Abstract
Since the discovery of NMDA receptor (NMDAR) dependent long-term potentiation (LTP) in the hippocampus, many studies have demonstrated that NMDAR dependent LTP exists throughout central synapses, including those involved in sensory transmission and perception. NMDAR LTP has been reported in spinal cord dorsal horn synapses, anterior cingulate cortex and insular cortex. Behavioral, genetic and pharmacological studies show that inhibiting or reducing NMDAR LTP produced analgesic effects in animal models of chronic pain. Investigation of signalling mechanisms for NMDAR LTP may provide novel targets for future treatment of chronic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hippocampal long-term potentiation (LTP) is a well investigated form of synaptic plasticity in the central nervous system. In the hippocampus, LTP is an activity-dependent form of synaptic plasticity in which increased synaptic transmission follows brief, high-frequency stimulation of input pathways. Three different major types of glutamate receptors have been found in the hippocampus: N-methyl-d-aspartic acid (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) and metabotropic glutamate receptors (mGluRs). While AMPA receptors (AMPARs) mediate normal synaptic transmission, activation of NMDA receptors (NMDARs) is required for the induction of LTP. Pre-treatment with the NMDAR antagonist d-2-amino-5-phosphonopentanoic acid (AP5) prevents the induction of LTP. Subsequent pharmacological and genetic studies have confirmed that NMDAR LTP in the hippocampus play important roles in spatial memory of the hippocampus [1]. New mechanism for the synaptic plasticity in the hippocampus has been cumulatively reported (for example, see [2]). In this article that contributes to the special issue for the discovery of NMDAR LTP by Prof. Graham Collingridge, we will review the roles of NMDAR LTP in pain systems, especially in the condition of chronic pain.
Basic Circuitry of Pain: From Periphery to the Cortex
Glutamate is the fast excitatory transmitter of first sensory synapses. Peripheral noxious stimuli activate nociceptive afferent fibers (Aδ and C fibers) and incoming action potentials trigger a release of glutamate in the spinal dorsal horn. In addition, some neuropeptides such as substance P (SP) and neurokinin A (NKA) are also released in the spinal dorsal horn. Glutamate and neuropeptides activate spinal dorsal horn neurons, including those that send projection terminals to supraspinal structures. Neurons in the thalamus play key roles in relaying these ascending inputs to different cortical area. Among them, anterior cingulate cortex (ACC) and insular cortex (IC) are believed to be critical the unpleasantness of pain. As protective responses to noxious stimuli, endogenous pain modulatory systems are also activated. Descending modulatory systems are biphasic, including descending inhibitory and facilitatory systems. LTP has been reported in synapses located in spinal cord dorsal horn and cortex that are related to pain.
NMDAR LTP in Spinal Dorsal Horn
Glutamate is a major transmitter between primary afferent fibers and spinal dorsal horn neurons, whereas neuropeptides (e.g., SP and NKA) mediate slow excitatory postsynaptic potentials (EPSPs) at synapses between small-myelinated Aδ and unmyelinated C fibers and dorsal horn neurons [3,4,5,6]. Fast excitatory synaptic transmission is mainly mediated by AMPAR and kainate receptor (KAR) [7]. In some synapses (or called silent synapses), synaptic responses can be mediated by pure NMDARs [4].
