1). 5-CT is a non-selective 5-HT7 agonist, showing high affinity to 5-HT1A [17, 30, 31]. Despite their tumor origin, human neuroblastoma SH-SY5Y cells are widely used in neurotoxicity and neuroprotection studies as a reliable and cost effective neuronal-like screening platform [32, 33]. Because these cells have dopaminergic phenotype independent of their state of differentiation, they are a common cellular model to study molecular mechanisms and new possible treatments of PD [34, Full size image
The Impact of Differentiation and the Type of Experimental Medium on 5-HT7 Receptor mRNA Expression in SH-SY5Y Cells
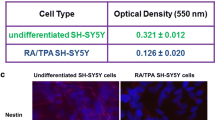
The RA-SH-SY5Y cells incubated in DMEM medium showed a significantly higher level of mRNA expression for the 5-HT7 receptor than the cells maintained in NB. However, UN-SH-SY5Y showed no significant difference in mRNA expression depending on the type of experimental medium used. Furthermore, in case of cells cultured in DMEM there was significantly higher level of HTR7 expression in RA-SH-SY5Y compared to UN-SH-SY5Y (Fig. 1B). Additionally for UN- and RA-SH-SY5Y incubated in the NB medium the additional statistical analysis was carried out using the t-student test, which showed a significant increase in HTR7 expression in RA-SH-SY5Y cells, when compared UN-SH-SY5Y cells (p < 0.01).
Evaluation of 5-HT7 Agonists Biosafety Profile in UN-SH-SY5Y Cells Cultured in DMEM and NB Experimental Medium
The incubation of UN-SH-SY5Y cells maintained in DMEM medium for 24 as 48 h with 5-CT and AH-494 in wide range of concentrations (0.1–80 µM) did not affect cell viability and cytotoxicity when compared to control groups (Figs. 2A, B, 3A, B). AGH-238 at concentration 20 µM showed an increase in cell viability after 24 h (~ 20%) and 48 h (~ 30%) (Fig. 2C), and in LDH release test this compound at concentration 80 μM after 48 h evoked a significant (~ 30%) increase in cytotoxicity level (Fig. 3C). For AGH-194 we observed a concentration- and time-dependent decrease in cell viability (~ 30–100%) with maximum damage of cells evoked by its higher concentrations (40 and 80 μM) (Fig. 2D). Moreover, for concentrations 20 and 40 µM of AGH-194 there was a greater decrease in cell viability after 48 h compared to 24 h. In the cytotoxicity assay an increase in LDH release was observed for concentrations 40 and 80 µM (~ 2–4- times) after 24 h incubation but also for concentrations 10–80 µM (~ 1.5–3 times) after 48 h. In addition for 20 and 40 µM of AGH-194 there was a significant increase in LDH release after 48 h compared to the level obtained after 24 h. Whereas in the case of 80 µM higher level of released LDH was observed after 24 h than after 48 h (Fig. 3D).
Biosafety profile of 5-HT7 agonists in UN-SH-SY5Y in the DMEM experimental medium. Cells were incubated for 24 and 48 h with 5-CT, AH-494, AGH-238 and AGH-194 at concentration range of 0.1–80 µM. Viability of cells was measured with MTT reduction. Results were normalized to control group and are presented as a mean ± S.E.M. Data from 3–6 independent experiments were analyzed two-way ANOVA and Duncan’s posthoc test. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control groups of particular time points; #p < 0.05 and ###p < 0.001 24 h vs. 48 h for particular concentrations
Biosafety profile of 5-HT7 agonists in UN-SH-SY5Y in the DMEM experimental medium. Cells were incubated for 24 and 48 h with 5-CT, AH-494, AGH-238 and AGH-194 at concentration range of 0.1–80 µM. Cytotoxicity was measured by LDH release assay. Results were normalized to control group and are presented as a mean ± S.E.M. Data from 3–6 independent experiments were analyzed two-way ANOVA and Duncan’s posthoc test. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control groups of particular time points; #p < 0.05 and ###p < 0.001 24 h vs. 48 h for particular concentrations
Twenty four hours incubation of UN-SH-SY5Y cells maintained in NB medium with 5-CT, AGH-238 and AH-494 at concentration range 1–20 µM did not affect cell viability (Table 2). However, AGH-194 at concentration of 20 μM evoked a significant decrease of cell viability at (~ 20%) (Table 2). An additional statistical analysis by t-test for AGH-194 at concentration 20 μM between cells maintained in DMEM and NB experimental medium revealed no significant differences between tested groups (p = 0.477).
