Abstract
Background
The current study aimed to investigate the stimulatory effect of beta-adrenergic receptors (β-ARs) on brain derived neurotropic factor (BDNF) and cAMP response element binding protein (CREB).
Methods
Human Müller cells were cultured in low and high glucose conditions. Cells were treated with xamoterol (selective agonist for β1-AR), salmeterol (selective agonist for β2-AR), isoproterenol (β-ARs agonist) and propranolol (β-ARs antagonist), at 20 µM concentration for 24 h. Western Blotting assay was performed for the gene expression analysis. DNA damage was evaluated by TUNEL assay. DCFH-DA assay was used to check the level of reactive oxygen species (ROS). Cytochrome C release was measured by ELISA.
Results
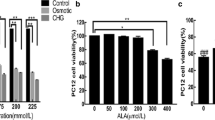
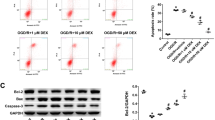
Xamoterol, salmeterol and isoproterenol showed no effect on Caspase-8 but it reduced the apoptosis and increased the expression of BDNF in Müller cells. A significant change in the expression of caspase-3 was observed in cells treated with xamoterol and salmeterol as compared to isoproterenol. Xamoterol, salmeterol and isoproterenol significantly decreased the reactive oxygen species (ROS) when treated for 24 hours. Glucose-induced cytochrome c release was disrupted in Müller cells.
Conclusion
β-ARs, stimulated by agonist play a protective role in hyperglycemic Müller cells, with the suppression of glucose-induced caspase-3 and cytochrome c release. B-Ars may directly mediate the gene expression of BDNF.



Similar content being viewed by others
References
Tan SY, Mei Wong JL, Sim YJ, Wong SS, Mohamed Elhassan SA, Tan SH, Ling Lim GP, Rong Tay NW, Annan NC, Bhattamisra SK, Candasamy M (2019) Type 1 and 2 diabetes mellitus: a review on current treatment approach and gene therapy as potential intervention. Diabetes Metab Syndr 13:364–372
Safi SZ, Qvist R, Kumar S, Batumalaie K, Ismail ISB (2014) Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. BioMed Res Int 2014:1–18
Coucha M, Elshaer SL, Eldahshan WS, Mysona BA, El-Remessy AB (2015) Molecular mechanisms of diabetic retinopathy: potential therapeutic targets. Middle East Afr J Ophthalmol 22:135
Kusner LL, Sarthy VP, Mohr S (2004) Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase: a role in high glucose-induced apoptosis in retinal Müller cells. Invest Ophthalmol Vis Sci 45:1553–1561
Subirada PV, Paz MC, Ridano ME, Lorenc VE, Vaglienti MV, Barcelona PF, Luna JD, Sánchez MC (2018) A journey into the retina: Müller glia commanding survival and death. Eur J Neurosci 47:1429–1443
Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC (2016) Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res 51:1–40
Sorrentino FS, Allkabes M, Salsini G, Perri P (2016) The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci. https://doi.org/10.1016/j.lfs.2016.08.001
De Melo Reis RA, Ventura ALM, Schitine CS, De Mello MCF, De Mello FG (2008) Müller glia as an active compartment modulating nervous activity in the vertebrate retina: Neurotransmitters and trophic factors. Neurochem Res 33:1466–1474
Fitzgerald ME, Vana BA, Reiner A (1990) Evidence for retinal pathology following interruption of neural regulation of choroidal blood flow: Müller cells express GFAP following lesions of the nucleus of Edinger-Westphal in pigeons. Curr Eye Res 9:583–598
Oshitari T, Roy S (2007) Common therapeutic strategies for diabetic retinopathy and glaucoma. Curr Drug Ther 2:224–232