Unlike with the hippocampus, studies of spinal LTP are limited by the technical difficulty of spinal cord slices and the complexity of the spinal local neuronal network. Electrophysiological experiments using intracellular or whole-cell patch-clamp recordings from the spinal dorsal horn neurons generate some important findings related to spinal LTP. Strong tetanic stimulation (100 Hz, 1 s for three times at 10 s intervals) of the dorsal root induces long-lasting enhancement of synaptic responses to presynaptic stimulation [8, 9]. The enhancement is relatively long-lasting (ranging from 25 to 90 min) and input specific. Postsynaptic depolarization of dorsal horn neurons is critical for the induction of spinal LTP. Pairing postsynaptic depolarization with synaptic activity also induces long-lasting enhancement of synaptic responses. Interestingly, the level of postsynaptic depolarization may be important in determining whether synaptic transmission will be potentiated or depressed. In some experiments, synaptic depression can also be induced by pairing synaptic activity with modest postsynaptic depolarization. The induction of spinal LTP requires activation of NMDARs and/or the SP (NK1) receptors. Activation of NMDARs in spinal dorsal horn neurons leads to increases in intracellular Ca2+ [10]. Pre-treatment of spinal cord slices with an NMDAR antagonist AP5 prevents the induction of LTP. The contribution of neuropeptide SP to spinal LTP may act by enhancing NMDAR mediated currents in spinal dorsal projecting neurons [8]. The intracellular signal pathways of spinal LTP remain to be fully mapped. Evidence from other studies indirectly indicates that several protein kinases may be important for spinal LTP, such as phospholipid-dependent protein kinase C (PKC). Phorbol ester induces long-lasting facilitation of evoked EPSPs or EPSCs (excitatory postsynaptic currents) amplitude to stimulation of presynaptic fibers [11, 12]. One possible mechanism for PKC-dependent spinal LTP is through the recruitment of spinal silent synapses or the insertion of AMPARs. Brain-derived neurotrophic factor (BDNF) can induce NMDAR LTP in spinal dorsal horn via different signaling pathways [13].
Early Genetic Studies of NMDAR GluN2B (Also Known as NR2B) in Pain-Related Cortex
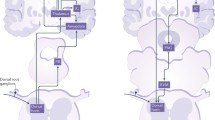
In addition to the spinal cord, early studies suggest that NMDAR in supraspinal structures may contribute to persistent or chronic pain. Direct evidence for the contribution of NMDARs in forebrains to behavioral nociceptive responses comes from genetic studies of NMDAR GluN2B transgenic mice. Tang et al. generated transgenic mice with forebrain-targeted GluN2B overexpression, and the normal developmental change in NMDAR kinetics was reversed [14]. GluN2B subunit expression was observed extensively throughout the cerebral cortex including the ACC and IC. In both the ACC and IC, GluN2B expression was significantly increased, and NMDAR mediated responses were enhanced [15]. Interestingly, while transgenic mice and wild-type mice were indistinguishable in tests of acute nociception, GluN2B transgenic mice exhibited enhanced behavioral responses after peripheral inflammation. These findings provide the first genetic evidence that forebrain NMDARs play a critical role in chronic pain.
NMDAR Dependent Postsynaptic LTP (Post-LTP) in the ACC
Stimulation Protocols
Among sensory-related cortical areas, LTP is well investigated in the ACC. In the ACC slices of adult animals, different stimulation protocols can be used to induce LTP using field recording and whole-cell patch-clamp recording techniques [16, 17]. For field recording from adult rat or mouse ACC slices, glutamatergic synapses in the ACC can undergo LTP in response to theta burst stimulation (TBS), a paradigm more closely to the activity of the ACC neurons. The potentiation lasted for at least 40–120 min [18]. Unlike the hippocampus, strong tetanic stimulation in the ACC did not cause reliable LTP. Whole-cell patch-clamp recordings allow better investigation of synaptic mechanisms for LTP in the ACC [16]. LTP can be induced using three different protocols, including the pairing training protocol, the spike-timing (spike-EPSPs) protocol, and TBS protocol [16, 19]. LTP induced by the pairing protocol is mainly triggered by the activation of NMDARs, but not through L-type voltage-gated calcium channels (L-VGCCs) [16].
NMDAR Dependent ACC LTP
In the ACC, NMDAR containing GluN2A or GluN2B subunits contribute to most NMDAR currents [16]. Bath application of a NMDAR GluN2A antagonist (NVP-AAM077) and GluN2B antagonist (ifenprodil/Ro compounds) produce almost completely blockade of NMDAR mediated EPSCs. Application of NMDAR GluN2A or GluN2B antagonist reduces ACC LTP, without complete abolishment of LTP. LTP is only abolished after the co-application of both inhibitors [16]. It is noted that LTP induced by spike-timing protocol seems to be more sensitive to NMDAR GluN2B blockade as compared with effects on LTP induced by pairing training protocol [16].