The Effect of 5-HT7 Agonists on SH-SY5Y Cell Proliferation
We did not observe any changes in cell proliferation rate measured by MTT reduction test after 24, 48 and 72 h of incubation of SH-SY5Y cells with 5-CT and AH-494 in wide range of concentrations (0.001–80 µM) in DMEM cell culture medium containing 10% FBS (Figs. 4A and B). However incubation with AGH-238 at concentration of 40 µM showed a transient increase in cell viability (~ 25%) after 48 h (Fig. 4C). This effect was significantly different when compared to 24 or 72 h time points. The same compound at concentration of 80 µM after 24 h induced a transient viability decrease (~ 30%), and that effect disappeared after longer incubation times (48 and 72 h) (Fig. 4C). AGH-194 evoked a time- and a concentration-dependent reduction in cell viability (Fig. 4D). Maximal cell damage was observed for concentration 80 µM after 24 h and this effect was similar after 48 h and 72 h. Concentrations 10–40 µM of AGH-194 evoked a concentration-dependent decrease in cell viability (~ 20–60%) after 48 h and these effects were significantly higher to relevant concentrations at 24 h time point. We noticed that AGH-194 at concentration 40 µM induced significantly higher reduction in cell viability (~ 20%) after 72 h when compared to 48 h (Fig. 4D).
The effect of 5-HT7 agonists on SH-SY5Y cell proliferation. The cells were incubated for 24, 48 and 72 h with 5-CT, AH-494, AGH-238 and AGH-194 at concentration range of 0.001–80 µM in DMEM culture medium containing 10% FBS. Cell proliferation was measured indirectly by MTT reduction test. Results were normalized as a percentage of control and are presented as a mean ± S.E.M. Data from 3–6 independent experiments were analyzed by two-way ANOVA and Duncan’s posthoc test. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control groups of particular time points; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. indicated time points for particular concentrations
Evaluation of Neuroprotective Potential of 5-HT7 Agonists Against Cell Damage Induced by H2O2 in SH-SY5Y Cells Cultured in DMEM and NB Experimental Medium
While UN-SH-SY5Y cells were incubated with H2O2 (375 μM) in DMEM experimental medium, the damage factor induced about 50% cell viability decrease as well over 3.5-fold increase in LDH release when compared to control group. These effects were significantly decreased by antioxidant NAC (Table S1). In UN-SH-SY5Y cells cultured in NB experimental medium we found that H2O2 (150 μM) evoked around 30% decrease in cell viability and about fourfold increase in LDH level which was significantly decreased by NAC (Table S1). Similar observations were done for RA-SH-SY5Y cells cultured in NB experimental medium where H2O2 (200 µM) evoked about 35% decrease in cell viability and fourfold increase in LDH level which in the former assay were significantly reversed by NAC (Table S2). The biochemical results for UN- and RA-SH-SY5Y cells incubated with DMEM or NB medium and treated for 24 h with H2O2 and NAC was also confirmed at morphological level using DIC light microscopy method. After treatment of cells with H2O2 we observed an increased number of rounded cells without protrusions with weaker adhesion to the surface which were prevented by NAC (Fig. S1).
In UN-SH-SY5Y cells incubated in DMEM medium 5-CT at concentrations of 0.01–1 µM did not affect the H2O2-induced decrease in cell viability (Fig. 5A), however in cytotoxicity test, concentrations of 0.1 and 1 µM of this compound significantly decreased the level of LDH induced by H2O2 (Fig. 5B). AH-494 at concentrations of 0.01–1 µM showed small but statistically significant increase (~ 10%) in cell viability when compared to the H2O2-treated cells (Fig. 5C), whereas in cytotoxicity assay we did not observe any protective effects for AH-494 (Fig. 5D). The incubation of cells with AGH-238 as well as AGH-194 at concentrations of 0.01–1 µM did not significantly affect the extent of cell damage evoked by H2O2 in both used screening assays (WST-1 and LDH release tests). (Figs. 5E–H).