Safi SZ, Noreen M, Imran M, Waheed Y, Imran M, Shah AMUH, Muhammad N (2017) Alteration in ocular blood flow and its effect on the progression of glaucoma. Int Eye Sci 12:394–398
Walker RJ, Steinle JJ (2007) Role of β-adrenergic receptors in inflammatory marker expression in Müller cells. Invest Ophthalmol Vis Sci 48:5276–5281
Safi SZ, Qvist R, Yan GOS, Ismail ISB (2014) Differential expression and role of hyperglycemia induced oxidative stress in epigenetic regulation of β1, β2 and β3-adrenergic receptors in retinal endothelial cells. BMC Genom 7:1–13
Walker RJ, Anderson NM, Jiang Y, Bahouth S, Steinle JJ (2011) Role of β-adrenergic receptor regulation of TNF-α and insulin signaling in retinal Müller cells. Invest Ophthalmol Vis Sci 52:9527–9533
Safi SZ, Shah H, Qvist R, Bindal P, Mansor M, Yan GOS, Ismail ISB (2018) Beta adrenergic receptors stimulation attenuates phosphorylation of NF-κB and IκBα in hyperglycemic endothelial cells. Cell Physiol Biochem 51:1429–1436
Safi SZ, Qvist R, Ong G, Karimian H, Imran M, Shah I (2017) Stimulation of β-adrenergic receptors plays a protective role via increased expression of RAF-1 and PDX-1 in hyperglycemic rat pancreatic islet (RIN-m5F) cells. Arch Med Sci 13:470
Hatefi A, Zare Shahneh A, Ansari Pirsaraie Z, Alizadeh AM, Atashnak MP, Masoudi R, Pio F (2021) The stimulation and inhibition of beta-2 adrenergic receptor on the inflammatory responses of ovary and immune system in the aged laying hens. BMC Vet Res 17:1–13
Walker RJ, Anderson NM, Bahouth S, Steinle JJ (2012) Silencing of insulin receptor substrate-1 increases cell death in retinal Müller cells. Mol Vis 18:271–279
Albert-Garay JS, Riesgo-Escovar JR, Salceda R (2022) High glucose concentrations induce oxidative stress by inhibiting Nrf2 expression in rat Müller retinal cells in vitro. Sci Rep 12:1–12
Dyer MA, Cepko CL (2000) Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci 3:873–880. https://doi.org/10.1038/78774
Aartsen WM, van Cleef KW, Pellissier LP, Hoek RM, Vos RM, Blits B, Ehlert EM, Balaggan KS, Ali RR, Verhaagen J, Wijnholds J (2010) GFAP-driven GFP expression in activated mouse Müller glial cells aligning retinal blood vessels following intravitreal injection of AAV2/6 vectors. PLoS ONE 5:12387. https://doi.org/10.1371/journal.pone.0012387
Muto T, Tien T, Kim D, Sarthy VP, Roy S (2014) High glucose alters Cx43 expression and gap junction intercellular communication in retinal Müller cells: promotes Müller cell and pericyte apoptosis. Invest Ophthalmol Vis Sci 55:4327–4337. https://doi.org/10.1167/iovs.14-14606
Wu M, Yang S, Elliott MH, Fu D, Wilson K, Zhang J, Du M, Chen J, Lyons T (2012) Oxidative and endoplasmic reticulum stresses mediate apoptosis induced by modified LDL in human retinal Müller cells. Invest Ophthalmol Vis Sci 53:4595–4604. https://doi.org/10.1167/iovs.12-9910
Reusch JE, Watson PA, Pugazhenthi S (2006) Disruption of CREB regulated of gene expression in diabetes. Adv Mol Cell Endocrinol 5:211–231
Qi G, Mi Y, Wang Y, Li R, Huang S, Li X, Liu X (2017) Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct 8:4421–4432
Ye Y-L, Zhong K, Liu D-D, Xu J, Pan B-B, Li X, Yu YP, Zhang Q (2017) Huanglian-Jie-du-tang extract ameliorates depression-like behaviors through BDNF-TrkB-CREB pathway in rats with chronic unpredictable stress. Evid Complement Altern Med 2017:7903918
Yego EC, Mohr S (2010) siah-1 Protein is necessary for high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation and cell death in Müller cells. J Biol Chem 285:3181–3190. https://doi.org/10.1074/jbc.M109.083907