NMDAR Dependent Calcium Influx
Calcium (Ca2+) signaling is critical for the induction of NMDAR LTP [16, 20]. Using the two-photon imaging method, it has been shown that NMDARs contribute to postsynaptic Ca2+ signal increases induced by different synaptic stimulation in ACC pyramidal neurons. Furthermore, LTP inducing protocols also triggered postsynaptic Ca2+ influx, which were NMDAR dependent [19]. These studies provide the first direct study of Ca2+ signals in the ACC and demonstrate that NMDARs play important roles in postsynaptic Ca2+ signals (see Fig. 1). As a result of postsynaptic increase of Ca2+, Ca2+ binds to calmodulin (CaM) and leads to activation of Ca2+-stimulated signaling pathways [21]. In support of the role of Ca2+ in LTP, postsynaptic injection of 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) completely blocked the induction of LTP [16]. Furthermore, a study using electroporation of mutant CaM in the ACC suggests that Ca2+ binding sites of CaM are critical for the induction of cingulate LTP [21].
NMDAR dependent LTP in the ACC. A Neurons in the ACC receive sensory afferents from the thalamus. Synaptic LTP is believed to be the key cellular mechanism for chronic pain in the ACC. LTP can be blocked by NMDAR antagonist AP5. B NMDAR mediated postsynaptic calcium signals in the ACC neurons. Representative calcium transient waveforms and average traces of fluorescence changes (ΔF/F) in responsive spines evoked by puff-application of glutamate in the control artificial cerebro-spinal fluid (ACSF), presence of CNQX (20 µM), and AP5 (50 µM) in the ACC, respectively. C ACC microinjection NMDAR antagonists reduced chronic pain. The NR2B receptor antagonists Ro 25-6981 and Ro 63-1908 microinjected into the ACC significantly reduced mechanical allodynia in the CFA injection 3 days mice.
Downstream Intracellular Signaling Pathways
Cyclic AMP (cAMP) signaling pathways are important signaling pathways in biological systems. Among more than 10 adenylyl cyclase (AC) subunits, AC subtype 1 (AC1) and subtype 8 (AC8) are two AC subtypes that respond positively to Ca2+–CaM [ Adenylyl cyclase Anterior cingulate cortex α-Amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid d-2-Amino-5-phosphonopentanoic acid 1,2-Bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid Brain-derived neurotrophic factor Calmodulin Cyclic AMP (cAMP) 6-Cyano-7-nitroquinoxaline-2,3-dione Ca2+ permeable AMPA receptors 3-(R-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid Conditioned taste aversion Excitatory postsynaptic currents Excitatory postsynaptic potentials Insular cortex Kainate receptor Long-term potentiation Metabotropic glutamate receptors 1-Naphthyl acetyl spermine Neurokinin A N-Methyl-d-aspartate Postsynaptic density-95/Discs large/zona occludens-1 Protein kinase C Protein kinase Mζ Paired-pulse facilitation Substance P ζ-Pseudosubstrate inhibitory peptide Bliss TV, Collingridge GL (2013) Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol Brain 6:5 Wang J, Lv X, Wu Y, Xu T, Jiao M, Yang R, Li X, Chen M, Yan Y, Chen C, Dong W, Yang W, Zhuo M, Chen T, Luo J, Qiu S (2018) Postsynaptic RIM1 modulates synaptic function by facilitating membrane delivery of recycling NMDARs in hippocampal neurons. Nat Commun 9:2267 Li P, Zhuo M (2001) Substance P and neurokinin A mediate sensory synaptic transmission in young rat dorsal horn neurons. Brain Res Bull 55:521–531 Li P, Zhuo M (1998) Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature 393:695–698 Li P, Calejesan AA, Zhuo M (1998) ATP P2x receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. J Neurophysiol 80:3356–3360 Yoshimura M, Jessell T (1990) Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol 430:315–335 Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M (1999) Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature 397:161–164 Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J (2003) Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299:1237–1240 Randic M, Jiang MC, Cerne R (1993) Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci 13:5228–5241 MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL (1986) NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 321:519–522 Li P, Kerchner GA, Sala C, Wei F, Huettner JE, Sheng M, Zhuo M (1999) AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nat Neurosci 2:972–977 Hori Y, Endo K, Takahashi T (1996) Long-lasting synaptic facilitation induced by serotonin in superficial dorsal horn neurones of the rat spinal cord. J Physiol 492(Pt 3):867–876 Zhou LJ, Zhong Y, Ren WJ, Li YY, Zhang T, Liu XG (2008) BDNF induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Exp Neurol 212:507–514 Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ (1999) Genetic enhancement of learning and memory in mice. Nature 401:63–69 Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M (2001) Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci 4:164–169 Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M (2005) Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47:859–872 Wei F, Li P, Zhuo M (1999) Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. The J Neurosci 19:9346–9354 Wei F, Qiu CS, Liauw J, Robinson DA, Ho N, Chatila T, Zhuo M (2002) Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci 5:573–579 Li XH, Song Q, Chen T, Zhuo M (2017) Characterization of postsynaptic calcium signals in the pyramidal neurons of anterior cingulate cortex. Mol Pain 13:1744806917719847 Zhuo M (2008) Cortical excitation and chronic pain. Trends Neurosci 31:199–207 Wei F, **a XM, Tang J, Ao H, Ko S, Liauw J, Qiu CS, Zhuo M (2003) Calmodulin regulates synaptic plasticity in the anterior cingulate cortex and behavioral responses: a microelectroporation study in adult rodents. J Neurosci 23:8402–8409 **a M, Sreedharan SP, Bolin DR, Gaufo GO, Goetzl EJ (1997) Novel cyclic peptide agonist of high potency and selectivity for the type II vasoactive intestinal peptide receptor. J Pharmacol Exp Ther 281:629–633 Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, Pineda VV, Xu HM, Chen ZF, Storm DR, Muglia LJ, Zhuo M (2002) Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 36:713–726 Liauw J, Wu LJ, Zhuo M (2005) Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J Neurophysiol 94:878–882 Chen T, O’Den G, Song Q, Koga K, Zhang MM, Zhuo M (2014) Adenylyl cyclase subtype 1 is essential for late-phase long term potentiation and spatial propagation of synaptic responses in the anterior cingulate cortex of adult mice. Mol Pain 10:65 Bliss TV, Collingridge GL, Kaang BK, Zhuo M (2016) Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 17:485–496 Toyoda H, Wu LJ, Zhao MG, Xu H, Zhuo M (2007) Time-dependent postsynaptic AMPA GluR1 receptor recruitment in the cingulate synaptic potentiation. Dev Neurobiol 67:498–509 Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287:2262–2267 Passafaro M, Piech V, Sheng M (2001) Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci 4:917–926 Song Q, Zheng HW, Li XH, Huganir RL, Kuner T, Zhuo M, Chen T (2017) Selective phosphorylation of AMPA receptor contributes to the network of long-term potentiation in the anterior cingulate cortex. J Neurosci 37:8534–8548 Bredt DS, Nicoll RA (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40:361–379 Song I, Huganir RL (2002) Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25:578–588 Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103–126 Carroll FY, Stolle A, Beart PM, Voerste A, Brabet I, Mauler F, Joly C, Antonicek H, Bockaert J, Muller T, Pin JP, Prezeau L (2001) BAY36-7620: a potent non-competitive mGlu1 receptor antagonist with inverse agonist activity. Mol Pharmacol 59:965–973 Toyoda H, Zhao MG, Zhuo M (2006) NMDA receptor-dependent long-term depression in the anterior cingulate cortex. Rev Neurosci 17:403–413 Zhuo M (2016) Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience 338:220–229 Zhuo M (2014) Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B 369:20130146 Liu MG, Kang SJ, Shi TY, Koga K, Zhang MM, Collingridge GL, Kaang BK, Zhuo M (2013) Long-term potentiation of synaptic transmission in the