Neuroprotective effects of 5-HT7 agonists against the H2O2-inducedcell damage in UN-SH-SY5Y cells cultured in DMEM experimental medium. The cells were pre-treated for 30 min with 5-CT, AH-494, AGH-238 and AGH-194 at concentration range of 0.01–1 µM followed by 24 h treatment with H2O2 (375 µM). Cell viability and cytotoxicity were measured by WST-1 and LDH release assays, respectively. Results were normalized to control group and are presented as a mean ± S.E.M. Data from 4–5 independent experiments were analyzed by one-way ANOVA and Duncan’s posthoc test. ***p < 0.001 vs. control group; #p < 0.05 and ###p < 0.001 vs. H2O2 group
5-CT at concentrations of 0.01 and 0.1 µM significantly increased (9–11%) UN-SH-SY5Y cell viability cultured in NB medium when compared to H2O2 group (Fig. 6A). The same compound at concentrations of 0.01–1 µM decreased level of H2O2-stimulated LDH release about 68–110% (Fig. 6B). AH-494 at concentration of 0.01 µM significantly increased (~ 10%) cell viability as well as decreased amount of released LDH (94–57%) in comparison to results from H2O2 group (Figs. 6C–D). For compound AGH-238 at concentration of 1 µM an significant increase in cell viability was observed (Fig. 6E) as also decrease in cytotoxicity for concentrations of 0.01–1 µM (64–105%) when compared to the H2O2 treated group (Fig. 6F). AGH-194 at the level of cell viability assessment did not affect the extend of cell damage evoked by H2O2 (Fig. 6G), although in cytotoxicity assay, at concentration of 0.1 µM it partially attenuated the H2O2-evoked increase in LDH release (Fig. 6H). Neuroprotective effects of 5-HT7 agonists found in biochemical assays were confirmed also at morphological level (Fig. 7) where we observed partial improvement of cell morphology after treatment with 0.1 µM 5-CT, AH-494, AGH-238 or AGH-194 in comparison to the H2O2 group.
Neuroprotective effects of 5-HT7 agonists against the H2O2-inducedcell damage in UN-SH-SY5Y cells cultured in NB experimental medium. The cells were pre-treated for 30 min with 5-CT, AH-494, AGH-238 and AGH-194 at concentration range of 0.01–1 µM followed by 24 h treatment with H2O2 (150 µM). Cell viability and cell cytotoxicity were measured with WST-1 and LDH release assays, respectively. Results were normalized to control group and are presented as a mean ± S.E.M. Data from 6 to 9 independent experiments were analyzed by one-way ANOVA and Duncan’s posthoc test. ***p < 0.001 vs. control group; ##p < 0.01 and ###p < 0.001 vs. H2O2 group
In RA-SH-SY5Y cells cultured in NB medium we did not observe any protective effects of 5-CT, AH-494, AGH-238 and AGH-194 at concentrations of 0.01–1 µM against the cell damage induced by H2O2 (200 µM) as confirmed by cell viability and cytotoxicity assays (Table 3). Furthermore, in the LDH release test there was a significant increase of cytotoxicity evoked by AGH-238 at concentration of 1 µM when compared to H2O2 group (Table 3).
Effects of 5-HT7 Agonists on H2O2-Induced Caspase–3 Activity in UN-SH-SY5Y Cells Cultured in NB Experimental Medium
In order to explore some of mechanisms which could be engaged in 5-HT7-mediated neuroprotection against the H2O2-evoked cell damage, we measured activity of the main apoptotic executor protease, caspase-3 [38]. Incubation of UN-SH-SY5Y cells cultured in NB experimental medium for 9 h with H2O2 (150 µM) induced approximately fourfold increase in caspase–3 activity which was fully inhibited by caspase–3 inhibitor, Ac-DEVD-CHO (20 µM) but not by any of the tested 5-HT7 agonists (Fig. 8).
The impact of 5-HT7 agonists on caspase–3 activity induced by H2O2 in UN-SH-SY5Y cells cultured in NB culture medium. The cells were pre-treated for 30 min with 5-CT, AH-494 and AGH-194 at concentrations of 0.01 and 0.1 µM and with AGH-238 at concentrations of 0.1 and 1 µM followed by 9 h of treatment with H2O2 (150 µM). Caspase-3 inhibitor, Ac-DEVD-CHO (20 µM) was used as positive control for the assay. Results were normalized to control and are presented as a mean ± S.E.M. Data from 2–6 independent experiments were analyzed by one-way ANOVA and Duncan’s posthoc test. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control group; ###p < 0.001 vs. H2O2 group
Evaluation of 5-HT7 Agonists Neuroprotective Potential in Other Cells Damage Models in UN- and RA-SH-SY5Y Cultured in NB Experimental Medium
In order to verify a neuroprotective action of 5-HT7 agonist in the PD cellular model we incubated UN-SH-SY5Y cells with 6-OHDA (75 µM) in NB medium which induced approximately 55% decrease of viability in comparison to control group, and this effect was significantly attenuated by NAC (Table S2). In case of RA-SH-SY5Y cells after 24 h of treatment with 6-OHDA (150 µM) 35% decrease of cell viability was observed and this effect was also significantly reversed by NAC (Table S2).