Kim ME, Ha TK, Yoon JH, Lee JS (2014) Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Res 34:701–706
Beaudouin J, Liesche C, Aschenbrenner S, Horner M, Eils R (2013) Caspase-8 cleaves its substrates from the plasma membrane upon CD95-induced apoptosis. Cell Death Differ 20:599–610. https://doi.org/10.1038/cdd.2012.156
Moujalled DM, Cook WD, Lluis JM, Khan NR, Ahmed AU, Callus BA, Vaux DL (2012) In mouse embryonic fibroblasts, neither caspase-8 nor cellular FLICE-inhibitory protein (FLIP) is necessary for TNF to activate NF-kappaB, but caspase-8 is required for TNF to cause cell death, and induction of FLIP by NF-kappaB is required to prevent it. Cell Death Differ 19:808–815. https://doi.org/10.1038/cdd.2011.151
Scott FL, Fuchs GJ, Boyd SE, Denault JB, Hawkins CJ, Dequiedt F, Salvesen GS (2008) Caspase-8 cleaves histone deacetylase 7 and abolishes its transcription repressor function. J Biol Chem 283:19499–19510. https://doi.org/10.1074/jbc.M800331200
Liu Y, Li L, Pan N, Gu J, Qiu Z, Cao G, Dou Y, Dong L, Shuai J, Sang A (2021) TNF-α released from retinal Müller cells aggravates retinal pigment epithelium cell apoptosis by upregulating mitophagy during diabetic retinopathy. Biochem Biophys Res Commun 561:143–150
Kriisa M, Sinijarv H, Vaasa A, Enkvist E, Kostenko S, Moens U, Uri A (2014) Inhibition of CREB phosphorylation by conjugates of adenosine analogues and arginine-rich peptides, inhibitors of PKA catalytic subunit. Chembiochem. https://doi.org/10.1002/cbic.201402526
Lee CS, Jang WH, Park M, Jung K, Baek HS, Joo YH, Park YH, Lim KM (2013) A novel adamantyl benzylbenzamide derivative, AP736, suppresses melanogenesis through the inhibition of cAMP-PKA-CREB-activated microphthalmia-associated transcription factor and tyrosinase expression. Exp Dermatol 22:762–764. https://doi.org/10.1111/exd.12248
Yoo JM, Lee BD, Sok DE, Ma JY, Kim MR (2017) Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol 11:592–599
Ashkenazi A (2008) Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov 7:1001–1012
**e W, **e Q, ** M, Huang X, Zhang X, Shao Z, Wen G (2014) The beta-SiC nanowires (~100 nm) induce apoptosis via oxidative stress in mouse osteoblastic cell line MC3T3-E1. Biomed Res Int 2014:312901. https://doi.org/10.1155/2014/312901
Acharya JD, Pande AJ, Joshi SM, Yajnik CS, Ghaskadbi SS (2014) Treatment of hyperglycaemia in newly diagnosed diabetic patients is associated with a reduction in oxidative stress and improvement in beta-cell function. Diabetes Metab Res Rev 30:590–598. https://doi.org/10.1002/dmrr.252
Hsu HC, Chiou JF, Wang YH, Chen CH, Mau SY, Ho CT, Chang PJ, Liu TZ, Chen CH (2013) Folate deficiency triggers an oxidative-nitrosative stress-mediated apoptotic cell death and impedes insulin biosynthesis in RINm5F pancreatic islet beta-cells: relevant to the pathogenesis of diabetes. PLoS ONE 8(11):77931. https://doi.org/10.1371/journal.pone.007793
Safi SZ, Batumalaie K, Qvist R, Mohd Yusof K, Ismail IS (2016) Gelam honey attenuates the oxidative stress-induced inflammatory pathways in pancreatic hamster cells. Evid Complem Altern Med
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, material preparation, data collection and analysis.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Safi, S.Z., Saeed, L., Shah, H. et al. Mechanisms of β-adrenergic receptors agonists in mediating pro and anti-apoptotic pathways in hyperglycemic Müller cells. Mol Biol Rep 49, 9473–9480 (2022). https://doi.org/10.1007/s11033-022-07816-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07816-0