adult mouse insular cortex: multielectrode array recordings. J Neurophysiol 110:505–521 Escobar ML, Alcocer I, Chao V (1998) The NMDA receptor antagonist CPP impairs conditioned taste aversion and insular cortex long-term potentiation in vivo. Brain Res 812:246–251 Mizoguchi N, Fujita S, Koshikawa N, Kobayashi M (2011) Spatiotemporal dynamics of long-term potentiation in rat insular cortex revealed by optical imaging. Neurobiol Learn Mem 96:468–478 Qiu S, Chen T, Koga K, Guo YY, Xu H, Song Q, Wang JJ, Descalzi G, Kaang BK, Luo JH, Zhuo M, Zhao MG (2013) An increase in synaptic NMDA receptors in the insular cortex contributes to neuropathic pain. Sci Signal 6:ra34 Yamanaka M, Matsuura T, Pan H, Zhuo M (2017) Calcium-stimulated adenylyl cyclase subtype 1 (AC1) contributes to LTP in the insular cortex of adult mice. Heliyon 3:e00338 Clem RL, Huganir RL (2010) Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330:1108–1112 Lu W, Chen H, Xue CJ, Wolf ME (1997) Repeated amphetamine administration alters the expression of mRNA for AMPA receptor subunits in rat nucleus accumbens and prefrontal cortex. Synapse 26:269–280 Cooper DM, Crossthwaite AJ (2006) Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci 27:426–431 Cooper DM, Karpen JW, Fagan KA, Mons NE (1998) Ca(2+)-sensitive adenylyl cyclases. Adv Second Messenger Phosphoprot Res 32:23–51 Willis WD (2002) Long-term potentiation in spinothalamic neurons. Brain Res Rev 40:202–214 Robinson DA, Zhuo M (2002) Glutamatergic synapses serve as potential targets for controlling persistent pain. Curr Anaesth Crit Care 13:321–327 Kristensen JD, Svensson B, Gordh T Jr (1992) The NMDA-receptor antagonist CPP abolishes neurogenic ‘wind-up pain’ after intrathecal administration in humans. Pain 51:249–253 Lagraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R (2010) Spinal cord mechanisms mediating behavioral hyperalgesia induced by neurokinin-1 tachykinin receptor activation in the rostral ventromedial medulla. Neuroscience 171:1341–1356 Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M (2005) Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci 25:11107–11116 Guillaud L, Setou M, Hirokawa N (2003) KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci 23:131–140 Wong RW, Setou M, Teng J, Takei Y, Hirokawa N (2002) Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc Natl Acad Sci USA 99:14500–14505 Setou M, Nakagawa T, Seog DH, Hirokawa N (2000) Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 288:1796–1802 Tao YX, Rumbaugh G, Wang GD, Petralia RS, Zhao C, Kauer FW, Tao F, Zhuo M, Wenthold RJ, Raja SN, Huganir RL, Bredt DS, Johns RA (2003) Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci 23:6703–6712 Yang JX, Hua L, Li YQ, Jiang YY, Han D, Liu H, Tang QQ, Yang XN, Yin C, Hao LY, Yu L, Wu P, Shao CJ, Ding HL, Zhang YM, Cao JL (2015) Caveolin-1 in the anterior cingulate cortex modulates chronic neuropathic pain via regulation of NMDA receptor 2B subunit. J Neurosci 35:36–52 Sacktor TC (2012) Memory maintenance by PKMzeta—an evolutionary perspective. Mol Brain 5:31 Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M (2010) Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 330:1400–1404 Han J, Kwon M, Cha M, Tanioka M, Hong SK, Bai SJ, Lee BH (2015) Plasticity-related PKMzeta signaling in the insular cortex is involved in the modulation of neuropathic pain after nerve injury. Neural Plast 2015:601767 Shema R, Sacktor TC, Dudai Y (2007) Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science 317:951–953 Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y (2011) Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science 331:1207–1210 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. Li, XH., Miao, HH. & Zhuo, M. NMDA Receptor Dependent Long-term Potentiation in Chronic Pain.
Neurochem Res 44, 531–538 (2019). https://doi.org/10.1007/s11064-018-2614-8 Received: Revised: Accepted: Published: Issue Date: DOI: https://doi.org/10.1007/s11064-018-2614-8Abbreviations
References
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keywords