Twenty four hours of treatment of UN-SH-SY5Y cells cultured in NB medium with 5-CT, AH-494, AGH-238 and AGH-194 at concentrations of 0.01–1 µM and neurotoxin 6-OHDA did not change the cell viability (Figs. 9A, C, E, G) and cytotoxicity (Figs. 9B, D, F, H). Similar results we observed in RA-SH-SY5Y cells in the same experimental medium (NB) where 5-HT7 agonists did not show any protection against the 6-OHDA-induced cell damage (Table S3).
The effects of 5-HT7 agonists against the 6-OHDA-evoked cell damage in UN-SH-SY5Y cells cultured in NB experimental medium. Cells were incubated for 30 min with 5-CT, AH-494, AGH-238 and AGH-194 at concentration range of 0.01–1 µM followed by 24 h exposure to 6-OHDA (75 µM). Cell viability and cell cytotoxicity were measured with WST-1 and LDH release assays, respectively. Results of absorbance measurement for each group were normalized to control and presented as a mean ± S.E.M of 6–8 independent experiments. Data were analyzed by one-way ANOVA and Duncan’s post hoc test. ***p < 0.001 vs. control;
Forty eight hours of treatment of UN-SH-SY5Y cells with MPP + (1 mM) evoked about 50% reduction in cell viability which was not changed by 5-HT7 agonists at any tested concentration (0.01–1 µM) (Table S4). Similar results we found in RA-SH-SY5Y cells where 5-HT7 agonist did not show any protection against the MPP +−induced cell damage (Table S4).
Twenty four hours treatment of UN- and RA-SH-SY5Y cells in NB experimental medium with Dox (1 and 2 µM for UN- and RA-SH-SY5Y, respectively) evoked approximately 40–50% decrease in cell viability as measured by WST-1 test (Table S5). 5-CT, AH-494, AGH-238 and AGH-194 in none of the concentrations used (0.01–1 µM) had a significant effect on UN- and RA-SH-SY5Y cells viability decrease evoked by Dox (Table S5).
The Effects of 5-HT7 Agonists on Neurite Outgrowth in SH-SY5Y Cells
To examine the impact of tested 5-HT7 agonists on neurite outgrowth, the SH-SY5Y cells were treated for 24 and 48 h with 5-CT, AH-494, AGH-238 and AGH-194 at concentrations of 0.01–1 µM. 5-CT and AGH-238 at concentrations of 0.01–1 µM did not significantly affect neurite outgrowth in SH-SY5Y cells when compared to control group at any of tested time points. AH-494 and AGH-194 at concentrations of 0.01 and 0.1 µM after 24 h of incubation significantly increased the neurite length to the level which was achieved by positive control RA (10 µM). After 48 h of treatment we still observed an increased neurite length by RA, but we did not find significant effects for any of tested 5-HT7 agonists. However, some tendency in increase of this parameter could be noted for AH-494 (0.01 µM) and AGH-194 (0.1 and 1 µM) (Fig. 10).
A DIC (Differential Contrast Images) microphotographs of SH-SY5Y cells after 24 h of treatment with AH-494 (0.1 µM). Retinoic acid (RA, 10 µM) was used as positive control for the assay. Neurite outgrowth was measured in ImageJ program in the way which is presented for AH-494 compound (24 h). B The effects of 5-HT7 agonists on neurite outgrowth in SH-SY5Y cells. Cells were incubated for 24 or 48 h with 5-CT, AH-494, AGH-238 and AGH-194 at concentration range of 0.01–1 µM. Retinoic acid (RA, 10 µM) was used as a positive control. Neurite outgrowth was measured in ImageJ program with Simple Neurite Tracer plug-in. Results are expressed as percentage of neurite outgrowth relative to control for each time point and are presented as a mean ± S.E.M. Data for each time point were analyzed separately by one-way ANOVA and Duncan’s post hoc test. *p < 0.05 and **p < 0.01 vs. control group for each time point